AS
0B-2
Oetober 28.
tr999.
Hearing
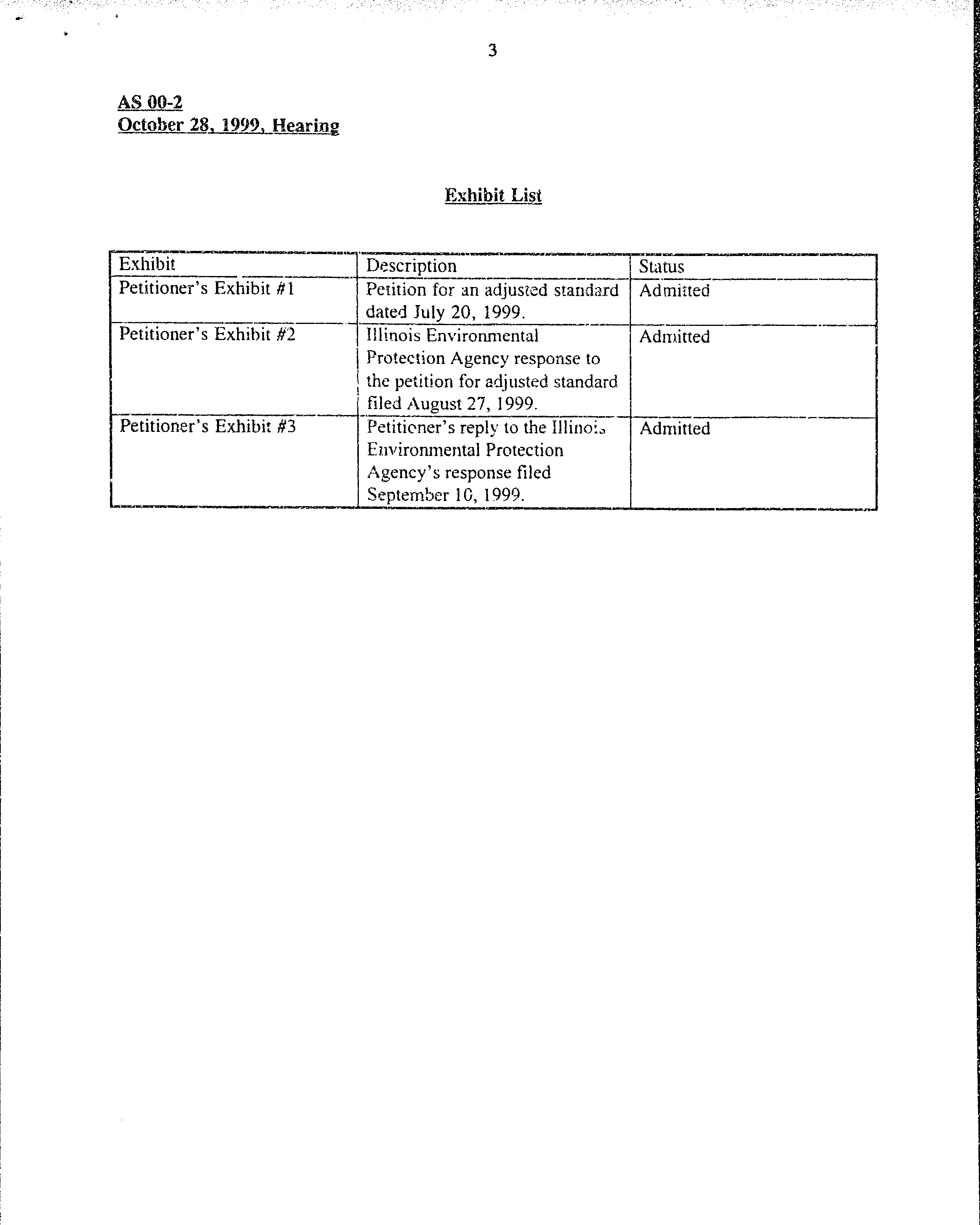
Exhibit
Petitioner's
Exhibit #1
Petitioner's
Petitioner's
Exhibit Lisl
Descriotion
Petition
for en
adjr:sted
stanciard
dated
July 20, 7999.
I
ilinoi
s
Environmental
Protection
Agency response
to
the
petition
for adjusted standard
filed
r\
27, 1999.
Fetitiener's
reply
to
the Illilroi,
Elrvironmental
Protection
/rgency's
response
fi led
September
I C,
1999.
Jr")
#3
j'::
--
-
.':l':::,'1':
.:1
"
4
CERTIFICATION OF SERVICE
it is hereby certified thiat true copies cf the tbregoing order
were
each of
t'e
rollouring
on Ncvember i6. 1999:
first class,
ttr
Paul E.
Gutermann
John N. N'loore
Akin, Gump,
Strauss-
Hauer
&
Feld Larv Offices
cf John
N.
lvloore
i333 New
Hrmpshire Ave,
N,\H.
200
North LaSaile
Street
V/ashington.
IDC 20036
Suite 2200
Chicago,
IL
60601-1095
Peter E. Orlinsky,
Assistant Counsel
Robert T. Lawley,
Chief kgal Counsel
IEPA, Division
of Legal Counsel
lllinois Department
of
Natural Resources
1701
Sourir First Avenue
Suite 600
N{avrvood. iL 60153
524 South Siecond
Street
Room 400
Springfield, n- 62701-11
87
it
is herebv certified
that a
true
cc.p)'
of the
foregoing
order
was hand deiivered to the
follorving on lJovember
16, 1999:
Dorothy Iu{.
Gunn
lilinois
Pollution Control Board
James
R.
'Ihompson
Center
100
W. Randolyrh
St., Ste.
11-500
Chica-eo.
l.llinois
60601
.,,"
/
,,///
.' /
,/
.., L/
\
y'<5lur
Kninle
/
Hearrng Officer
'
Illinois Pollution
Control Board
Janies R. Thompson
Center,
Suite
11-500
100 West Randolph
Street
Chicago, Illinois 60601
312.814.3473
/
."2
PI,.A!NTIFF'S
s .
EXHtBt'r
itr
-,
r
\--
H'-i-
11
'
J
fi=:--J-----
v
;.ii1i
il6TE:
$
R;EL'
x'
iiiJh'-iff
Ex
H
le'ejsrss
PETITIOI{
FOR
AN
ADJUSTE'D
STANDARD
Submitted
to
ihe
ILLINOIS
POLI-T-]TION
CONTROL
BOARD
By
FIORSEI{EADRESOUR'CEDEvELoPh,IENTCotsIPAN\',INC.
Date:
JulY
20,
1999
---
=1:=;
-l
t
I
I
I
;
3
n
g
&
E
E
tE
ln
Ia
Adiusted
Standard
No'
99-
(R.cRA)
$
€
#
ffi
&
&
&
g
w
I
E
fr
E
E
#
fi
fi
g
a
'5^r,+it'v+,+v.+.t
JABLE
OF CO_r\TE_NT'$
NiTRODUCTION
LEGAL BASIS
FOR THIS PETITION
3
A.
The Regulation of Ceneral Applicability.
B.
Reasons and Basis
lor
the Adjusted
Standard.
...........5
C.
F{RJ's
Operations
and
Control
Equipment.
....... ......
......6
II
APPLICATIO}.I
OF THE ADruSTED
STANDARD CNTERIi\
DETIONSTRATES'THAT
CZO
IS COMMODITY-LIKE A}ID
,\joT A
\\'ASTE.
............9
A.
CZO Has
Undergone
SubstantialProcessing.
............... I I
L
Direct feedstock in
the zinc
protluction
process.
.
. ..
.
l3
2.
Dircct
feedstock
for calcining
....... 15
3.
Ineredient in
the
production
of
mieronutrients..............
.
................ l7
4.
Summar\,.
................
l8
B.
CZO
Has
Sr:bstantial
Value.
..... ..........
.l8
L
CZQ
is
produced
and
scld
worldwide
as
a
process
substitute for
zinc
concentrates
produced
from rnined
ore............
.................... l9
2.
The economic value
of HRD's
CZO
is
substantial
and
quantifiable
......................21
3.
Summary.
...............22
C.
CZOIs
Si:nilar
toZincConcentratesProduceo
fromMined
Ore............... 23
D.
End Markets
Are Guaranteed
for
CZO.
...................24
E.
CZO
is
Handied
to Minimize
or Eliminate
Loss........
.................2d
l.
Handling
of
CZO from
production
through
ort'-site
shipment....
16
HE
HE
HI
H!
g1
m
ts
2.
l.
i
2.
Handling
during
processing
into
ziric
metal'
.27
3.Handlingduringprocessingintronticiorrutrientingredient......,.'...21
Other
Relevant
Factors
"""""2E
i.
The
Big
Rivcr
Zirrc
adjusted
standard
for
crude
zinc
oxide'
ta
Other
variances
from
the
definition
af
solid
waste'
""""
30
An
adjusted
standard
support's
statutory
resource
recL)very-
and
waste
mininlization
mandates "'-
""""
31
.
.33
CO*-CLUSION
Eu-lsug
I.
Facilin'
Process
florv
diagram'
3
HT\{R
Feedstock
1998
monthiy
composites'
i CZO
1998
monthlS'comPosites'
,1
ZCrl,',\lonaca,
Pennsylvania
process
florv
diagram'
5.
Excerpr
from
Pehlke,
unit
Praqtiqes
oGx3lac-tiye
Melalluigy'
5
Zinc
Calcine
1998
monthly
composites'
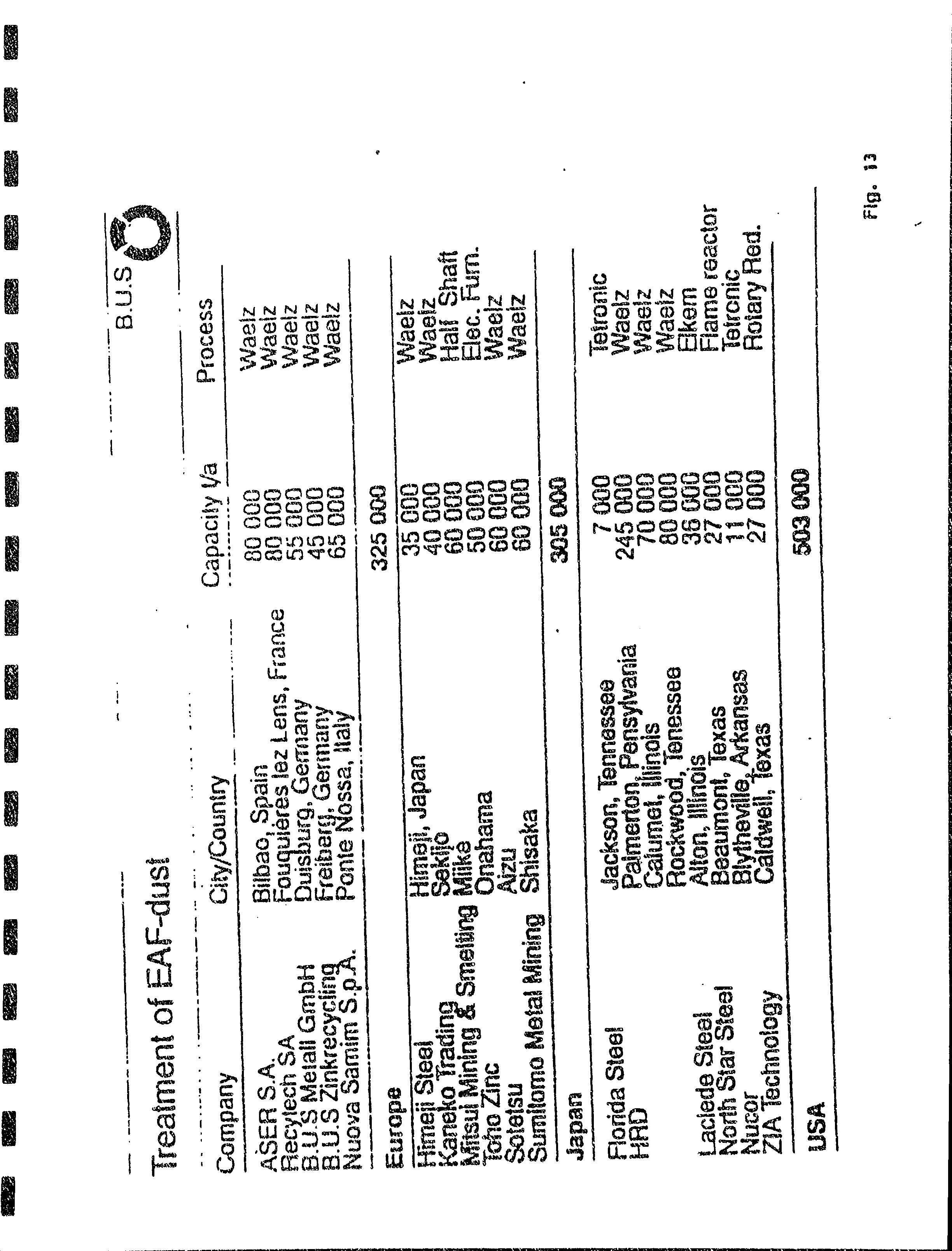
7.(a)SummaqyofEAFdustprocessin-gcapac.itiesinEurope,Japar,,,aridtheUnitedStates,and
iui
r-*n.r
frbm
Ling
Wcng
lo
Tom
Theobaid'
8. HRD
1998
invoices
to
Zinc
i'lacional
for
sales
of
CZO'
g.
F{RD
1998
invoices
to
zinc
corporation
of
Anrerica
for
sales
of
cZo'
10.
Typical
Mined
Zinc
ConceRtrate
Assays'
H
ffi
B
ffi
n
ffi
I I
. Opinions
an<J
Orders
of
the
lllinois
Pollution
Control
Board
in
In
re
Petjtiori
of
Big
-Ri"'gt
Zinc
Corpcrati0I
1999,
amended
MaY
5,
1999),
AS
99-3'
a
A
il
Tertnessee
Depanntcrtt
oiErtvirorirttent and
Consen'ation decision
granting
rariance
from
ciassificaiion
as a solid
\\aste
to AlteriS,eel Dust
Processing
Di',ision
lor
cn-rde
zinc
oxide
ffi
(Sept
I l.
lees)
l3 Ercerpts
front
"A
Pocket
Guide
to Zinc" and
related
irrforrnation
provided
by
the
ffi
lniernarionai Zinc
Associarion.
a
B
g
fr
F
n
g
F
a
a
n
a
a
il
w
*
n
a
E
&
fr
ffi
a
fi
fr
a
I
fr
w
a
n
n
n
BEFORE THE
ILLINOIS
POLLI.''TION
CO.\TROL
BOARD
Ni THE
}l.{TTER
OF:
PETITIOn*
CF I{CIRSEI{EAp
RESOURCE
DEITELOPIIENT
COTTFANY,
IHC.
FOR AN
ADJUSTtrD
STANDA,RD
UT,{DER, 35 II,L.
ADlt.
CODE
720,131(c)
AS 99-_.__
{Adjusted
StanCard
-
RCRA)
PE'TITION
FOR
4_N
ADJUSTE]|}
SJANDARD
lNlRSDllCrrOry
Horsehead
Resource
Development
Company, Inc.
("[{RD")
herebl,petitions
rhe Illinois
Pollution
Control Board
("Board")
foran
adjusted
standarci
under
35
lll. Adm.
Code
720.131(c)
for
crude
zinc
oxide
C'CZO")
produced
by HRD
at
its
Chicago
facilitv
(hereinafter
referred
ro as
the
"Facility.'')
SectionT?0.131(c)
authorizes parties
lo
petition
for
a derermination
rhar
"[m]aterials
that
have
been reclaimed
but
must
be
reclaimed
lurther
before
recoverv
is
comalered
are not solid
r'.'astes
if, after
initial reclamation,
tbe
resulting
material
is
coarmodity-tike."
I-[tD's
CZO,
like
C7O produced
elsewhere in
ihe Unite<i
States and in
orher countries,
has
substantial economic value
and
is
sold
for valuable
ccnsirJeration
in
lnarkets rvorldrvide.
it is
used
to
p:'oduce
zirrc
and
other
nretal
products,
often as a direct
process
subsritute
for
zinc
concentrates
pioduced
from
mined
ore. HRD
produces
CZO
from
the recycling
of
electric
arc
furnace
("E41=";
riust,
a hazardous
waste,
along with
significantly
srna!ler
quantiries
of
other
metal
bearing
feedstocks,
in
FIRD's
high
temperature
metal
recovery ("HTMR") process.
Recyciing EA-p
dust
results
in
multiple
environmental
benefits, inclucling
a
reduition
in
the
volume
of EAF dust
that
cthenvise
rvould
be
rvastefully
disposecl
of in landfitls,
consen.arion
of
non-rene\\'able
natural
resources
(e.g.,
zinc
clre), and
saving
energy
by'reducing tlre rieed
tor
:ffi,ffi:
;tocks,
orzi'c
including
ores
cZo
virgin
rhererore
zinc
ores,
is
preferabre
and
irs
r
ro
rnany
other
primary
and
dergf
gpnlgnl.
-'
LJ'
3rr'
tts
use
promotes
susrainable
::-#::;ffi
,:::,1;:'d
sran'ljaid
roiEAF
dusr
zinc
oxide
p:-ocesseci
by
Bis
lee
in
re
Periri^.
^F"'-.- ^,
;,;J:"1;
,Code
720
ti
lic.)
(Aprit
lS,
t
999,
n&end€d
hfay
6,
I
999)"
AS
ard
l-jnder-
35
Ilr
Adn
;::,lllll;s;f
sanaar.,
rfrL^
-
laeilities'
functicn
to
contain
rhe
EAF
similar
dusr
concr:ntrations
zirrc
oxide
processed
by
BRe.
Bosh
;,:
nrareriars
;:.;::"":::naure
are
produced
by
Hrj\{R
zinc
and
other
p;'oducts
Moreo
ver'
czomeets
of
zinc
and
rhe
se*ion
other
constituents,
72o.r3r(c)
ancr
adju.sted
are
used
to
produce
lor
s!n'lilar
reasons
as
the
EAF
zinc
oxide.
Therefore,
rhe
BRZ
adjusred
standard
"randar',
prc'ides
cnteria
a
unguestionably
As
con:pelling
explained
precedential
in
detaii
in
this
basis
Perition,
for
the
Board
czc,
ro
rikethe
granr
EA.r'dust
rhis
Ferition.
zinc
see
oxide
pan
processed
II.F.
r.
of
by
rhis
Bi€,
peririon.
commodity-liI,e
meets
material
the
for
criteria
the
following
in
section
reasons:
720-r3r(c)
for
an
adjusred
srandard
for
a
l.
CZA
is
subsraii
,
o
a ;,
;
" ",i
p,Jj
x,i
i
:r,
i:
"xH
:i
[T
i
ffii:
TJ,1,;;,,T
:
;
l;1
fi
:,
_.,
2.
CZO
has
a
docr
averagemarket
lmented
hislory
of
su'
o"'.it..,
jt,:*::l*f
*,*t'i[T';lffi
';.':ffJ:ilill:,ilJ,ilf;
l,n
3
CZA
issimilar
ifi
comnn.;r;^_ _
co
n
ce
n
r ra
r
e;
;;;il;1,il?:j
;|ff
:
j
eq
u
i'a
t
e
n
t i
n p
r-o
c
es
s
s
u
i
r
a
b
i
r
i
i
l,
r
o
zi
n
c
4
CZO
has
guaranr.eed
erd
markers,
and
all
CZO
produced
b
snrpped
off-site,
inro
rhe
rtr;;;;';'f
.;;'1n'erce,
;",","air,*ri:.xyrj:j:lijl,
r<::
Seo
ts
Periri
etrtiOn
I
T
E
fi
ffi
ffi
ffi
$
tr
ff
I
u
g
ffi
w
E
ffi
a
H
ffi
g
E
F
F
g
H
B
g
a
n
5.
CZO
is
ntanaged
in an environntentally
protecti!'e manner.
6. An adjusted
starrdard
for
CZO
is consistent
rvith
variances for
commodity-like
rnaterials
issueel by the Board
and other
regulatory
agencies, and
supports
the
nrandates
of Illinois and
federal
law
prioritizing
recycling over disposal.
Part i of
this Perition,
rvhich
sets
forth the
legal
basis
for ihis Petition
for an adjusted
standard,
ciescribes the
regulaticn
of
generai
applicability,
states the
reasons and
basis
for
the
adjusred
srandard, and summarizes
HRD's operations
and control
equipment. Part
il of the
Perition
cjemonsrrates that
F{RD's CZC
meets all of
the criteria
in
Section
i20.l3l(c)
t-or
determining
rvhen
a material
is
"commodity-like"
and not a solid
waste. Based
on the
information
conrained
in
this
Petition,
HRD respectfi.rlly
requests
that the
Boai-d
grar:t
HRD an
arJjusted
srandard
froi-n the
definition
of solid
waste for
CZO
produced by
HRD
at
the Facility
T.
LEGAL
BASIS FOR
THIS PETITION.
Section
28.1
of
rhe
IllincisEnvironmental
Protection Act
("Act")
authorizes the Board to
granr an
adjusred standard
frcm a
regulation
of
general
applicability upon
request
of
persons',rho
can
justifl'rhe
adjustecl standard.
4l5ILCS 5/28.1(a).
The regulation
of
general
applicabiliti'
from
n'hich
FIRD seeks
an
adjusted
standard
is 35lll.
Adm.
CoCe
721.102
(definition
of solid
rvaste). .4s explained
in Part
II
of this
Petition, the criteria
to be
used in
justifoing
the adjusted
standard
are established by
Board
regulation.
Secrion
28.1(d)
of
the
Act sets forth tlie basic
procedural
requirements
ior an
adjusted
standard,
and
the
Boaid's
implementing regulations
include more
specific
requirements
applicable
to
RCI(A adjusted standards in
particular. Seg 35lll.
Adm.
Code
106.410
et seq.
Those
regulations
require
the
following
information
to be
provided.
(,a)
Identification of
the regulation of
gerreral
appii:abiiir;'for
which
FIRD
seeks an adiusted standard.
A
*ritten
statenlent
outlining
the scope of the
"evaluation,"
the
nature of,
the
reasons
for and the basis
of tbe
adjusted standard,
consistent
rvith
tlie level
ofjustihcation
contained
in
the
reguiation
cf
general applicability;
The
nature of
i{RD's
opeiations
and control
equipment;
and
Any
additional
information
which
nray be
required
in the
regulation
of
general
applicabil
ity.
5eS
35
lll. Adm. Code
106.413.
HRD addresses
the first three
lactors below.
The fourth
iactcr
isnorapplicablehere,sincethe"regulationcfgeneral
applicability"(35
Ill.Adrn
Code721
102)
does
not require
any such
aclditional
information.
A.
The
Regula(ion
of General
Applicability.
lllinois larv,
like federal
lau,,
classifies
non-prcduct
materials
derived
from
the
reclamarion
or
other
treatment
of
"listed"
hazardous
wastes
as soiid
and hazardous
*'astes.I
Neither
Illinois
larv nor federal
larv, horvever,
regulates as
solid or
hazardous
\\'aste
all
rnaterial
produced
from the reclarnation
of haz,ardotis
waste. Products,
refined
materials,
and other
non'
\\'astes
produced
fiom
the reclamation
oiiisted
hazardous
waste
and that
are
used beneficially'
are
not
solid
or
hazardous
wastes:
"l'{aterials
that
ate
reclairned
lrom solid
wastes
and
that are
used
beneficial!y
are
nolsolid-ivaslg5
and
hence
are not
lrazgtd-Q.us
wastes
ttnder
this
provision
unless
the
reclaimed
material
is
burned
for energy
recovery
or used
in a manner
constituting
disposal."
35lll. ACm. Code
$
721,103(eXl)
(emphasis added)
When
the Ui:ited
Siates
Environmental
Protec{ion
Agency
("U.S,
EP/r")
promulgated the
identical federal equivalcnt of
rhis
rule in 1985,
u.S. EPA explained that
its
purpose
was to
make clear that
fully
reclairned
i
See35lll.Adm.Code72l.l02(c)(3)(cross-referencingcolumnioftlietableirr.\ppendirZ)
and72l
l03(eXl)(lliinois
larv);
ses also
40
C
F
R
S
261
?(c)(3) and
$
?61
3(cX:)(i)
(fedc'rrl
Ia*').
(b)
g
ffi
ffi
ffi
ffi
ffi
E
F
E
E
ffi
ffi
ffi
ffi
H
g
(c)
(d)
w
H
ffi
'#
&
8
&
products
arc
,lot
\\asres,
eyen
if
lhe
produ
conrntercial
vaiue:
..
..."
yruuucis
subsequently
are
r"eflned
to
jncrease
their
fr
ffi
IC]onrnrercial
pr_odu
\\.asres,
ano
so;;;;::t:':clairned
rronr
h
regenera:ed
,u,"*n,r'jlU.;..t
ro
i,iii
i'ltidous
lvastes
are producrs,
nor
*fft*.',ffi/'ffi
lc,
are
not
g
fi
50
Fed.
Reg.
6
14.
634
(lan.
4,t985)
(emphasis
adcled).
B.
Reasons
and
Basis
for
the
Adjuster!
Standard.
As
esplaineel
in
cetail
iater
in
thi's
Fetirion,
lxRD
fundarnentaf,y
transforns
a
lo.,v-zinc.
high-iron
hazardous
ivasre
feedstock
into
a
high-zlnc,
row-ir
on
czoproduct.
cZo
and
other
crude
zinc
oxides;lre
commodities
thar
are
used
and
sord
thror,:ghout
the
rvorld.
As
a
resuir,
FIR'D
has
alr'ays
unciei'stood
that
irs
Cza
isa
fi-r,y
reclaimed
product
and
is
nor
a
nr:nirnary,
pt'ocessed
or
partially
:'claimed
material'
The
Itincis
Environmentaiprotecrion
Agency
("lllinois
EPA"')
has
talien
the
position
r'at
cZo
may
not
be
a
fury
recraimed
produci
and,
therefore,
rvouid
be
subjecr
ro
regulation
as
a
solid
and
hazardous
rvas(e.
Illinois
larv
provides,
hoi'r'e'er'
that
even
a
parriailv
reclaimed
materiar
can
be
excli.rded
from
reguration
as
a
sorid
r*aste
if
ir
is "conmcdity-!ikc."
35
Ir.
Adrn.
code
72o.r3r(c).
Therefore,
to
resor'e
any
question
that
may
exist
regarciing
cZo's
reguratory
status,
FlrD
is
firing
rhis
petition
for
an
adjusted
srandard'3
An
adjusted
-ctandard
for
czowirr
resorve
any
porenrial
regurarory
issue
rhar
nay
exist'
and
it
wi'further
€ncoufage
recyclingof
renervable
natural
resources.
EAF
dusr
v.
r_/rr
oust
and
reduce
deplef
ion
of
non-
fr
g
fr
#
n
r
t
t
I
I
oi
'
The
at
anyrime
filing
of
has
rhis
or:j":l
I
should
not
be
en,
a
soiiJrr;;;;".ue
coDSrflrBd
as
an
adntission
of
lau,or
iacr
rl*r
CZO
is.
,aa
_:
.:.:'
-1
-.
'.-:,:.:'.'
I
.
'
:
1
:
:r:;
.:-T;:::-ili::--..llT::T:::"T::-T-:r-::---:iTTTTT
C.
HRD's
Operations nnd
Control Equipment.
I-IR,D is ihe largest
cperalorof
HTNfi,
faciiities
in
the
Unired
Srares,
and is
the
largesr
recvcler
of
inorganie
hazardous \vastes.
Historically,
foraimost
sixty
years,
the
priorowners
of
ffiD's
"\\'irelz"
rotary
kilns
in Palrnerlon.
Pennsylvania,
operated
the kiins
to produce
zinc-
based products
from
oxidized zinc
oies
and
similal
zinc-bearing
secondar-y
r:rateriais.l
irr
the
nrid'1970s,
as
mirte
reserves
\\'ere
being
depteted,
the
operarcrs
of the Wae!z
kilns
explcred
other
t"aw
mate!-ial
sources
of zinc
for
the
\-Vaelz
kilns,
and
iound
that steelmaking
dusts,
including
E.A.F
dust,
coulci
serve
as an
effective
aiternativc
to the
oxidized zinc
ores.
Zinc
is
an
abundant
cons:ituent
in EAF dust;
its
concentration
ranges
from
five to
forty-trvo p€rcent,
or up
to
eight
times
more
zinc than
in
rarv
ore. Lead
and
cadmiunr
also
are
present
in recoverable
quanlities
in
EAF
dust.
EAF
dust
was processed
lor netal
recovery
in
Palmerlon
before
U.S.
EpA
lisred
rhe
materiai
as K06l in
1980,
and EAf
dust
resource
recovery
efforrs
accelerared
thereafter.
The
reci'cling
of
EAF
dust
in
\\tae!z
kilns
has
served
as a nationat
model
of resource
recovery
and
tvaste
minimization.
Significantiy, lJ.S.
EPA relied
on rhe proven performance
of
HTlr{R
technologies
wherr
it
designated
I-lTlvIR
as
ihe
Best
Demoiisrrared
Available
Technotogy
("BDAT")
for K06l
under
the
Resource
Conservation
and R-ecovery
Act's
("RCRA")
land
disposal
restrictions program.
U.S.
EPA
concluded
that rhc
H';MR piocess
ccnsen,es
natural
resoutces
by recycling
zinc
and
other
metals
recovered
from
the EA.F
dust
that
otherrvise
*'ould
be m!ned,
and recycles
the
K06t
into
non-rvasre
procluas.
53 Fed.
Reg.
31138,
Jl162
(AuE.
lg,
I
rhe
\\'aelzing
process
deri'es
its
name
lrom
the Gernran r.erb.,rr.aelzen
tr'rndle
or roll, aptly
describing
the rolling
movenrent
of rhe feed nraterial
of
the kiln
,"
rrhicir
nlesns
ic
along
the insidr-
slcpe
.a.:-a.:-
ffi
ffi
ffi
ffi
H
H
g
H
fr
ffi
H
H
ffi
H
w
B
ffi
H
w
lgSS).!
EPA
alsg
has
designated
Hfit{R as
the BDAT for othei
metal-bearing
rvastes. Seg l'7
Fed
Reg.37l9.1.312A7
(Aug 18, 1992)(F006);
63Fed Reg
28556
2856C1
(May26,1998)
(rogerhe;rr.ithsrabilization,fornon-lisredrvastes).
LIRD'SFaciliryislocated
at27Ol
ll46St:eet
in
Chicago,
anci
rvas
first
peri''iitiedby the lllinois
EPA
Division
of
Land
to
operate
a solid
tf'a.ite
rnanagernent
faciliry
in 198'9
'l
lre Facility
emptoys
trvs
Waelz kiln
HTlr{R unitst
and accepts
for recy,cling
K061 and smailer
quantities oicther
hazardous
and non-hazardous
z!nc-bearing
feedstocl:s.
h{ore
than
ninety
percent
of
the feedstock
consists
of
EAF dust.
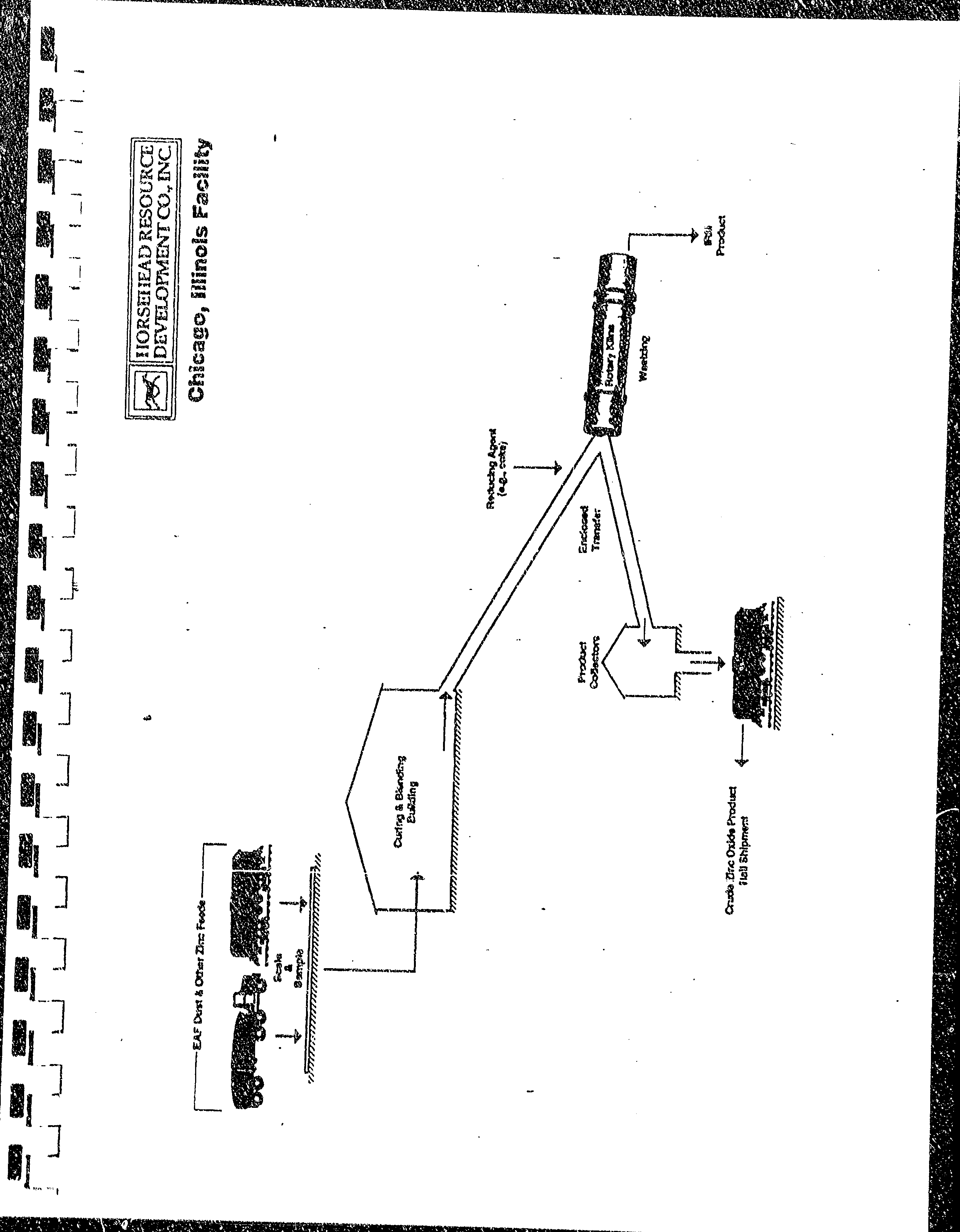
A
process
florv
diagranr
of rhe
Faciliry
is
included as
trrhibit /. Notably,
all
phases
of
I-[RD's feedstock
management
occur
in an enclosed,
negative
pressure
environment,
anC all
nraterial
lransfer
points
are
equipped
rvith
collcction equipment
and
baghouses
to
prevent material
loss and
to
recl'cle the
collect*rJ
maierial.
HRD
receires
EAF dust
arrd
other
zinc-bearing
feedstocks
frorn of'!'-site
by
enclosed
railcar
and
rr.rck.
Up,tn
arrivai at the
faciiity,
tlre
tbedstocks
unclergo
confirmatory
testing
Bnd
sampling.
This
:;ampling and resting consists
of
visual
inspection
for
nonconforming
material,
tests
for radioacrivity
and the collection
of
generator-specific
samplss
for metal
content
analy'sis
The
feeclstocks
are then
untoarled for direct
introduction
into
the recycling
process,
rvithout
J
U.S.
EPA
origina!ly drrsignated
HTMR
as
BDAT
in
the
so-called
"First
Third" rulemaking,
in
which U.S.
EPA also establishcd
rccycling
as the
required
treatment
method
fbr K051.
5i
Fed
Reg.3il38,3ll63(Aug.
17, 1988). Althoughthe{J.S.CourtofAppealsfortheD.C.Circuit
subsequentty
vacated
and
remarrded the
treatment
method
dctermination;API-YJ,SI:PA
906
F.Zd
'l'29
(D.C.
Cir.
1990), the BDAT designation
*,as
not challenged
and, therefore,
was
not an
issue
in the
litigation.
Nelther the rulemaking
follorving
the
remand,
56 Fed.
Reg.
4l161
(Aug
19.
l99i),
nor
the decision
uphotd,ing
the rule,
SMA
V.
U.S.
EPA
2,7
F.3d
642'
(D
C Cir.
199{),
affected
the
BDAT Cesignarion fc,r
K061.
Although
the Li.S.
EPA nrore
recently
also
has
designated
sraL,ilization
as a
BDAT
(along rvith
FITh{R)
for metal-bearing
\\'astes in
general,
the
agency's
findings
regarding l{Tlv'lR's resource
recoveD'benefits
remain tnte
todal'
storagei in tiie
Curing
and
Blending
("C&8")
Bu,:ding
(illinois EPA
approved
rhe
design
o!'ihs
f
ft'B Bu$Snr>
DDlr>aDt
lD l
DEDDi')
)EliliDrr $atE$
Ouaber 1$. t99'r
)
lnn$i>reip
ipa>
unloading
in the
C&B
Bu.lding. feedstocks
sre conoitioned rvith water
to achieve
a moisrure
conient
of approximatelS' l0
percen:,
iareC,
and
blended
before:ransfer
to a
fced hopper
for
transport
to
the HTI'{R
prccessing
area.
These
prepdratoq/
steps achieve
a unifc,rm
feed
composition ficr processing
i,,
rhe HTIvffi.
rrrrits
to achieve
optimal efTiciency.
The
C&B
Bui(ding is "enlcd acd cquip2ed with
collestion
equiprnent
antl a baghoute
to
prevenl
natcr:al
ioss
and lo recycle
the
collected mate:ial.
'the
C&B Building
is operaled
undei-
negarive
pressure
to
pi:event
fugitive
emissions.
The blencieri
zinc-bearing
feedstock is conveyed
l-''y fully enclosed
belt
cunve,r'ors lrom
r.he
C&'$
Builciing
to Ged
bins
that suppii' the
\i/aclz
l:iln
HT,\{R
units.
From
the feed
bins, rhe
feedstock
is metered
in
proper proportion
rvith
a carbon
source, for
example, coke
(added
as
a
reducing agent)
CIilc a eorrtpiereiy eneloted
eoh\re\l6i hnd tianifciied
to
lhe HT\R
untr,
ln rhe
liTN,'{R
reci'cling
process,
a complex
series
of chemical
o;tidation
and reduction
reacrions
concenlrare
the
non-ferrous
metals
of the feedstocks
into
CZO.
The
leedstocks
are
first heated
ro
a
temperature
high enough
(approximately
1200u
C)
to chenrically
reduce
nanferrous
merals
Then,
these constituenls are
reoxidized
in a
countercurrent air srream,
ar,d the resulting
product
is
ccoled and
collected
as
CZO. The HTtvfR
process
also
produces
rhe
lron-Rich
Material ("tIL\f')
prorJuct,
which
is
a
coarse aggregate
The IT{M
is
sold as an asphalt aggregate,
an
iron
source lbr
cement production,
or
as
an
aggregate
for
conltruction
use. The
process produces
no
rvasres
and
no
water
dischargt:s
:
q'O emptoys
t\\'o t)'pes
of I{T}rO(
units:
a) rotaiy
\\'aelz
kilrrs in
Palnrcrrcn.
Pcnnsr.lr.ania,
Chicago,
Illinois,
and
Rockrvood,
Tenrressee,
anC
b) a
flanre
ref,clor
in Bear,rnronr.
Tcras
Ssc
also
40
C.F.R.
0
261 3
(idenrif5'ing
r1,pes
of
HTN,TR
units).
ffi
H
g
H
H
$
6
H
g
B
H
F
H
B
w
ffi
a
B
w
',-12t:,
H:,
fi
a
n
&
a
g
w
a
n
lfr
la
lE
iu
lH
lF
ln
la
IE
CZO
produced
fronr rhe
HTil{R
recl'cling
process is
collected
coniinuously in
product
coliecrors
and
rail car
loading
tanks.
The
eollected
CZO
is
then
transferred
by encioseci
screw
eon\'e),or
to
fi-rlly,-enclosed
pressure
differer:tial
rail cars
for shiprnerlt <1ff-site
U.
APPI-ICATIOiV
GF
HE .ADJUSTED
STANDAR.D
CRITERIA
DEh1OiiSTRATES'THAT
CZO
IS CONN{ODITY-LU<E
AND
NOT A
\\'ASI'E.
Section
720.13!(c)
authorizes
a
delermination
that a
ntaterial
is not
a soiid
rvaste
if the
rnarerial,
aF.er
initial reciamation,
is commodiry-!ike.
Section
720.131(c)
provides
in
full thar:
"l'he
Board
will
determine
that those
materials
that have been
rec!aimed
bur
rnus!
be
reclaimed
further befbre
rccavcry is
completed are
not
solid
\\'aste5
if, after initial
reclanration,
the
resulting material
is
commodity-like
{even
though
it is not
yet
a
commercial
product,
and
has
to
be reclaimed
firrther).
This determination
is based
on
the follorving criteria:
l)
The degree of
processing the
material
has undergone
and the
degree of
further
processing
that
is required,
2)
The
valuc
of the
material
after
it
has been
reclaimed;
3)
The degree
to
r.vhich'.he
reciaimed
material
is like an
arialogous
raw material;
4) J'he extent to
which
an end
marhet
for
the
reclaimed material
is
guaranteeci;
5)
The extent
to
rvhich
the
reclaimed
material
is
handled
to
minimize
loss; arrd
5) Other
relevant
factors."
35 Ill.
Adm. Code
720.131(c);ge
als! id.
720.1,30,720.133
(procedures
for determinations).
(.These
criteria
are identical to
the federal
criteria
for
a
commoclity-like
variance.
Sqe
a0
C.F.R.
$
260.31(c)).
As
ciiscussed in detail
belorv, CZO
unquestionably
meets the
criteria
for
an
acljusred r-tandard
for a commodity-like material
because
CZO:
(i)
is
substantiall;'
reclainred
fronr
hazardous
\\'aste;
(ii)
has
substantial
value,
(iii)
is
a substituie
for
zinc conccntrales
produced
from
mined o:'e;
(iv)
tras
guaranteed
end markets; and
(v)
is
LranCleci
to
elinrinate
or
ntinintize
proCucl
lo s. Orher
relevant
faitors also support an
adjusteri
standard.
One
r,uch
factor
is
the
Board's recentl)'-pronruigated
adjusted
standard for
EAf
zinc
oxiCe
processed
by BRZ. The
BRZ
adjusted stanciard
is conp:lling and favcrable
precedent
because it confirn,s
the
commodit5'-like nature
of CZO.
HRf) dernonstrates
in
this
Petition that
CZO rneets
the
adjusted
standard criieria for substantially
the
same
reasons as the EAF zinc oxide
in
ihe BPJ adjusted
standard.
A
related
factor
is
consistency
with variances
frorn
the definition
of solid
u,aste
promutgated
by other
regulatcry agencies
(including
a
variance issued
b-v
Tennessee
tbr
the
sane
EAF dust
zinc
cxide
inaterialthat
rvas
the basis for the
BRZ
adjusted standard).
/tnother facior
is
encouraging
recycling oflEAF
dust
and the conservation ofncn-renervable resources, thereby
prcmoting
sustainable
developnrerrt.
!
HILD
thereiore
respectfulll' requests that an
adjusted
staniiard
lronr the deflnition
cf solid
waste
for HRD'.s CZO
be
granted.?
Irr the remainder of
this
Petition, HRLr apl
lies
each criterion
for
a.n adjusted
standard
fcr
e comrnodit5,-iike
material to
CZO to
denronstrate that
CZO
is
commodity-like
and noi
a
solid
\i'asre
The
adjr.rsted starrdard regtiiations are substanlively
identical
tc the
federa!
regulations ar
10
C.F.R.
$5
260 30-26C.33.
U.S.
EPA
precedent
therefore
is relevant
to
interpreting
and
appii'ing
the
commodity-like
adjusted
standard criteria,
and HRD
addresses
U.S.
EPA
precedenr
in this Petition
where
appropriate
$ee
Rec:els_teEhnalag,re.9, AS 9?-9,
siip.
op.
at 6
("[T]he
Board
has
referred to
USEPA
preamble
langrr6gg
interpreting
the
federa! counierpart
to
the
E
U.S.
E?A first
promulgated
tlre
grrovisions
on
rvhich
the
Board's
adjus'red standard authority is
based
in
the context
of the redefinition
of solid
wastc. 50 Fcd. Reg 614
(Jan.4,
1985).
One
of
the
principa.l
purpcses
underlying
these
regulations
was promoting
appropriate
rec5'cling,
therebl'
rendering
this factor
"re'levant"
to FIRD's Peiition
for
an
adjusted srandard.
2
in
addition
to
the
BPJ
adjusted
standarrl, rhe
[Joard
has decided trr'<l adjusted stancla;d
petitions
under 9ection
'120.131(c):.ln
re
Pedtiouof Rec],clc'fechnoiogies.
lnc
for
f
djus!_ed
Standard
Under
35
lll
Ari_ln
Code
120
l3l(c)
(Sepr
3, 1998),
AS
97-9;!n
re Petirion
of
ffi
H
H
$
H
H
ffi
ffi
fr
ffi
H
H
H
B
m
tr
ffi
w
ffi
l0
n
E
n
v
a
a
u
n
fr
g
&
g
a
fr
8
fr
F
H
il
Board regulations at
issue.").
U.S. EPr\ clearly' inlended
that the
criteria be applied
te
individua!
nraterials in a conrnron-sense
n'rilnner.
"The
Regional
Administrator
(or
an
authorized
slate)
ma,v
rveigh
these factors as slre sees fit, and
may
rely on any or
alloithem ro
reach
a decision." 50
Fed.
Reg. al655. Even though
not
ail the criteria
nnust
be
relied
on
in
making
a decision, IIRD
demonstrates
that
CZO
rneets each and
every criterion
for
an
adjusted
standard
for a commodity-
iike material.
A.
CZO
IIas
Undergone
Substantial Processing.
The
tlrst
factor to
be
considered is the
degree of
processing
that the material
has
undergone
and
the
degree
of further
processing
that is required. According to the
Board,
the
"more
substantial
the initial
processing,
the
nroie likely the resulting materiai is
to be
commcrdity-like
"
.&cgi:gle-kgb!91ag,ies,
AS 97-9,
slip o?.
ar7
(quoting
50
Fed. Reg ar
655)
HRD
recycles EAF dust
and other rnetal-bearing
feedstocks
in its I{TiMR process
to
produce
CZO
in
a complex
series of chenrical reduction
and oxidation reactions. These reactiorrs
iundamcntally
transforrn relatively
locv-zinc,
high-iron
wastes
that are
incapable
of
being
processed
at a zinc re fineryE
intc
the high-zinc, iow-iron
CZO
product
ihat requires
only'
minimal
additional
processirrg.
The
HTMR
process
also
prodirces
the high-iron
product
iRlr{. The
HT\{R
process
results
in
substantial
processing
for
the
follorvirrg reasons:
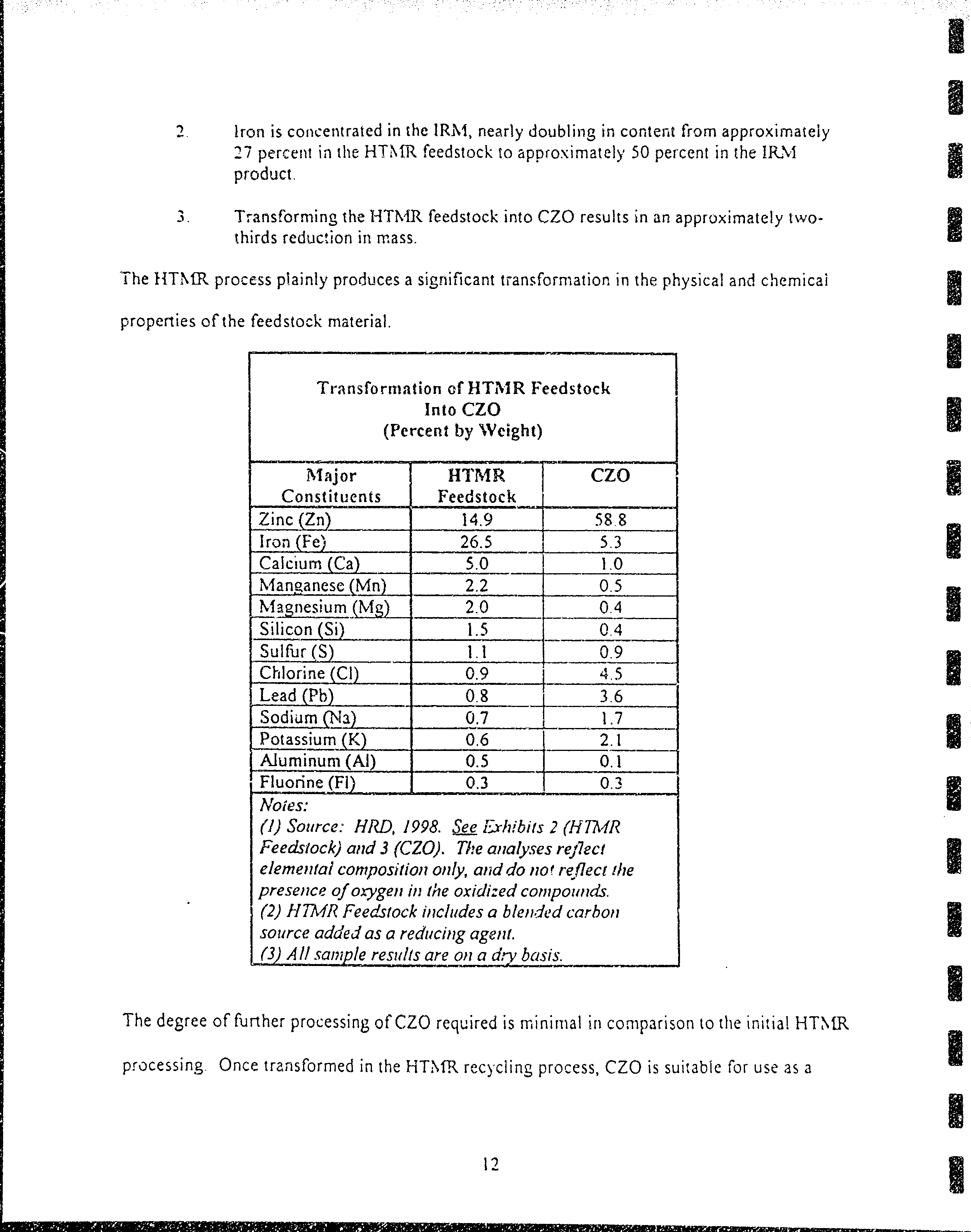
l.
Zinc is
concentrated in
the
CZO,
quadruplin;
in
content
fronr approximately
l5
percent
in
the
blended HTMR feedstock
to approximately 60
perceirt
in
the
CZO
produA.
Chemetco.
Inc f'ut&iu:!sd-Standard
from 35lllldrn-.]eoc!e:2Ol3l(a) and
iS)
ft{arch
19,
1998), AS
97
-2.
s
Zinc
t'efineries are
incapable
of
recy,cling
EAF
tiust
and sinrilar loi','-zinc
\\'astes
becruse tlre
refinery equipment is neither
designed nor
built
ro
remove
thc si.gnificant
levels of ncn-zinc
constituents
(e.9,
iron)
in
steel inCustry
\\'aste
feedstocks.
Sgg
pages
l3-15 belorr for a dc'tailed
description af
zinc
refinerl'equipmenr
used lo
process
CZO.
l!
?
lron is
concentrated
in the lRtr{,
nearly
doubling in conterrt from approximately
27
percerrt
in
the HTI\{R
feedstock
to aporoximately
50
percent
in the IR\I
produet.
3.
Transforming the
I-ITN'{R feedstock
into
CZO
results in an
approximately two-
thirds reduction in mass.
The
t{Tlt{R process plainly produces
a significant
transfornration in the
physical
and chemicai
properties
of the feedstock
material.
Tra nsfo
rmation of HTilt R F'eedstock
Into
CZO
(Percent
by
Weight)
ilIajor
Constit uents
HTMR
Feedstock
CZO
Zinc
(Zn\
14.9
588
Iron
(Fe)
26.5
53
Calcium
(Ca)
5.0
I.0
N'lanqancse
(Mn)
2.2
0.5
l"{€l..tu11(Mg)
2.0
04
Sr!,c,oq(!i)
1.5
0.4
Sulfur
(S)
l.l
09
Chlorine
(Cl)
0.9
4..5
Lead
(Pb)
0.8
36
Sodium
fNs)
0.7
1.7
Potassium
(K)
0.6
2.1
Aluminum
(Al)
0.5
0.1
Fluorine
(Fl)
0.3
0.-1
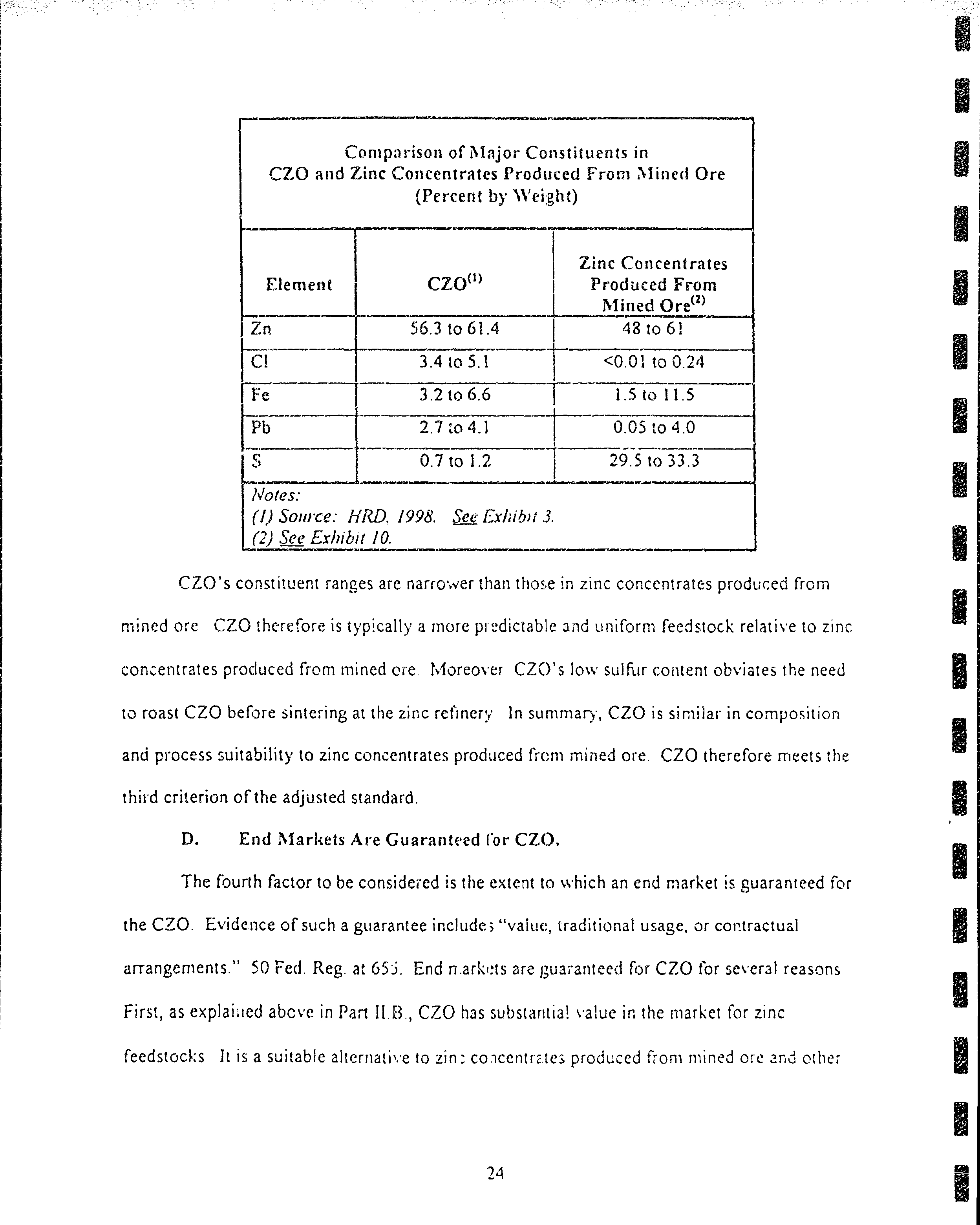
Noies:
(l)
Source:
HRD, 1998. See ishibits
2
(HTMR
Feedstock)
and
3
(CZO).
The analy.ses relilecr
elemenlal
compositiotr
only, and do not re{lect
the
presetrce
of
orygen
in
the
oxidized
compounds.
(2)
hITMR
Feedstock includes a blen,Ted
carbon
source
adde,J
as
a reducing ageilt.
(3)
All
sample
remlts
are
on
a dry
basis.
The
degree of
further
processing
of CZO required
is
minirnal
in conrparison
to rhe inirial
HT\1R
processing
Once transformed
in
the HTi\fB. rec5'ciing
process,
CZO
is
suirabie
for use
as a
H
6
ffi
B
B
E
ffi
ffi
H
g
ffi
g
fr
fr
B
H
$
ffi
w
t2
t3
'f'"t
""
&
n
&
&
&
ffi
u
fr
a
n
fr
a
w
n
fr
&
a
E
direct
fleedstocl.:
in
zinc production,
a"
a direc't
feedstock
for
calcining,
or
as an
ingreclienr
in
the
production
of nricrcnutrients.
(CZO
is not
used for
lerrilizer.)
Each
use
is
desci'ibed
in
furiher
detail
beiorr'.
l.
Direct
feedstock
in
the zinc productiarr
process.
FRD's
CZO
is
used
as a direct
feedstock
in
zinc
production.
CZO
is
a
high-quality
feedstock
substitute
for
zinc
oresthat
have
been
mined
and
processed.z
CZA
is
a
more
predictable
and
uniform
feedstock
than
the
zinc
concentrates
produceC
irorn
miled
ore,
since
CZO's
constituent
ranges
are
typically
narro\t/erthan
the
constituent
ranges
in
zinc
concentrates
produced
f?orn
mined
ore.
See table
belorv
at
page
24.
Since
CZO is
already
high
in
zinc, litrle
additional
processing
is necessary.
HRD
sells
CZO
to Zinc
Corporation
of America
("2C.4")
for use
as a
direcr
tterjsrock
in
ZCA's
zinc
production
orocess
in
Monaca,
Pcnnsylvania. (ZCAand
HRD
are
separate
companies
orvned
by
Horsehead
Industries,
Inc.)
ZCA's
zinc
refinery processes.r,arious
zinc-
ccntaining feedstocl<s
to
produce
zinc
metal
slabs
and ingots.
The refinery
ieedstock
ty,picalll,
includes
zinc
concentrates
produced
from
mined
ore,
purchased
zinc-bearing
secondar;,materials
such
as
CZO,
and other
zinc
oxides.
ZCA's
processing
of
CZO into
zinc
meta.l
consists
af sintering
and
thernlal
reducrion.
These two
processing
steps
are
summarized
below,
ancl a
flowchart
of the
ZCAzincproduction
process
rs
included
at
Ex-hibit
4.
2
Sulfide
zinc
ores
extracted
from
the ground
typicaliy
contain
three
ro
fir.e percenr
zinc.
This
mined
ore is usually
beneficiateci
at
the
mine
to concintrete
ihe marerial
containing
zinc
(and./or
other
valuabie
metals).
The
beneficiated
ore is referred
ro
throughout
rhis
petirion
as.'zirrc
ccncentrates
produced
from
minecj
cre."
Prelinrinarily,
it
is
rvorth noting that
virgin
zinc
ore
is subject to a
number
of
processing
operations e"'en
before
it reaches
the
quality
of CZO and
thus
becomes
a
suitatrle
feed
for zinc
procluction.
These
cperations,
rvhich
are
necessar)' to
concent!'ate the
zinc
content, include
extraction
and
beneficiation
processes
such
as
mining,
crushing,
milling,
sequenlial
fiotatioa/separation,
dervatering,
and drying. Subsequent to these steps,
zinc concentrates
produced
from mineci cre
also must be
"roasted"
at high
temperatures
in air
to
produce
roasted
zinc
concentretes and recover sulfur
as sulfur dioxide
gas.
The resulting
roasted
zinc concentrate
is
then
sent
to the
sinter
plant. The
sulfur
dioxide
is
converted to sulfuric
acld !n
another
process
and
soid
to
third
parties.
By comparison, CZO
is
lorv
in sulfur
content
and already
of
sufliciently
high-grade
that
it
dces
not require any
pre-sintering
processing
steps.
(i)
Sintering
-CZA,
as
rveil
as
roasted zinc conceritrates and other
lorv-sulfur zinc
oxides,
must
physically
be
agglomerated into a
coarse,
larger-sized
materiai
before charging to
the electrothermic fi;rnace
.
l'he
sintering
process physically
prepares (i.e.,
densifies
and
hardens) the zinc oxides, and
reduces
siightiy the
rrther
mil;or
constituents in the
zinc feed.E
The zinc
oxides are mixed
with
a
carbon source
(for:i.rel)
anci
with
silica ta
bind
the materials
rogether
The
sintering machine
operates at approximately
900-1200o
centigrade.
Sinterirrg
results in two
materials: zinc
sinter and a
lead
concentraie.
The
zinc
sinter
is
an agglomerated
material that is hard and
porous
in
physical
composition and
is the
feed for
the
electrothermic
furnace.
The
lead
concentrate
produced
from sintering senes as a feedstock
in
anolhei
processing
circuit.
&
More
technically,
sintering is
"the
process
o{'heating
fine
particles
to an
eievated
tenrperature
ivithout
complete
fusion such that the
small
solid
particles
in contact
ri'ith
one
anorher
adhe
re
and agglcmerate
into
larger,
more useful
particles."
Robert D. Pehlke, Unit Pracriges
gf
Ertraaive
lr{etal.Iurgy
(1973),
at
16.
See
Exhibit
5.
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
H
ffi
ffi
&
ffi
H
ffi
ffi
&
g
ffi
l4
(ii).lhgll]]a!-reduction.-Z\ncsinterislreatedinanelectrorherrnicfurnace,r.r,hich
r.aporizes
and
condenses
rhe
sinrer
feed,
resurting
in
zi.c
metar
and
a
non'hazardous
siag'
The
purpose
of
the
thermar
reduction
is
to
remove
oxygen
and
the
remaining
n:iri!"
constituents
in
rhe
zinc
sinter.
The
thermar
reduetion
step
resurts
in
prime \{esterrr
Grade
zinc
metal
suitable
for
direct
sale
or
production
of
specialty
zinc
products'
2'
Direct
feedstock
for
calcining'
cZO
produced
ar
the
Faciriry
is
also
sert
to
IIRD,s
fac*ity
in
Farrnerron
for
c.alcining
carcining
furrher
purifies
the
cZo,
and
resurts
in
a
zinc
calci*e
product'
"l'hc
zinc
calcine
is
then
soldtoT]f[r,,:hereitissinteredrviththeotherzincfeedstockstoproduceaphysicallyuniform
and
agglomerared
feed
for
thermar
reduction.
zinc
calcine
ranges
from
60
ro
65
percent
zinc'
compared
to
less
than
60
percent
in
the
CZO'
and
lead
and
chlorine
are
reduced'
n
B
I
rg
r5
E
g
E
*
ff
tr
tr
ffi
g
&
g
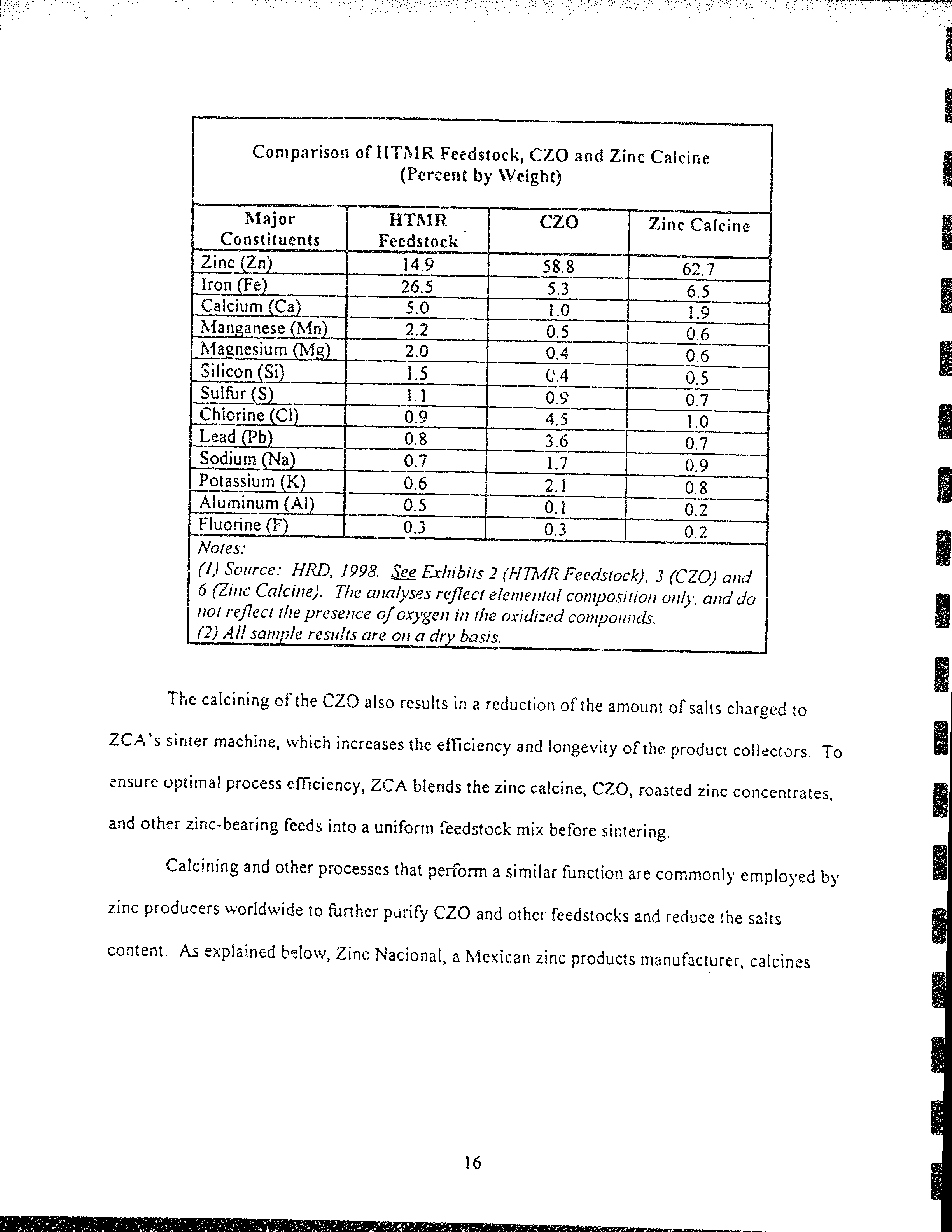
The
calcining
of
the
CZC
also
results
in
a
reduction
of
the
amounr
of sahs
charged
to
ZCA's
sirrter
machine,
rvhich
increases
the
efTiciency
and iongevity
of the
producr
collecrors
To
ensure tlplimal process
efTiciency,
ZCA
blends
the
zinc
calcine,
CZo,
roasted
zinc
concenrrates,
and othar
zirrc-bearing
feeds
into
a
unilorrn
feedstock
mix
belore
sintering.
Calctning
and
other
pfocesses
that
perform
a
similar
function
are
commonly,
emplol,ed
by,
zinc
producers
worldwide
to
funher
p,irify
CZO
and
other
feedstocks
and
recluce
:he
salts
content'
As explained
t'elorv'
Zinc
Nacional,
a N{exican
zinc
products
manufacturer,
calcines
Conrpariso:: of
l{TfrtR
Feedsiock,
CZO
ancl
Zinc
Calcine
(percent
by
tVeighr)
Stajor
Cons(ituents
HTfr{R
Feedstoek
czo
Zinc
Cale ine
4nc
(Zn)
t49
s8.8
62'l
Iron
[Fe)
26.5
5.3
6.5
Calcium
(Ca)
5.0
t.0
t.9
lr{aneanese
{i\,{n)
2.2
0.5
0.6
l'{aenesium
ffo{s)
2.A
0.4
0.6
Silicon
(Si)
1.5
C4
0.5
Sultur
(S)
t.t
0.e'
0.7
Chlorine
(Cl)
0.9
4.5
t.0
Lead
fPb)
0.8
3.6
07
Sodium
[Na)
0.7
t.7
0.9
Potassium
(K)
0.6
2.1
08
Aluminum
(Al)
0.5
0.i
o?,
Fluorine
(F)
0.3
0.3
02
Nales:
$)
source:
HRD.
1993.
see Frhibirs
2
(HTMI?
Feeclstock),
3
(CZo)
artd
6
Q.inc
Calcine).
Thc
analyses
refiect
elernetttal
contpositiott
ottl1,,
atvl
do
ttot
reflect
the
presence
of
ox\'gen
i,
rhe
oxidircd
conipo,ttris.
(2)
All
y11ple results
dre
on
a dry
basis.
CZO, as
do
other foreign
nietal nranuiacturing
facilities.!
l-iker,,ise,
BRZ, which
produces
zinc
products
front
crude zinc oside
purchased
from
other
producers,
rvashes
the
salts frcm
the
zinc
oxide
to
purif1'the
material and
prevent
corrosion
of BM's refiningequipnrent.
,Sge B_rS.Rty,sr
ZincJCgrp.orati-on
(April
I
5, 1999).
AS
99-3,
slip
op. at 8-9.
More
generally,
catcining
and
similarprocessesarenotuniquetothezincrecyclingindustry.
Zincproducersthatusczinc
concentrales
produced
fiom mined
zinc ores typically
calcine
or otherwise process
the
cOncentrates
lo
rernove
naturaliy-occurring
salts,
thereby frrrrher
purifying
the
product.
Like
sintering, calcining
also
may serve
to
densily
and
harden
the
feedstock into
a
lnore
easily
rnanaged pellet-like
material.
Indeed, ZCA's
sintering
process
is similar
in
function
to HRD's
calcining process,
andZCA
-q.inters
nearly
all
of
its
zinc feedstocks,
including
zinc
concentrates
produced
from mined
zinc
ores, before
final
processing
in
the electrothermic
firrnace.
3.
Ingredient
in ttre
production
of micronutrients.
HRD sells CZA
to Zinc
Nacional,
a
pyronretallurgical
facility
located
in
Monrerrey,
N{exico. The
CZO sold
to Zinc Nacional
is
used as
an
ingredient
in
the
producriorr
of
micronutrients
for
animal
feed
products.
HRD
transports
the
CZO by
pressure
differenrial
rail
car to the Mexican
border rvhere
Zinc Nacional
takes tiile
to the
proCuct.
At
Zinc
Nacionat's
facility,
the
CZO
is unloaded
within
a fully enclosed
building. The
operating areas
of the
plant
are
aiso
equipped
with
collection
equipment
and baghouses
to
prevent product
loss
and
to
recycle the collected
material.
Zinc Nacional
transnorts
the
CZO
pneumatically
in
an
enclosed
conveyance to a
cone
pelletizer.
The pelletized
CZO
is
then fed
via
covered
conve!'or
belt to a
"
S.J
alsg
Exhibit
T,tetter
from
Ling
Wong
to
Tom
Theobald, s,hich
srares
rhar
three
of four
Japanese companies calcine
CZO
(referred
to as
"\\,aelz
oxide" in ihe
letters)
into
calcirred
zinc
concentrate.
lr7
t\\'o-staqe
calcine
process
tlrat
volatilizes
certain
nretal
compounds, ren'roves
salts,
and
prcduces
a
zinc
oside,
',r'hich
is sold
to the agriculture
industry
as
a rnicronutrient
for
animal
feed
prorjucts
4.
Summary.
F{RD's
t'lTi\{R
process
substantially transforms
the hazarCous
waste
feedsiock
from a
lou'-zinc,
high-iron luasle
mixture
to the
high-zinc,
!ow-iron
CZO
product
suitable
for
direct use
in
the zinc
production
process,
for calcining
in
HRD's
calcining
kilns,
and
as an
ingredient
in rhe
Production
of
micronutrients.
The
CZO
results
from
substantial processing
of the feecJstock
and
i'equires
oniy minimal
additional processing
to
produce
zinc
products.
CZO therefore
meers
the
first
criterion
cf
rhe adjusted
standard fbr
commodity-liiie
products.
B.
CZO Has
Substantial
Value.
The
second
criterion
to
be considered
is
thevalue
oithe
material
after it
has
been
reclaimed.
According
to
the
U.S. EPA
guidance, "[o]bviously,
the
more
valuable
a mater-ial
is
after initial processing,
the more
likely
ir is to
be commodity-tike."
50
Fed. Reg.
at 655.
The
HTN{R process
transforms
material
rvith
negative
eeonomic
value
into
a material
rvith
sr.:bstanrial
positive
economic value.
More
specifically,
the hazardous
wastes
used
to
produce
CZO
haye
ne.gative
economic'value
because
generators
rnust
pay
lor
the rnaterial
to
be
either
Cisposed
of
or
reeycled.
indeed,
EAF
dust's
high iron
content
and
relatively
lorv zinc
content
prevent
zinc
produaion
facilities
from
using
EAF dust
directly as a feedstock.
The processing
of EAF
dusr
and other feedstocks
in
HRD's
HTMR
process
produces
the comnrodity-!ike
product
CZO,
u'hich"
along
u'ith
other zinc
concentraies,
is
part
of
the
rvorldrvide
market
in zinc
commodities.
CZO
is
a
valuable
product
because
it is
high
in
ziric and lorv in
consiituenrs
like
iron that
cannor
be
processed
at
zinc
production
lacilities
Zinc'sprice
is esrablished
bl,suppl;.an,J
dc'nrand
on
the London
\fetal Exchange
("LME")
The
long
rernr
average
Lj\{E
price
for
z_inc is
ffi
ffi
ffi
ffi
ffi
g
H
ffi
ffi
H
ffi
ffi
ffi
ffi
I
ffi
w
ffi
ffi
IB
t9
;3
Fs
Es
f;m
lm
ilE
l€
ls
rg
€
g
F
fr
g
t
a
n
approlin]rteii'5s
cents/pcund,
but
in
the past
ten
),ears
the
LN{E
zine price
has uaried
from
a
lo$'of
39'7
cents/pound
in
seprenrber
l99i
ro
a high
of
g3.7
ccnrs/pound
in
h{arch
lggg.
The value
of ntost
zinc-bearing
mate:"ials,
including
czo,isbased
on
a tbrmula
that
is
generalll'
accepted
in
the
rvorldrvide
indusrry.
The generic
iormula
is
lypically
to
pay
rhe
LI,IE
orice for
a
fixed
percentage
of
the
zinc
contained
in
rhe
marerial.
The
buyer
(e.g.,
a
zinc
refiner),
mdi'3!so
re'ise
the
fc;^mula
to
inclucie
and
deduct
a
"processing"
charge
from
rhe
zinc paymenr,
r'hich
represents
an
approximate
overall
cost
to
process
the
material
in
the
zinr:
producrion
proeess.
The
processing
charge
rvi!l
increase
or
decrease
with
the price
of
zinc,
so
that
the
mine
and
the
zinc
producer
share
in
the
risk
associated
rvirh
fiuctuations
in
the
zinc price
Finat!y,
credits
and
debits
may
be paid
by
the
buyer
(or
applied
to
rhe
selter-)
lor
cerrain
non-zinc
:onstiiuents
in
the
zinc-bearing
material.
As
explained
below,
CZo
is
produced
and
sotd rvorldrvide
as
a process
subsrirure
for
zinc
concentrates
produced
from
nined
ore.
h{oreover,
the
economic
value
of
HRD,s
CZo,
lii:e
oiher
cZo
end
z1'nc
concenlrates
produced
from
mined
ore,
is
substantial
and
quantilrable
czo
iherefore
meets
lhe
"\'alue"
criterion
of
an
adjusted
standard
for
commodity-like
producrs.
I'
concentrates
CZo
is
produced
produced
and
sc,ld
frorn
worldrvide
mined
ore.
es
a
process
substitute
for
zinc
Hundreds
of
thousands
of tons
of
cza
and
sinrilar
zinc
feedsrocks
are
sold
*.orldr'ide
as
a
process
substitute
lbr
zinc
ccncentrates
produced
from
mined
ore
because
they
contain
higlr
zinc
content
and
are
suitable
forprocessing
at
zinc
manufacturing
facilities.
CZo,s
economic
value'
like
any
other
valuable
zinc-bearing
marerial,
depends
rargely
on
its
zinc
contenr
and
ihe
LN{E
price
for
zinc.
The
same
is
true
for
other
zinc
concentrates
or
secondan,
materiars
pr.r;'chased
by ZCA
and
other
zinc
producers
i
I
t
I
I
I
I
I
I
I
I
]
!
a
I
E
E
E
g
H
I
I
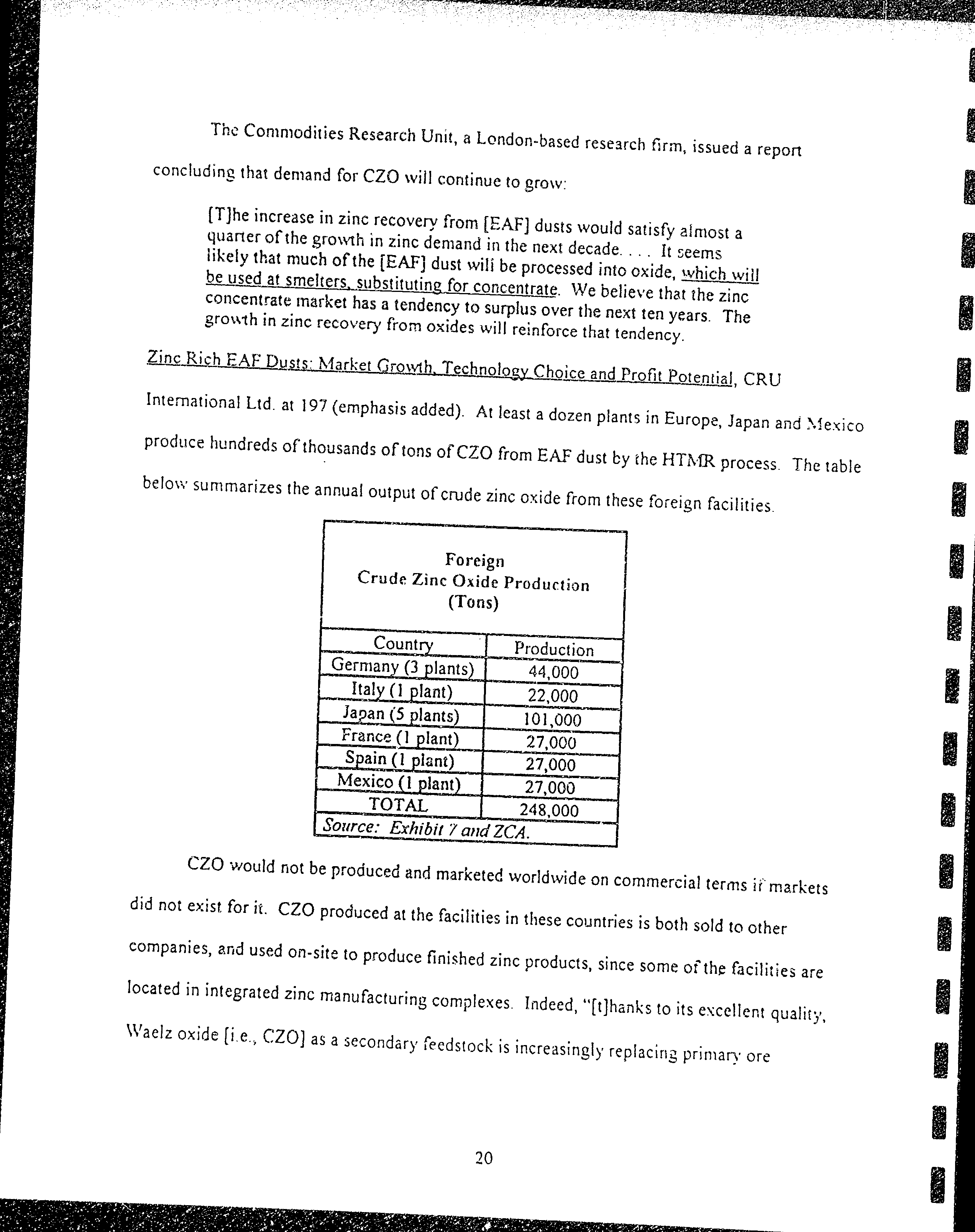
Thc
conrnrodities
Research
unit,
a
Loncon-based
research
f;rm,
issueci
a
repon
concluding
rhat
denrand
for
CZO
rvilt
continue
to
gro\v:
gror*rh
concentrate
U"r
iikely
quafler
[TJhe
that
increase
in
of
zinc
the
muc-h
market
gro*,rh
recovery
in
of
zinc.recovery
the
has
in
tEAFi
a_tendency
fronr
zinc
d-en:andln
oxides
dust
from.
to
wiri
wirr
TEAFJ
surpru,
u.
trr.
relnforce
pro..rr.J'iito
n*^ia*.uae
dusts
**,
.
We
rvourd
tlre
that
belie'e
next
tendencv.
satisfy
.
o*id.,
.
ren
rhar
Ir
years.
seems
arrnost
rvhic_h
the
zincThervitf
a
$
tr
ffi
tr
ffi
g
ffi
ffi
rv,,,,
v'
vvurJ
\. rlg
I
cg
a
n
o
l,ro
I
[_
pot
e
n
t i
a
l,
CR
U
International
Lrd'
at
I9? (emphasis
added).
Ar
teast
a
dozen
prants
in
Europe,
Japan
anc
),1exico
produce
hundreds
of
rhousands
of
tcns
of
CZo
from
EA*F
dust
by
rhe
HTi\.{R
process.
The
rable
belori'summarizes
the
annual
output
of
crude
zinc
oxide
from
these
foieign
facilities
cZo
'"rould
not
be
prociuced
ancl
marketec
worldwide
on
commerciar
terms
ii.rnarkets
did
not
exist
for
it'
cza
produced
at
the
facilities
in
these
counrries
is
both
sold
ro
other
companies'
and
used
on-site
to
procluce
finished
zinc
products,
since
some
of
the
facirities
ar-e
Iocated
in
integrate
d
zinc
manulacturing
complexes.
Indeed,
"[t]hanks
to its
excelrent
quarit1,,
\\/aelz
oxide
Ii
e.,
czo)as
a
secondarS,feedsrock
is
increasingry,repracing
prinran,ore
ffit
I
ffil
ff$
Foreign
Crude
Zinc
Oxid-e
production
(Tons)
Prcduction
France
(l
fur*t
tAW,
f
"rd
ZCA.
lF
a
fi
g
8
t
faciliries
con!'entrlles
or
used
in
tlte
on-sire
European
b1'zinc
zinc
ntanufacturers,
and
lead
snrerrers."r
it
is
an
econonricaily
11,;-,.,
her
czeis
and
environmentaril,
sord
ro
zir;cprodu*ron
desirabre
subsrirute
fbi'zinc
concentrates
pioduced
from
mined
ore.
z.
The
econonric
varue
of
HRD,s
CZO
is
substanr:at
and
guantifiabre.
CZO's
economic
value
is
c,rant!fied
by
its
transaction
price.
The
commercial
the
summarized
transactions
esrablishea.econonric
belo*''
for
czQ
The
sold
transacrion
by
FIRD'
as
price
rvel!
of
as
at
for
least
rransacrions
srio
per
ton
for-
for
other
cZo
zinc
ctearry
oxide
demonsrrates
products,
are
value
of
CZO.
E2l
ffiffi
mfi ** ..
;i;tltU!:JL"Jfj,"*,?:;:lllljJl;j".*
of
B
U
s
,
Ber.zirius
Un,*eir.sen
ice
AG
llu":t:r:tr ,t!e!
satesjigttres.
--%-r%+%-
',;
,"::/;i\i;{,f,:;:
#;"f;x:,;;,;:;,,i::,x,::::::::{?:
i::.,t1,,,,,,,
,,.,,darctu
I
Economic
Oxides
Transactioi
FIRD
CZO
sale
ro
Zrnc
Nacional
4pr,'o'i"t{68ffiiffi,
(tfexico)
fRD
CZO
sale
ro
ZCA
AmeriSteel
(crude
zinc
oxide)
sale
River
Zinc
Cor"poration
to
Big
-S111
of
"ir-ni
ca
l
"
zinc
concenrrare
ploduced
from
mined
or*rii6'i
Each of
r}:ese conrnrercial
transactions
denlonstrates
that
CZO
and similar
zinc oxides
have
substantial
econontic
valtte:
.
HRD sells CZO
produced
frorn
its I{TIUR
facilities to
Zinc
Nacional
in
Monterrey,
Ir'lexico.
Zinc Nacional
refines the CZO into
an
enriched zinc
ptoduct
suitable
fbr use
as a
micronr.rtrient
for animal feed
products
(zinc
is an essential human and
animal
nutrient). Dccumentation of the
Zinc Naciona! 1998 saies
transaciiorrs is included
at
Erlilbit 8
.
[-IRD
sells CZO
to ZCA for refining irrto zinc
rnetal and oxide
products
Docurneniation
of the
ZCA 1998
sales transactions
is included
t Evhibir
9.
n
BRZ has
agreed to
purchase
crudr: zinc oxide
produced
by AmeriSteel for
at least
5200
per
ton.
See
Blq,-Rlyer
ZltE-C-orpqiallan
(April
15, 1999), AS
99-3,
slip
op
al
17. AmeriSteel's
crude
zinc
oxide,
rvhich
is
produced
lrom
EAF dust,
is
virtualll'
identical in
source,
composition
and
process
suitability'to
HRD's
CZC
prcduct.
'
The
value
of
zinc concentrates
prociuced
irom nined
ore on
the
open nrarket
prci'ides
a usefi-rl comparison
because these concentrates
and CZO are
both marketed
"r'or,drvide
for
their zinc,ralue. As shown
in
the table, typical zinc ccncentrate
pro<luced
from mined
ore,
assuminga45f.llb. LIr{E Special
High
Grade Zinc Price
(1998
average), is vaiued
at
approximately $266
per
ion.
3.
Summary.
CZO
has
substantial
economic
value,
as demonstra'ied by
an established history
of
commerciat
transacrions
and
its important
rotc
in
zinc cornmerce
rvorld*'ide
CZO's
precursors
cannot be
processed
directly
into
zinc
produc:s
and they,have
qeeati'.'e
econonric
value
The
CZa
produ
ced by recl'cling
lhese
rvastes
in
the tlTi\tR
proccss
lras
substantirl
econornic
:,':
H
g
ffi
ffi
B
H
ffi
tr
H
ffi
ffi
ts
g
g
B
$
w
H
ffi
22
'#
g
tr
$?
l..rlue
{!J,
CZo
corrrpares
far.oi
"'alue
rc'larri.e
ro
otrier
a,n,
,ourttolf
in
'alue
fo
ofher
zinc
oride
producrs
reedsrock
cZo
js
conrnrodirl,
;:;',r::,::::;"'ed
rir'c,ushour
rhe
,
as
dernon'st*':ed
b;'irs
ti'orid
as
a
zinc
rd;usted
srandard.
,_
.,v,
d
rvaste.
CZO
thus
rneets
the,,value,,criterion
of
the
C..
CZO
IsSimitar
rr
The
thirri
facto,o
o..onu::::l.
tttttt"trates
Produced
rram
i\rinec
r)re.
:;:,,
.:,
# :j:j*i:li::"
;,;
;:l
"".,;jl"'s
.
a
ra\
to
be
commodirl,-like.,,
50
Fed.
*.lntttnt*
as
a
ibedstock
ro
a
primarv
oro"*lut*t'al
can
respc,cf
to
CZO
J'
al
6'55.
U.S,
EpA,l
"is'
it
i-t
rnore
liltell'
because
Czo
issimirar
,n
".,,..1^*.
:tnt
t
guidarlce
is
drre'ly
on
pr.,inr
*.irh
consriruenr
zinc,
and
"".,:':
rs.stmilar
in
conrpositiorr,
pi
o,*e
,
rr
hich
.r.
,
uno
esui'alenr
in
process
,r,,;;;,;;;
:|,nt*'*t'
"vith
respe'-r
te
:lie
crirical
;-
;";#;'::::'::i'"
c'nceni
b:es
n;
ca;
cZo
ihereror.,-.,::::
:;
"'"'
i,
I997,
approxirnarety
I0*,,,"1
_"oir1'-rike
produ*s.
ore
r.\
ere
produce-drhrn,,^r-^
.' .'"
"'!::tc)n melrjc
rons
of
zin
(e
g
,
lead
^rn
"^o^l)),'^t*.'t
rhe
rvorld
for
use
in
the
nrantic
concenti'rtes
produccd
fr-orn
minecl
majorr:onsrirr:enrs.nium
oxides
and
rnetat,
,""r:;:;;t:-t'ure
orzinc
and
allied
precrucrs
dernonsrrares
rhe
,rnn
rrowirh
rhose
,n
r,.r,
,r,ruric
acid).
A
comprriscn
of
rhe
ra
zii:c
producrs
tirariries
of
rhe,-";;;,"ff,
ffi:,:;;.o'arges
of
the
rabilit;,
for
direct
processing
inro
8
&
&
&
g
fr
fr
8
8
g
I
t
-___
--__-
,l
ia
I
I
I
I
E
G
Conrprrisorr of ilIajor
Constituents
in
CZA
and
Zinc
Correentrates
Produced
Fronr
i\lined
Ore
(Percent
by
\\'ei'ghl)
Element
cz,CI(J,
Zinc
Concentrates
Froduced
From
illined
Ore(l)
7.n
56.3 to 61.4
48
to 6l
(:!
3.4 to 5.1
<0.01
to 0.24
fre
3.2 to
6.6
1.5 to I 1.5
Pb
2.7
to
4.1
0.05
to
4.0
:;
0.7
to l.?.
29.5 to
33.3
J'lotes:
(l)
Source: HRD.
1998.
Sstlixliibtr
(2)
See
Exhiht 10.
,3,
CZQ's
constituent tanges
are narro',ver
than
thos,e in
zinc
concentrates
produced
from
niined ore
CZC
therelore
is
ty,pically
a rnore
pr:dictable
and unifornr
feeCstock
relative
to zinc
concelrtrates
prccluced
frorn
rnined
ore l"loreover CZO's
lo*'sulftrr
coittent
obviates
the
need
to roas(
CZO
befirre
sintering
at the zinc retineryi
ln
summarl
,
CZO
is
similar
in
compo-sitiorr
anci
process
suitability to zinc concentrates
produced
licnr nrineC
ore CZO therefore
nreets
ihe
rhiid criterion
of the adjusted
standard.
D.
End illarl<ets
Are
Guarante'ed
lbr
CZO,
The fourth factor
to
be
consideied
is
the exte:rt to
rvhich
an end nrarket
is
guaranteed
ior
the
CzO.
Evide nce
of such
a
guarantee
includc;
"vaiur:,
traditional usage. or cor:tractual
arrangements."
50 Fed.
Reg.
at 65j.
End
n.ark':ts are
tguaianteed
for
CZO
lor
several
reasons
First, as
explairred abcve
in
Part
II 8.,
CZO
has substantia!
r'aluc
in
the
nrarket
for zinc
feedstocl:s
It
is
a suitable
allernatr,.'e
to
zin: corcentra,tes
oroduced
fi'onr nrined orc and
otlrr'r'
g
$
ffi
ffi
H
g
H
H
H
g
ffi
H
H
H
B
ffi
w
aA
#
I
E.
CZO
Is
Hrrndled
io ilIininrize
or Eiinrinare
Loss.
The fifth factor is the extent to
rvhic.h
the
CZO is handled
ro minimize
or eliminate
loss.
CZO is ntanaged in att
environmentally
protective
manner
ihroughout
all
phases
of its life
cycle
from generation
to
prodr:ction
of
zinc
meta!.
[-[RD
and ZCA
carefrriiy
manage
CZO to elirninarc
loss
to
the
greatest
possible
extent
lor economie
and
envircnmental
reasons.
Seg
F.ecycls
TgehnEiogtes,
AS
97-9,
slip op. at l
l
("The
Beard notes
that
[Reeycle
Tectrnologies]
has a
financial
incentive
not
!(,lcse the
filtered
used
antifreeze
itl it loses
rnaterial,
it
has less
to sell
back to
custorners.")
FITLD's
handling
of
CZO therefore saiisfies
the
fifth
ciiterion
of the
adju
st ed st andard
for
cornmod
ity-i
ike
produet
s.
l-
Handling
of CZO from
productionr
through
otT-site
shipment.
HR"D
carefully
rnanages
CZO in
an
environmentalli,protective
manner
from
the rime
ir
:s
produced
through
the
tinre
of ofT-site
shipment
into
the
stream of commerce.
As
<Jescribeci
in
the
nexl
paragraph,
all of FItr'.D's
unloading
and conveyance
ot,erations are
enclosed
to
prevenr
an),
product
loss. These
operations
are under negative pressure
ro
elinrinate porential
fugitive
enissions. Fffi.D
is in compliance
rvith
all
air
permits.
CZO is
pneumatir:ally
conv'eyed
from
the
product
ooltectors
to enclosect
prsssure
differential
rail
cars
lcr
off-site
shipment
immediately
after
productior'.
(I{FS
does nor
store
CZA.\
The rail
car
loading
tank,
into which
the
CZO is
trnnsferred
after
prociuction,
is
insicje
an
enclosed
building
that is r:quipped
with
collection
equipmenr
and
a
baghou:;e
to
prevenr
product
loss
The rail car
loading
tank
empties
CZO into pneumatic
discharge rail
cars
through
a
pipe
that extends
down into
the
rail
car.
-ZQ
is
transported
tc its
destination
in
compliance *,ith
t)epartment
of Transoortation
regulations
for
class
9
substances.
ff
ffi
ffi
ffi
ffi
H
ffi
H
g
H
H
H
B
g
ffi
B
ffi
a
H
26
'1#
8
n
E
F
a
27
WN
*
o
**--
2.
Itandling
during
processing
into
zirrc
rnetnl.
building
slored
from
\lonaca
raiicars
in
cZo
are
ancJ
orocess
vented
ar
through
also
FIRO,s
bins
is
ro
managed
pneumaiic
collecrors
in
calcining
the
enclosed
in
to
an
conveyances
facility
prevent
en'ironmentaliy
sintering
in
product
palrneno
that
buirding.
prectude
ioss.
n.
protective
CZ01
AJr
environmenta!
product
har
rnanner
arrives
transfer
et
in
ZcA,s
exposure.
Monaca
points
refinery
in
is
cZO
off_loaded
thein
is
to
p:'ertent
surge
a
product
bins
product
czct
and
collector'
transporred
rnetered
loss'
all
The
into
conveyance
to
conditioned
HRD's
a
mixer,
ealcin!ng
sysrerns
where
cZo
it
is
are
facility
is
fed
conditioned
totarly
into
in
Falmerton
trie
encrosed,
carcine
wirh
water
and
is
kirn
pneumatical!y
the
by
prior
surge
an
to
encrosed
carcining.
bins
unloaded
are
ventedTo
inro
emissions
coliectors
con'e)'or'
from
is
rvhich
recycled
both
has
ends
Flgirive
to
the
of
process'
ki!n.collectors
Inside
cn
the
the
system
calcine
transfer
kirn,
pressurizecr
s),stenr
points.
sears
eriminate
Any
czoin
potenr:ar
rhe
3'
Handring
cruring
proccssing
!nto
micronutrient
ingrerJient,
'
operating
all
facility
con'eyances
Zinc
in
areas
Mexico'
Nacional
to
are
prevent
Zinc
encrosed,
also
Nacional's
product
handles
and
the
ross
HRD's
materiais
faciiity
and
--'r
to
cZO
has
r'Ee
recycre
receiving
in
cotecticn
wv'sLriun
an
the
environmentary
and
corected
equrpment
e
preparation
materiar.and
protecrive
areas
baghouses
are
F.rry
manner
in
encrosed,
a,
at
of
irsirs
n
summary.
product
e*ent
possible
CZo
management
produced
dur:ng
systems
all
at
phases
the
Facility
for
of
czoto
its
is
life
handled
enslrre
cycre.
in
rhar
FIRD
a
manner
it
is
has
rnanaged
invesred
that
mininrizes
so
nritiions
as
to
pre'ent
ross
of
doilars
to
rhe
the
grearesrescape
in
of
this
va!uablc'ntaierial
into the enlironnrent.
The
environmentaily protective
management
of
this
nraterial
therefore
satisfies
the fifth criterion
of
the
adjusted
standard.
F.
Other
Relevant Fnctors.
Three
other factors
support I{RD's
Petition
for
an adjusted
standard
for irs
CZO
produa.
First,
the Board
recently
granted
an adjusted
stanCard
under
Section
72O.l3i(c)
ro
BRZ
for
a
crude
zinc
oside that is
virtually
identical
to
FiltD's
CZO
in
source,
composition
and funcrion.
The BRZ
adjusted
standard represents
an
indistinguishable
prece,denrial
basis for
HRD's
perition,
and
virtually
mandates
the conclusion
that HRD's
CZO is
a commodity-like
marerial
when
processed
into zinc
products.
'the
BRZ
aCjusted
standard
demonstrates
conclusively
the
existence
of an ac.tive
ntarket
for
CZO
and
provides
independent
confirmation
of
rhe commotlitS,-
like
nature
of the
material.
The
fortuitous
timing
of rhe BM
and
HRD
petitions
gre;rrly
simplifies
the Board's
task here.
Another"
related
lactor
is consistency rvith
variances
from
the
definition
of
solid
$'aste
fcr commodity-like
zinc products
promulgated
by
oil-er agencies,
including
one
for
the
same EAF zinc
oxide rhat
rvas
the
basis for
the
BRZ
adjusted
standard.
A
third
factor
is consistency
with
the
resource
conservation
and
rvaste
minimization
mandares
of
Illinois and
federal
law.
Recycling
EA-F
dust
into
CZO
produces
several
major
environmenral
benefits, including
recycling
rather
than land
disposal
of the vatuable
consriruenrs
in
EAF
dusr,
and reduction
in the
mining
and processing
of scarce
and non-renewable
natural
res,rurces
(and
the
asscciated
energy
savings).
ffi
H
I
ff
B
ffi
ffi
g
ffi
w
F
B
B
B
H
w
g
ffi
ffi
38
ffi
&
w
g
ffi
ffi
g
tr
g
r'
The
Big
Ri'er
Zinc
adjustcd
st*ndard
for
crucre
zlnc
oxide.
on
April
I 5'
1999,
rhe
Board
granted
a
perition
submitred
by
ERZ
for
crude
zinc
oxide
material
produceci
fronl
the
IJTL{R
processinEl
of
EAF
dust.u
(The
Board
amencje,J
the
adjusred
standard
orr
r\{ay
6,
1999,
by
removing
the
constituent
concentration
specifieations
and
revising
the
zinc
oxide
sampiing
requirements.)
See Exhibit
! I
forccpies
of
rhe
decisions.
The
Board,s
firrdings
in
rhe
BRZ
acljirsted
standard
opinion
and
order
are
rerevant
precedent
for
HRD,s
cZO
because
cZo'
like
the
crude
zinc
oxide
in
the
BRZ
adjusted
standard,
is
produced
from
the
HTI'{R
processing
of
EAF
dusr
(and
smaller
quanrities
of
otrier
zinc-bearing
materia!).
CZo
and
BRZ's
zinc
oxide
are
similar
in
constituent
composition.u
czo
and
BRZ,s
zinc
oxide
are
u;ed
to produce
zinc
products'
Therefore,
the
BRil
adjusted
standard
for
crude
zinc
oxide
srrongly
suppons
l{RD's
peririon
fcr
an
adjusted
slanclard
for
CZO.
il{ore
specitically,
cZo
meets
the
adjusted
sranciard
eriteria
for
virrualiy
the
same
reasons
as
BRZ's
zinc
oxide:
'
'rhe
degree
of
HTMR
processing
of
cZo
is
substanriar,
and
significantly
increases
the value
cf
the recycied
EAF
dust.
The
degree
of processing
of
BRZ
crude
zinc
oxide
likewise
is
substantiar.
see Big
River
Zinc
corporario_n
(AS
99-3),
Aprir
ls,
1999'
slip
op
at l2
(Boareldetermination
that
FITMR
processing
is
substanriat
in
terms
of
both
the
process
and
irs
effect
on
EAF
dust).
El@iyerzin
refened
tc
as
"EAF'dust
zinc
n*ia.
ffiffiid:l;fii;ffi'"i;.,1
rs
lH
le
ir
g
$
B
n
n
r0e
ts
t{
-
;
":::".TirlJ,..,],L.
AmeriStee.t
EAF
dusr
zinc
oxide
included
in
BRZ,s
neriti
?il!r,";j,!,!y:,::i, : :,j
r".a,
LJ
e%
;rj;,rd*:l#:J;;'d
:ff;;,fH:l"T:'j'll,
:1,
99-3,
99-3,
slip
slip
op.
op.
at.7
ar.7
with
yllJth
Exhibi,
lirltih;,
-l
a
^i,r,i-i.,.,_
tr
ililL?i::iiil,;';
Ii'^,
"iif,ilp.t;rion
t>
"
Once
F{FJ)
reci'cles
EAF dust, its
value
is nearly
the sanie
(approximately
5200) as
rhe
purehase
price
for crude
zinc
oxide that
BRZ
rvill purchase
from
AmeriSteel S_ee
id at l3
(BM's
zinc o:tide
has
significant
value).
e
Fffi,D's CZO,
like BRZ's
zinc oxide, is
chemically
similar
to zinc
concentrates
produced
from mined ores,
and
both
rnaterials typically
require
removal
of some
constituents before
firral
processing
(i.e.,
roasting
the
mined
concentrates to
remove
sulfur, and
calciningCZO
to
remove
salts). See
id. at
l3
(EAF
zinc
oxide
is
similar
to mined
zinc suifide concentrares
and
can
be
substituterj
for the mined concentrates)
.
End
markets for
CZO are
guaranteed;
likervise, BRZ's
contract
rvith
ArneriSteel
provides
an
end
market for a camparable
zinc
oxide
nraterial.
Id
at
l3-14
o
Finally,
HRD's CZA
and
the
BP€
zinc
oxide are managed
in an
envirorimentally
proteciive
manner from initial
prcduciion
through
end
use to
guard
against
product
loss. Id.
at 14.
In
summary', since
HRD'S
CZO
and
the
BRZ
crude
zinc
oxide aie
similar
products,
used
similarly as substitutes
lbr zinc concentmles
produced
from ore,
and are managed in
an
environmentally
protective
manner,
the
Board's
adjusted standard
for
BRZ
strongly supports
this
Petiiion, and
confirrns
the
commoCity-like nature
of CZO.
2,
Other
variances;
from the definition of
solid
rvaste.
HRD
is
aware
of
two
other
promulgated
variances from the
R-CRA definition of
solid
u,aste
for
commodity-like
zinc
produas.
First,
in
September
1998,
the
I'ennessee Depanrnenl of
Environrnentat
Conservation
("TDEC"') granted
a
variance
to Ameristeel
for
its
crude zinc oxide
product
(which
is the
same
zinc
oxide
product
that
is
the subject
of
the
BR7-
petition
for an
adjusted standard).
TDEC
evaluated ,crmerrsteel's
crude
zinc
oxide
rvhen
sold
"for
funher
ffi
ffi
g
ffi
ffi
ffi
ffi
ffi
ffi
H
H
ffi
ffi
H
ffi
ffi
H
ffi
ffi
30
tii,..'i.
*
fr
F
ffi
*
E
g
u
g
fi
*
E
*
a
fi
H
t
F
a
i:,tt':
f,.1.:
.
.''.t.
processing
into
irigh-ggrade zinc oxirii:."
See
Exltibit
12. TDEC
cietermined
rhat
the crucje
zinc
oxide
satisfied
all applieable criteria
for
a comnrodity-like
marerial,
and
the
crireria
applied
to
ArneriSteel
are identica!
to tlre criteria
applicable here.
fjince
AmeriSteel's
crude
zinc
oxide and
HRD's
CZO
are similar
products,
usr:d
similarly
as substitutes for
zinc
concentrates
produced
frOm
ore, and
are
managed in
an environmentally protective
manner,
TDEC's
variance
foi-
Ameristeel's
crucje
zirrc cr:ide
suppcrts
FR-D's
Petition
for
an adjustecl
standard
for
CZO.
In
the
third rel,:r'ant
variance,
the
U.S. EPA. applied
the federal
variance
criteria
(which,
as
noted
in
this
Petition,
are
identical
to the criteria
applicable
here) in
1991
ra promulgate
a rule
that excludes
from
the definition
of
solid
\vaste
a material
knorvn
as
"splash
condenser
dross
residue"("SCDR").
SCDR
is
produced
from
the
processing
of
EAF
dust
in
HTNfi. processes
that contain
splash
condensers.
56
Fed R.eg. 4i
l64,
4l
l?3
(Aug.
19,
i99l).
Applying
rhe
criteria,
U.S EPA
determined
that
the
SCDR.:
(l)
results fronr
substanriai processin_q;
(2)
is
solcJ
for
value (or
reprocessed
c,n-site
lo r€:cover
additional
zinc;
(3)
contains
zinc
concentrations
compatable
to
olher non-lvaste
scurcr3s
(i.e.,
50
to 60
percent
zinc);
(4)
is
guaranteed
an end
market,
and
(5)
is handled
safely
up to the point
of finat reclamation.
tj.S. EPA's
exclusion
for
SCDR directly
supports
this
Petition.
Like
SCDII
CZO is
produced
from
the
processing
of
K061. Like
SCDR,
each
of the factors
set
forih
in
the
regulations
is
applicabie
to
CZO.
Therefore,
the
SCDR
exclusion
supports
HRD's
Petition for
an adjusted
standard
fbr
CZO.
3.
An
adjusted
standard
supports statutory
resource
recover),
and
u.aste
rninimization
mandates.
An
adjusted
standard
for
CZC| used
as
a sutlstitute for
zirrc
concentrates produced
from
mined
ore
supportsthe
resource
recovery
ancl
rvaste
minimization
mandates
of RCLA
and
the
r''ct by'encouraging
the recycling
of EAF
dust
and
other zinc-bearing
secondarl,nraterials
_Ss-e
4i5 ILcs
5/2(a)(iv),5/20(c);42
u.s.c.
g
6902(a)(6);
see atso
n 6,
supra, ar
page
l0
T'he
3l
e;lcoufsgenlenl
of
rec).ciing
is..relevant"
to
3n
adiuste'J
standard
proceeding
as
a
result
of
the
purposes
underr5'ing
u.s.
EpA.s
originar
pronrurgarion
of
rhe
provision
on
rvhich
rhe
Board's
authorit)'
is'oasec'
,
^,..,6?'tqnd
wast€
rnininrization
benefirs
in
clear
U'S'
EPA
has
stated
F{Th'lB"s
resource
recovery
and
ten-ns:
'stent
\f
ith
natioilal
poliey'
idenrified
in
rhe
use
of
HrMR
is
also
*l'';;;;;"
it,.
nu.nlitl"li[:'j""":1s
vatuable
Ll:l*::*F:";1i:::"#nt=1*'l*t:iilil.'*,i;{;
consiituents
frorn
$'aste
tnutltt
*uuiiing
fro*
t**lln-o,land
disposeo,
volurne
cf
the
waste
residuats
fi;',.e"l;.r.o
t1l-*.rrr,e;
this
saves
enerBy
add
it'io
n'
becau
se
me'*
als
"T^
iJJ?"*"o"'onttn''
itreY
co
not
have
to
be
Proces:
'na
oorruiion-"i
unorr'er
source'E
RecyciingzincfromEAJdustproducesser,eralclearenvironnrentalbene|lts-
eor,ef4milliontonsofEAFdusthavebeenrecycledtodatebyHl.ir{Rtechnologies
intheUnitedStates,reco..eringover600,000tonsofzincandotherr'aluablemetals
that
otherwise
*'oulci
have
been
rvastefully
dispose<i
of
in
landfills
e conseryarion
of
mirlions
of
tons
of
domestic
zinc
ore
reserves
annua';'
Sulfide
zinc
ores
contain
bet*,een
3
and
5
percent
zinc
on
average,
compared
rvith
an
a'erage
of
20
percenr
zinc
in
EAF
dusr
and
nearry
60
percent
zinc
in
cZo'
Thus'
one
ton
cf
CZocontainsapproximateiyasmuchzincasmorethan!0tonso|zincore.
c
Reduces
the
need
for
irnpoflation
of
zinc
concetttrates'
":::,.,inlowerenelgycostscomParedrvithminingandprocessingot.r,irginzinc
tt"'rtt-'*:fi["',:11;:'l'J,[Jff
Irff
*J.f
llt'?'1il!*';'"oDocun':en'l
ffi
q
5t
l:.'.:
a
a
a
a
e
a
a
w
n
w
E
E
ia
I
l8
lE
t
IE
I
ln
la
lr
ln
Zinc
rcc:5'cling
prontotes
sustainable
developntent, especially
because
zinc, unlike many
cther
ntaterialls, is
^"oable
of
repeated
recycling
rvith
little or
no deterioration of
its
chemical
and
ph5,sicat pr
r,,.,,.ts..u
Indeed,
36
percent
of the
rvorld's zinc
suppty comes from
recycled
zinc.
See
A
l'oqNet_Gujdg__tgl{qlldzi0!
(Exhibit
/3). In the United States,
the federal
goverrrnent
has
estimated
that,
rvitlr
increasing
recovery, recycled zinc
rvillaccount
for,:0
percent
of
total
consumption by
2000. An acijusted
standard
for CZO
viill
create additional
incentives
to
recycle
EAF dust
in
an environmentally
protective
manner, thereby further supporting
the
resource
consen'ation
and
rvaste
minimization
mandates of Illinois and
federal
law.
EQNCLUSTON
HRD's
valuable
C.ZO
product
is
produced
and sold as a
process
substitute
tor zinc
concent;atesproducedfrommined
are. CZC meetsall ofthecriteriaforanadjustedstandard
from the deflnition of a solid
\.\,aste
for
a
commodity-iike material because CZO:
(i)
is
substantiall.v
reclaimed from hazardous
waste,
(ii)
has substantia
value;
(iii)
is a substitute
fbr
zinc concenirates
produceJ
from mined ore,
(irJ
has a
guarantced
end market; anci
(v)
is
handled
ro
minimize
or
eliminare
prociuct
lo:ss. Moreover,
an
adjusted
standard tor
CZQ
is consistent
u'ith
the
BRZ acijusted
standard for EAF
zinc oxide, as
well
as
variances
from
the
definition
of
solid
waste
issued
by other
regulatc,ry
agencies.
An adjusted standard also
rvill
encourage
recycling
of EAF dust, conservation
of
naturai resources and
reduced
energy
consurnption
(thereby promoting
sustainable development HRD therefore respectfully
requ(rsls
the
granting
of
this Petition
fbr
an adjusted
standard l'ar
CZO
produced
from the recycling of
EAF dust, as
*'ell
I
Additional
information
lroln
the
International
Zinc Association ex1:laining
the benefits of
zinc
reci'cling
u'orldrvide
is included
at Exhihit
/-l
of rhis
Petirion
JJ
as
smar!er
quanriries
of
z-inc-bearing
hazardous
arrd
non-hazardous
waste
feedstocks'
at
HRD's
HTlrlR
Faciiit3''
ResPectfullY
submitted'
HORSEFIEAD
RES
OLTRCE
DEVELOPiVIENT
COlv{PA}iY'
INC'
Paul
E.
Gutermann
?H
Br
B
&
w
ef.in,
Cu*P,
Strauss,
Hauer
&
f..:!9:LlP
i;;'N;;
iianpshire
Avenue'
T{'w''
Suite
400
Washington,
DC
20036
(202)
887-4000
John
N.
h{oore
Law
Oflces
of
John
N'
Moore
ioo
Xonfl
LaSalle
Street,
Suite
2200
Chicago,
IL
6C645
(312)
?82-es03
Date:
Jul,v
20,
1999
J.{
of
its
AttorneYs
l:l';.
E
g
a
a
E
E
E
a
I
E
E
;
n
?
n
E
E
a
t
EgBITLW
I,
the undersigned,
on
oarh
state
that
I have
sen'ed
the
foregoing
PETITION
FOR
A-\
.{D;USTED
STA\DA-FJD
upon
the
follo,.ving
in
the
manner
indicated
be!or',',
this 20thday
of
July,
1999.
Dorothy
h{. Gunn,
Clerk
lllinois
Pollution
Control
Board
100
\Yest
Randolph
Street
-
I l'Floor
Chicago,
IL
60601
(HAND
DELI\ERY)
Robert
La*'ley
Chief
Legal Caunsel
Department
of Natural
Resources
524 S. SeconC Street
Springfield,
tL
6270l
(FtRST-CLASS l'{ArL)
Peter Orlinsky
Assistant
Ccunse!
Division
of
Legal Counsel
lllinois
Environmental
Proteaion
Agency
l70l South
First
Avenue,
#
600
lv{ayrvood,
IL 60l53
(FIRST.CLASS
MATL)
:'.:
-';
:::"
.'
n
a
a
8
n
u
a
a
f;
E
$
g
a
a
E
E
n
E
E
rIFJ).
CHICAGO.
IL
EXHIBTT i
I
i
I
ffiil
ff
il$$ilfi
ffil
ff
l--ffi|
^\
r*af
$ffr
mr
l
Fr
j
sr
j
{#'
tP
'a
n
a
8
n
ff
fr
EXHIBIT
2
u
&
a
n
i8
I
",
r,
r. r:
.
E
il',i, : : i
:
:,
:
E
?'
3
:
3
:
E,
:
:
:
:
: ?
:
3
:
:
i
:
*";";d^ido^o.'
.\;
.y\.
i;
:'ii
t : :,::::
:
::::E
::
:=:
-:
:
:
:
:i:
=
;:
:
:
J.i":-*eoN@c
!,
\).t
q{r
4.E
i i:: r::::g3:3=:'i:3::?:?
i:::
=i;;;":oo-os
.-
ar
!.
..,
"
q
{
{
ul
*
-t
: : 3l I
:3:
:Tj::
:::
=
:
:
3
:
::
::
;:
:
:
..:J;"i4qtPruec
a:
...;-..
::::
;:::;?
:
::33?=ii3:
i::i==::E;:
:
:
*
4 E
i
",
ii.::
:
:::::::3g'ii3
::3:i:::E
;:
:
:
^id*opee'aec
.v
vv
:3
:
5
:
::::=i3i:
=:3
5
:==:3
;:
:
:
H-
€
€
.
g
F6
!
e
;
:
:
:r
;
:
:
:
:
:
:
:
:
3
:
=
:
il
i=
3
:
:
3
:
?=
?
:
=
;
:
I
:
o,
o
0
NN
-
(:
E
=@IG
8:
n
.-i"
@
5-.6
LJs
h
dai
8*
F
Er
B
(n@
{i
HF
5d
>@
tF
F.l
o
]-
u.-
6.
ge
a
o
E
H
ss3!
e?H,"",
F
u
N
,**t,
ufJ-t,1
I
Nl{x:F:
,q:
,^
d,-
@ts
Q€
oo
N)
0
x
li
6
e
N
Ng
e€
oO
|.r
N
e9
o
g
at
N
r.{
Hg
iE[q:ei:qelqlqEeia
!!i;il!;I!!iii:ili
l{
H
l-..-t
I
l,__l
E
l3
qr
E
ql
*l
_;
ffiI'
ffii
r
qr
l&'l
g)
ur
E
aF
rE
Eti
6>,
gp
5E
t^l
tr
Ert
l!
.,s
F{
P.
:1
eJe
=
E
)
-t
-J
-l
)
-1
I
_l
--1
I
J
.-
-'t
I
I
ffi
ffi
ffi
Hl
H-l
H-l
n
-t
Hl
$l
ot
'rJ
H
E
5
EXHIBIT
3
sdee
dQ
h
0
N6!\
9{96
p$d
e\
v'fr
{
L\
6
u\
a;6.r
b6-
o
;
d
e
q
o
o €
o
ts
!'
e
o
d
R
a €
L\ oi\
6
-
e.noF{O:6N86;
+ee€a
g gO
O
N
dA
O
g
e O Ns1€
O
-
<
^r
aQ
9D
6€9
d o
e
@ e
6
o e
-r
Q
e
(r
o O
O 6
e
O
_r€
€ e e e O
..,
\.;
r.:r
'!,
'.'
T
!:
e
L\
t\
Ll
60*6ONN
NOANO@d
deOOOg-r
oi.r=a
K
v,
oo
6
6
moL-
€Y.v@
€€<
Lt
rt't'n
E*
15;
'.
. ot€5::tI$r
-1-i
-;':';
-;';
g:**E-gsEg33E:B
gE
BBRF:3qq
' '
"
:
F;.
$-?oLrooo;oiooeF@NQoo€eea6oo
e ee
oo'<
Lld
v,
u
'./'v
'Y
'-'
i
e
ut
oe
€eNd<mR
NO6afOe6
o@oe9cg
a
Ad'{o
oE
6
@6;i
€f:@€
6ro-'
-6-
vrL\
e,
-L
sd
E Nme
@*
$d
i
6{
€
O e
O
p
o € O
!:'
V'9
n€
o
NS
Etnv6
€
I r o
go
@
Gg
€oioGO
e€
o
-o-.4:
I
e
€
eQa!e
e
NQ
o
oe
o
e
@ a
v
do
o o
ea
e
6
E
O
e
I
m
OO
....:
s
O
o
€
e o\G
v.-. v
v'.'
-"
a;
@
@dda
o
crd
N
FoLl
dY'*e
+11-
{e
ts+v,riNrrdEoN€q
e
d
a*6e
s
eo
a !.1
a
6
-NNe
-6
>6
xir.
€6<
t
a;;
-ia
oi
6
oei
N€e
oo
No
e
c? q
-
o Fr
m
o
o r'
4
i{
;;l;;J;
rid;
rdr.i;;;
r r
r.i;
r
";
J
d
"i
r
";;
r.;
'-'
t/
Y
G
edav'
d
oa
Vt
Pl-tn
AtnHo
O::!
6
or5
N
v
e
N
d
s
o
ur
6 e
ri
ur
+ ir
m
:9
o
i5 e a
o
d
I €
::'
6 N
n
L\ Q
o
€
trlo
N s ?o
NdoS?ii5i-aiieo
ee6ee
ev
-o
o€
{6RL\oo
@
t;
a..-...
r
o
3;
c;;;d;c;ci<ixeeeeeoeNeoe€@ee€o
o€
EQeo
o
-f,d
€
i.t
.-'
tf
'.t
odio
f!
oo
vt
a
de91
ob1
ro
o!-1
a
L'
urN
9dt4rr6dLte€
o+E'egeN-!?q!:de''u\o€d
>,6
u,^r@o?
<esiiAio
veoo6-e
o
Q
d
?oood
o N€
oo
v
?t;
-,;
;-;-1
'.-;-,
.
,
f
A
@.,i;e.doee-i6ie€€6€<doQ€@€64€€o€@eoo
.-.
y
!.j
.,,
'.'
!i'
6
€dN€
€o+€ivLlay'rsg-6Y=L\
J
v€€h-FoLlri<ax;oeNQ.>o€odeedNo+NeHvl
k@
e6@eft
deii-6doatj-o,n-eepa<FqoNoNL\oe
$
Gb.-r-.-;
C-
6
d6*
€
v@qcticjdddgce
Ro6€eo€€oeo6e€
e€
e
qd
€
.r/
.\/
.J
.J
\!,
6
odd€
e
@h
6
s
uvL\
bioPo
eY:1
L\
ad
=
6li!i{-rts+ttisfioCt€9ffi5oeoqNeo:{F-e€6o*6
Ljr6
mseoE
-oi
pEEbi're=+E---;
-==
Q
=
G'r''=c
s
E
IEd
r
$
J;":;
j;;;I;i;
d
-'J.l;;
d d
JJ
d
d
J.i
-':;
J;
d d
'i
V V V
vV
V
oge
B*
f 6
-ssS::3ie;HsEsBEEeEHEEEeq€qqBeeEEe
'''
:
E;
d*oeGtociio'io€elclEioa€s@o@€edeooood
Irl
Fr
v.
Pr
VvV
V
V
ddN
v.
s6
<e
FNva
oi$
n
too€
*-3
tq
6qtpS
q
9:
o
*eq
9{d
q
1'
-.-!t-
I P
o
?
Er'
on
?
ts
\?
f,n a
s
e
dvr
e
o
L- 5
; 6 € 5
;
s
Q
"5
5
e
; o
a
e
6
Q
^-'
-
e o
o
..aa
Ee
? O € A O€ft
€ ds
cl€
e€d€
odci
eo
oo
ee
o o
60e
d
V
'v'
,,
"'
i'/
z
l+{ H
€
5
-r
6
S
@
n
a
t
g
o
I
? r
i:3
u
}
d
*3
e
r{
o
3
o
ES
3
5';
E
g
3
F !
!
n
3E
r
s
3
A f I !
-
@
-
*
;j
il
;
ii
;
{
ul
<
6
n N N
L\
6
m
-
A8
:> E)
il;fi
[F:H]:BnEEsE
s:TEBBE:,raEE
-sBti
q q{
4F
@ne
L1
w
F
x
l"E
{LtJ)
N
rr\O
e@.{
a€N€QOd
ooL\doQ@
9€pao9e
@
e*
6
6!
$
La€L\
Olt'$€
6hd
?
@
€ N il e
+
@
O Y
I
!\
e
d
€
*
€
o
a
F \r''
rr
o
I
*
6s€6
-r6
+
s
edr
e I
6 ooee
6O
O
t.
dO
Oo
€
-so
eee€<€
6
eo
e e o
N6
0 0 4t
oe
€
(f
e
I
od
O
OS
{
O
NCtLt
Aft
a
-f
<
b N Ll
R I € s YrN
C{
sr
€
d
e €
&69d64d€€€6€L\€60660
€Nv6€O@6HeSec3€60do9
'.' Y'
.v'./
l.r
lrl
G
6
$-
K
x
ES
hr"
ut
$r
\/
r,f
\a'
\,/
'.'
f;u
n
l*t
mC1
'*tJ
*,.
*
}.t}{ NNXN,{
XH
H
HN
XA
*
*
X}I
NN
}.l
N
}{
,{
f
;j t,3
I
"i
I
f
,fl
,J
J ffri
r
{
:" d
d
FI';
ci':'j'-
E
3
N
€
r
e
E
l+
H
q
E
E
G
r
€
a
!-
ry
Hl
lrts
EE
ffi
ffi
ffi
ffi
ffi
&
ffi
ffi
tr
w
ffi,
ffit
pt
FH
EtJ
D};
EH
i;H
HE
HE
Rm
H r.t
HE
R:
flq
=E
sl
)
g-
6€
H'r'
€-.
ffi1
ffit
ffi1
ffir
HI
$l
HI
$l
ZCA
.
IvTONACA"
PA
EXHIBIT
4
ii'..
a..:
.t
I
I
F
iJ
ft
xt
n
I
B
w
F
E
Is
Ia
la
flg
Im
IF,
$,
$,
8,
$$
8$
ff$
$
$J
ffiFg
ffim$sg
sm*
EffiWI
ffp
g
ffiffi
ff*
$
EsEim
+iir
f
$
ffif,
ffiffi
ffiu
ffiffi
ffir$$
ffi
$*
/fT
r
|
i
$$
-*
il
*
ffitr $$(iil II
ffim
ffi-
$
\LiJ
6 s lf
ll
ffip
ffi'
ffig
gft
-t\k
r*.i
ft,E
F
$
g
LPffi
I
if
Sffi
ffis d*s-ffiu
ffi$*$
ffi"
Iil-
ffiW$rr'$*fi
XNffi6
H
nq
gf!
+
tr
ffilHfi
Fugtr
3l
EAItrffiJ
IEffii
I
F
mie8
+
T:
ES
EEI
tEt
l-
$n'
|
re
I
lwt
*-{l
F-_ni--
.,f
$ti#'
i
lm {b
.1J
H$l
;
l*
tr'F*"$ffi
\1"
ret
tr
-#ffi-I
-r
t
I
ff'
"*ffi
g
-
H
g
s-
a $$i"
6
$g
g$
"r
u
'li.'
$$
$$
,
EI
,E
zt
xfi
IIl
tf uJ
'-t-
EXHIBIT
5
'..,.t,:
&
a
n
g
E
fi
g
n
a
iH
is
lE
lu
ln
Is
ln
Ig
ln
!n
E
fis
il
IE
E
E
IE
E
E
Ifi
I
lH
lE
ls
I
lI
fl
a
Uruff
FHNCESSESCtr
EXTRACT*ffi
^,H]:ALLLJffiGY
RCEERTN"
The
lJnivensity
F}EHLKE
of
n
tichigen
,Ann,Anbon,
Michid*..,
ccMfqANvrrvc.
AME:H'=AN
HL*EVIER
F]UBUffi
HNIG
New
yonk
Londen
Amstendarn
AI'{
ERICAN
ELSEvIER
PUBLISHIN6
Cor4.P+ryY'
ii{q.
52
vanderb'iit;;;;;'
NJ
Yortc'
N'Y'
lc{!?
E
LSEVIER
PIJBLISIIING
COF{PANY
3351;;
-
---
v;-
Amstcrdarrr,
Ctltntttaat'
The
P'O'
Ncttrcrlands
Box
2l I
il
g
fi
O
Amcriean
Elsevier
Publishing
Co''
lnc"
t973
All
rlghr
reserycd'
No
part
of
this
publicaiion
may
be
reproduccd'
stored
in a
retrieval
system'
or iransmitted
in
rny form
or
by
any
mcans'
eleetronic'
meehanic-al,
photocopying.
racording'
or
otherwise,
withsut
perraission
in
writint
from
the
PubliSer'
American
Elscvicr
Fublishing
Cotnpany'
ln+'
52
Vanderbilt
Avenue'
Ncw
York'
N'Y'
l00l?'
Library
of
Congrtss
Ca'.aloging
in
Publication
Data
PehLke,
Roberb
D
Unit
processes
of
extractive
metallurtry'
Includes
bJ'bliographical
referenees'
1.
HetallurgY.
I.
Tltle.
TN655,P/,22
669
72-872]-4
IStsN
C-l,1/*-oollo-r
l{anufacrurcd
in thc United
States
of
America
n
I
I
I
I
I
ro{!
!t6{lrlrc
inrt0
Eott
(
lgtrtr
t6
*
a
&
rtt9
rtrrm.trttl
lttlltll
s
rrln
trt{t
l0 l6tl
ItBovsl
RtEl
ie
ls
Ia
aa
l.ttnt
Prousses
o/
Erlrcclive
ifetallvrgy
*a
I
8
llBn
Fis.
2-?
Drigbt-Lloyd
Sintering
lviechirrc
Sotrrce:Lurq;.1!anuat,LurgiGe*llschsfteri'Frankfurt(trtain)Germany'June196l'p'150'
S:nw-roast
oflers
an
*dveotage
over
the
previously
described
roostitrg
pro-
cesses
io
tbat.
agglomeration
ol
tbe
roa^sted
-*t*tiui
is
accomplished'
The
blast
furnace
requires
*
,rri*tfv
farge
particle
size,
and
hence
iron
and
lead
s'uJf,de
ores
ere
sinter-roasted.
This
process
is
usually
"*c*d
cut.on
a D*'ight-Lloyd
sintering
mochine,
ss
shown
i"
ris
2-?.
Roasting
i"
".*o*panied'
by
ineipieot
ftsion,
s'hich
;;J;;
I
l,orour5
cinder-like
materisi
cslled
sinter'
The
Duigh"r;;;J
'i"i*t"g
machire,
yrhich
";as
developec
more
than
5o
]-e'rs
Bgo,
eonsists
oi
o
s"rie"
ci
patlcts
or
pate
mounted
on
Bn
endless
track
T6e
concentratc
is charged
lo
a depth
o{
aboit
S-20
io'
on
the
psliets'
wSich
move
over
wi:rd
boxes
-t i-a
iift"it.
Combrxtion
of
the
bed
is
iaitiated
on
its
surface
b]'
a
b'rner,
end
tbe
**U[*ioo
is
oreintai"e<l
and
carried
througb
the
mass
of
the
cberge
by
the
oir
aro*o
through
tt"
.o"oitrute
to
the
wiod
box
below,
which
is
eonneeted
t*"
*rrJgioo-f*o*fflr*tvely
high"temperetures
(9r00-1200'C)
are
de-
ve.lo5red
is
tbe
*"#;
;;;g
it.tt.f*se
ioto
o
ccnrpact
rne"ss.
After
tl^e
ainter
has
reached,
tbe
end
J-il;
macLiie
it
it
ait"Ustged,
eooled'
gud
size'l
to
provide
a
uniform
prorluct.
fino
f'o*
the
siziag
oe"t"ti"l^
*:-t:t1:otd
*s clrarge
meterial*
Iu
sinter-ro.Jttr;;
lu.
.*u-
k,
itte
ore
scts
as
a
fuel.
The
rclstively
high
temperatures
&nd;dt;;
conditions
usuelly
provide
low
sulf'r
coutents'
pnruicu-
larly
for
the
roa-stiii"f
ffit*
({e) *
py*Uitite
(FeS)' tn
the
csse
of
low
sulfur
or
oxicie
ores,
fuel
is-"adea.
The
lat'*ici"l
i"
tofttted
to
sirnpiy
as
sloteing
ard
is
used
in
particular
;;;;;;;"r-riou
of
charge
materiel
to
the
iron
blsst
fur,.ace'
2-3
Sintering
Tberequirementfqpggsrsechargematerialfortheblastfurngcenecessitstes
agglomeration
of
fine
orss.
oue
method'
for
agglomerating
6nes
is
by
sintering'
sintering
i-s
the
procBs
of
heotiog
fine
materiuls
to
"o
elevatnd
temper&Lure
without
corrplete
fuion
;;1L;
aUu
Julutt,"rotiJ
part'icles
in
coutact
vrith one
anot'ber
adhere
*ra.
"ggi;rate
itrtr,
f"tgut,
*ore
'sejg|
puticles'
The
predominaat
mecbanisrdg
in
the
action
of
sinterin6;;;*t
aiff*ioo
and
incipieut
fusion'
nnd
Uotl
ota*
in the
comrnercial
slotering
of
ore'
- -
.
i- rha anar
Tbe
sintcri;;;f
lil-
quantitiesil
matcrial
is ofteu
necess'Tv
in
the
operatton
ie
L7
6rr -'t
fr
w
ffi
u
a
#
*
ffi
ffi
t
ft
a
il
fi
g
il
a
fr
P'gr
am
etwlur
g g
[
: ft,
o as
t
ing
-
A
g
g
I on
er
oti on-
C
ol e)nel
iott
sf
a rr,staIurgical
plant.
This
process
provides
aa
oppdrtuhity
to
use
fine
msterial,
arrd
often
makes
a
particular
process
feasible
bi'
converting
availabie fine materials
i-o
ar, agglomerated
fornr for
use es
a charge
material.
Sintering
is sometimes
ccrtied
out
in rotary kilns,or
b.v batch
processing on sinter
pans
or
hearths. Flow
of
sir
through
the
eh^arge
mty be by updratt
or
donndraft
methpds'
but
the
predorrrioant
ind'.rstrial
technique
for sintering ore
is
on
a movir'g heati,h, as
rr-ith
the Drright-
Lloyd
continuous
sintering
machine,
As origin:lly
desi6ned
for
processing
copP€r
ores,
the
sulfide fines
were
distributed
in a
thin
layer aiong a
traveling beit made
up
of
gr-ata1.
Tne
charge
w*s
ignited
and
the sulfur
burned
qut
sf
the
ore s-s air E/Bs
dra*n through
the charge
by large
fans. The
fines
fused
together,
forrrring
a
sffong
silttr
cake that
wss
desirrlble
for charging
to
e blast firrnace.
The brsic diflcrence
between
the
processiag
of sulfide ores
end the
siatering
of oxlde'ferrous
ores
is
th.e
self<ontained
fuel of ttre
sulfide material.
In
the
processirog of hema[ito or megnetite
files, carbon
ia the
fornr
of
coai or
coke has
to be
added to
provide
fuel
for the
sintering
proeeis,
The
utilizeticn
cf
the
Dw-ight-Lloyd
rnschine
(Fig.
2-?)
in
the
processing
of
iroe
qres
i-s
e..entially
the sane
as for
nonlerrous
sulfide ore$. A schcmatic
diagfam
of un
iron
ore sintering
Flspr
is sheit1 ip
Fis" ?-B- Ie is eYid*nt
thst
s{i imBertsut
part
of
the
sinter
plant is thr
rnixlntr systern
that
blends the fine
ores,
Iimestone,
coke,
plue
r,be
fiues
returaed
from
t}e
einter
strarrd,
The charge
mix is loaded
onto
fhe
meving
grates of
the sinttring
txrachine,
u'here it
passes
under
a
burner
thst
ielrte.
the
bcd, Air !s
drasn
th'qugh
the brirning bed by
the suetiee
sy€kql
beiow',
a.tHi|lt
g.
a*6tG
€.ffi68
r.EA
xl@igaq!
'
fig. 2-8.
Ima
Qrc
$intering f'lent fo,
Prepratioo of Self-fiuring Sinter
Source:
Lurgi
ltanvt\ Lurgi Ceseltsohaften, Frankfurt
(Main)
Cetmmy,
June
1901,
p.
l5l.
.-
-,*_-q
! ,-
(-.n
-:J-J-
|
:H
r----*
wl
I
|
t
e
cb
o
f
o
6
o
G
t8
t*
la
le
ls
ls
la
lB
lffi
lz
il;
Unit
Proueses ol Eilratliue
ltf eto.llvrgy
and
at the
end
cf the
strand thc sinter
drops
ofl' the
pallets,
where it.
is
cooled
and
screened.
The
undersize
particles
ere
then
returned
to
the
sintering
Frocess
LS
rec-vcle.
The
Juel
requirenenl for
lhe sintering
of
iron
oxide ores
ranges
from
&-8/s
coal or coke, and is relatively independeut
of
the ma,teria.l
to
be sintered.
Tbe opti-
mum for the
fuel requirement varies slightly and depends
ulon
whether or
not
chemicd
reacrions
are involvod in
the
sintering
proc€ss.
Tbe
presence
of
apprmiable
amoun'r,s of limestone
or
water will require
additionsl
fuel,
anrl
may
depress
the
msdmum temp€rsture
gchieved-
Ysriation
in
the carbonete or
moisture
content
of
the
sinter
nir
will
give
a
varistion
in
the
widtb
of the hot sone thgt
moves
do*n
through
the
ore bed. In normal tiowndreft
sintering, the cornbustion of the
fue!
in
the sinter
mix is imtigted
in the upper
levels
of
the
sinter bed.
The hai. combustion
g&ses
are
pulled
doerward
through
the bed
and
preheat
the
sinter ch*rge.2
The
presence
of
rvater
iu the
sinter
mix
q'ill
limit'the
incregse
in
temperature
of
the
sinter bed until
the
water
is
veporized. The
presence
of cerbonate, such
as lirnestone
that
is
charged to self-flujng sinter
(see
P1'rometallurgy
III),
rv"ll
resuit
iu
s
broadening
of the
corrrbu-sion
frorrt
thet
follows
the
hest
front
do$.u through
the
bed.
Iemperulure,
oC
Fig. 2-9.
Tenperatule
Dlstribution
for
T*o Sint'er hfixes'
(Combustion
is
occurriag approxbnatcly
et tbc
midpoint
of tbe bed)
!8.
Eketorp,
8fE'6LMr{KING,
Thc Chipman
Confaerc,
p.
180,
\ttT
Press,
Cambtidge.
Mrss.,
1905.
a
n
&
a
#
Pyrometallurgy
I:
Roasting_Agglonrcrotian_Calcination
lg
iilustrated
hor
in
Figure
the
zone
cherge.
2-9
is
sho*'s
b.v
increased.
the
The
the
selid
peak
inftuence
iine
e
,.*p*r"iuLu'or
*"ifi"h"Jptun*.
i,'
F;.';-;:
of
the presence
,r,u
b.d
of
fron[
a
large
i;
;;;;ua
shouid
amount,
una
cf
calcium
rhe
u.idth
carbona,u
oi
the
v!Atra{
trotrE
snould
oecur
in
sintering,
as
often
or
-
arr
.Thc
can
rhrough
engineering
be
derermin$
porous
rlntionlh'ir-t
betis.
t
v
of
l"f*;;;;-rinterin6
puiti.J"r.importanc€
f;;;rr:i:af
stntering
,*L,
ii
the
,.i.".ommerciat
opercttcnsare
bed
permeabirity,
besed
on
n.hich
flow
r
to
BI torrs
of
materiar
operetions,
"",,
u"-"i*"X
per
squ&r.e
foot
of
hearth
srea
per
day
on
a
,il;::l-,tr:tJJ}';'"rt
'n"'hin;'
.'r
lJ,.ui".
in
ruer,;;;;;""ts
courd
be
achieved
such
i;-;;;;?:"i",il1ff.?rff
as
ffue
fas,
naturar
e*,
ii::#Ifi
;i-f-u,ur'Jit"iurnua
f,:.,T;*6,*:lJl?
in
*
t
oo'aliouu
tt,u
jtr;Hf
sinter
machine.
il*il
ilJ,'I
::::;ff;|illf
;';**;;;
mechanicar
."i
.r,.*i.ar
properties
or
the
uniform
metens'i
rmportance
.
contror
sinter
florv
as
of
and
mix
the
the
sintering
*i,^ur.
is,or
oroductivity
pt"p"rit"ri#
of
iron
or
ores
fr,"
by
iion-
o-f_rarr.
the.D*ight-Lr,ryd
urast,
-",.i^i,
frr;";;i";resses.
i"
process
provide
rhe
is
a
of
chernicartl,
contror
particurarof
requires
conr'ro!
of
the
i'rr,In
ryi#;;;;;:.
u""i,nu*
i,ffi;;"
or
rhe
sinter
strand
iillu'fh;"point-
r"rr,
*.r,"i'.lmbustion
just
wind
as
box
the
ofren
sinter
can
reaches
be
used
,h"
to
d;;;;i;;
monito?
,hiu
end
.,burn
of
the
strand.
Ti
is
compreted
rhrough,,
o"lrli.it*r-rure
in
the
8
T-f,RD
.
?ALT.IGRTON.
PiT
EXHIBIT
6
I
w
s
6
d
o@
e
b
o€g
do.*8o.oo-
eLt
|:JL@$N€€€dfiF@sod-€+oo€o{L-€<-'oNdd$
E;
F x
m€
.?
q
dw
"i
i:
6
i?'l?
o
0
g.qo
e tq':1
<o
ereG,
1
ctdocre
?9
i':
q
":
6
a
I
1
o
a
os
? ci
odoa
i":
i?:
a oo
HH
€
H
=
"'
L'Y'
@o
OR
U\
e
N€S
OL\ar€€@$i
Vt
+d
ov6euF*as@
qe€NsqNqo6oNbeodF6€ddN
;; ; e
;;
n eLts
o e;v,d
o e
s6o
G
c)vrG
o
eoa
eeo
oR
-r'e.l
t; c;
; cj v,
e
e € €
€
-.
6
€ e ar
o e 6
g
o 6 o
s o
e.? €
o
e o
q
d
v...
..,v
v
1./.,'
ct
<
^Sor.-*
E@
L\
F
ts6yr
AUlt
6
^ie
c,
A. tu f v. o
€
€ ft
-
c! o G
d d 6 V {5
O
6
Gt
5
ts
d
q
:i
cl
!l
c
d
6
v,
6i *
x x *o
6ut
6o
c\
g
vt a
o
eF
o
Q€
s s
o o
€e
e
oN
6
oo
taataa
fr
Oa
D
$
<r
(:
OO
O.<
O eeeOeAeO
g
A
OO
Q
o o
o
9e
ed
v'.'
v
\./
cld{t
6F
1.1 d
dsv,
oLte€o9.94
L\6dmOn&R-{O
6@+ssOao
€A
Q
F
tu
dq
-!1
-6e
=q
e@o€6
;d,
N iq
; d
q)s
H e
i
iiu
o
o o o
F
6
6 o
/n
e
q
g
€
E
o.{
6
o
N
rjci
;
cj
o o o@.{
oco
a @ o€do66
o
€ e €
o a
eo
e
o oo
i.j
..,
..,
v
Y
'.,
ta
atao
tat
L\
fl
9€U'
eU\ffAOmd+
st
4N
ts
gSNeQee+oa<ol'<ioNagaoo\aqi6oF'N*N
idto
;.God
4qou\r
eNouro€€6oo€o
€6e<2oqoN
@o6
r{:
E
;i
{o;ci
* ooo€G
"{€€o€(?oa€6
(rGo
eoGeooeQ€
v.
...
v
!/.
6
o9
oe
L\
@
vC?Yr
6LrSOOA"dtl
V\
@s
e{ i F v
N F €
v
\t
-
o
p
N
d
iO
e5
€ O
6 €
€
re
<
o
_ra
e
< e r
{
>.D
N;m;ft
oeu\oo;b€€ocdooo
oN€
oQeL\o
No€v'
:r6
S;
s-i.re$Eoe
dedect6€6s€GClo6oo6cls6oego
)
d
'.j
.-'!j
v'l
a9
6v.
$
F
*ttv
61.1
6€6$d{
Vt
0L\
s66Lrg,r{
d+
OSNOe-TQFOOSe
S d
d
G
d?
oe
d
Fa
trt e
ui'n
ra d
ar
a
ei
iq
ts
-
€;i
!'.
I
6
o ur
s €{5
cl
6
€t o e
€
Ll
€
N o o
@
3
6-
;
ocicir
ci
oooQ
d@€osasoo€69€
o e
eE€66€
ee
'v
..,
w
Vl
o<a
!\
e
6$!.i
\-r9\d6+9dd
v,
6t{
n
B
s
{
d a
r\
@
d s 6 N
q
a
E
ai e
e
€E
I
vr
!9
=
!l ].:m
6m 6 vv\
>d
+ vo
q
sooulr-a
; €e€€br€eoct
e o{geoul6
No
€N
i';
.-.
t
'
'
c
t
t
t
hX
;€dd$o6ooedeLre€oea€ooeo6oo€9ao€d
'
'.'v
€o
u
N€
e
fJ
fHU,
FL\G€o9tttd
N
Ovr
J
{v&ddqf+g+6o++ft€evae€egN_r€4Lr€G''Ne
F w
;
'ir
itii@
6e-A'
o
*;N
eao
s e
{t
oe'e€
e
qo
{ a
N
o 5J
+
f
S
d;
d d;.j
dd;
d":*:-'"iJ
"idd
{*
{
\'/
./
'r'V
dd,j.;
V
d
d.j.j-i
\/'J
i
d
i
d
-:
L1
cdq
GR
{
o
€es
€|.|rc!lf
q?99$I-'
{
66
=
aul(aFBaANEFQ*!m-de€?F€(:pac':t9:{6mo
dv'
H
U
s
n-
.ics;
4 a
q
n
ci
"r
*
q
6i
qqt
6€ rF
q
€
":
d
":1
aoosct
":
qq':eqc
glc!
cllGtc,
?
1:
olp
G@
q
?
e:
or?o€
t':
i
e
i:
e<
Err
€
w
'JV
V
V\'
T/!
od€
'-
!6
ts
6
eOU
Ottil(A€GtF*
6
6e
h.
NG$6FdF6€9NNm.!!leoEFei9Gddedfr
osFrN6
f, s
il;a;'i&
i.a,ar 6
vj
-
o
is ir
€ iso
(a
[^
c| Gr
69
cr
r.fr d ta
q
€Ll
o
d e e
m
q=
'
;
'
i o r r
'
t
'
'
E
6;
;acj
qi
o e
o66
c)..ct6oo
o
o cl
Gl€l/lt
G'
q,
ct<lctso
o E @
x
t4-,
{
l+
!, vV
o€
€@
u1.o
vr
No
G
6m
q
wl
6
m
g
i
€
d€t'
N
rr e 99 !?
cttJ\+f5
!l
6 ri
lAFdd
F Q
d
e
-'
6
--l>-M<o
n d N 6
ai ; a
;
N
G,
o
L\
d
ci
;:
;
rn
b er o
co c5 tt <'
tit
co
ct
€!
.J
ct
$ Gl
fl e 6
N
G€
€ €
{
Q
A€
*O
€O
6 O € O CtO €
€il o 6 c,
g
oGl
g
C:€
O e't
rv"J
A?
ldH t33
.i;j
Fii
sfisttr
L;
o
q
o L1
s
sfr9l
v,
{
N
@s
€s;33
e
e ii6;i
S
q
g
K;
3
3
3
EA{E E E!
'n
=
r{;
e
@$
!\
d €
r
AC'
dLr
?, ur
a
cr.*
a
€ F
6 o 6
d
o o ii i5 Ei
o o
e
6
d
q
='N
o
F
'<
N
-
n
-'d
ioo
-v,o€!rG
€
@
d
< oeoii
66€6
v
6 e
ee
€e
Nee
1
5GJ
-;:-;';--;--...
(i..
l{
I
.!t
s
f4
He
H5
v
F
Hg
8*
&
lr{
L!o
Eo
Ad
L!
h
il$
i{
rrr\rFtrrNt{N
NN},..
l:
g
H
q
f
.F
@
p
s
a
E
E
L{
H
E
E
LI
d
H
fr
&
o
fi,::y
)rc
}.l:.l
Pd
ffi
ffi
ffi
ffi
g
ffi
ffi
$
$
ffi
ffi
tr
H
ffi
ff
ffi
ff
ffi
t{l
i
*F
>^
E(J
tr>
gA
8",
5E
lrd
G
F{
TJ
4rl
F lir
r.t
flil
H
,qT
Yrn
t:t bl
VJ€
*f;
g
$
$x
EXHIBIT 7
H
ia
ln
lm
lB
ls
ls
lm
|;
ll
a
a
F
$
a
a
a
a
$
E
E
I
a
n
a
iE
in
I
it
n
Fnre
I
EXHTBIT
7
DcEstiptipn
Summary of
EAF
dust
processing
capacities
in
Europe, Japan,
and
the
United
States. Fronr
Kola,
"Steel
Industry Dust: Solving of a
Problem
by
Recycling'
(1993)
Letter
fiorn
Ling
Wong to Tom'l'heobald
OIou.
5, 1998).
tl
B
IL
d
@
m
-d
e
g
ery
H
G
<:
t3ti
$d@
?rl
H.g'
eE
'ErqruruF@'eF
i5-6fi-6
d
E
gS
.E_0JU_Gqx
fif
x
g
$BFBTNE#TT
(g
BEH
H
Hses.sg
s
$ffi$FFffi
Q}
F\
E
*0
Etr€
flq
€'i.d
es
0
#
ffiHF
".i
€q
*F
En
Effi
*€
rd_85$
8*
ffiE
Ju
aF.l
HEHHHHSH
*SR€ShrR
sl
€
(B
g
rtt
"?
ffi
tfil
ffi
$&EE$$
HHHBHH
S$8EEH
I
c3
!n
s.l
so
m
Jd
$
tr#
affi
g
(s
e
E
(D
F.
cs
'€
e
$
qrl
tr
T ]H
rr-
L;-
n
+t
lJd
00
ftl*H
#t€s
:q--Ssd-*11
ABQdE
F tr.y#.,G
[9"(9tff,(?
lJ.x;'d6$*
$
G[r
C}
h6
Rl
.&d
u
G
H
EE
e
G
0g
6
E
€
!o
(ng
(pE
SH
t.f}(t)
l&ffi
l(4
c0
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
ffi
$
ffi
ffi
$
$
H
gg$g$
q.l
gI
HHHHH
$i
aenwu
C):
G?
(n
03
t)
O
6
OJ
u
c5
Lr-
H
YtEEffi
&"o*
-t
€:g;:
$#$-f u
4$slsl
& +#ss
fi'e,$,flS
*gl4 1
ffi.G
et
,EB?l
#*$fi$
i
mr:ng
$
i
,Cn
0)*{*'l
5
I
(ffi{ncnH
I
I
I
:
i
I
I
I
:
!
FI
fii
ol
\al
(Jl
"ml
C
(g
a
fJ
i.
I
.as$
ff!
*l
.54
\J
I
L[*
{JJ
4
t{6
O
rts5r
l*
Q
F
!4.d
ff
rLa
tr
r*
Ftr6a4qEqq
strffirffiffi
1,
3.
4.
t
q
s"
Fr
ls
I
I
g
335
lv{cdisoa
Avea:rc
New
York,hIY
10017
Tet:
212-818-8186
Fan
21:,-81&8502
NovgPlex'5,
1998
To:
&{.r.
Tom
fteobsld
Zi"c
eorporn*ion
ot'
Amsrie
e.c,
Mu,
Mike
llehas
From:
Lbg
S/oag
ITCJCHU
Inrersriolal
R-E
: WAEIJ
OIGDE
we
rrs
plea.rd
tc
prqvide
rbe
daa
on
sarlz
oxide
prod,.xan
io Japcn
as
yeu
bave
reqt:estcd
$Pr?rlqstoSqtBI
h&Iin"Cs.
t
tfu
Shrlshiiqclls
l.
t
o{Ered
on
acs
ialqd
nerr
suitotu *irii
asnust *wtz
oxide ptoduction
of ?0,000
wMr.
9-qrdfg*duciag
2i,00o
DMT
ofzlrto
(zinc
cslchsd
conecutrce)
and nrpplying
I{srba
phal
r,,tme
eiac
actal
b being
reelrclod.
!{erbe'r
eqpaciry
k
S0,OCO
DhtT.
ltirtns
Suaitn*rno
plart'
Meat
locsced.*
Minisg
q$y
Co."
Lgd.
city,
Flyougo
Mecusc
(nc*r
Kobe),
ir owncd
by
Loce#d
in
FuhpbKyushu
wla
8!sBE
wadz
oxice producdm
of 70,000
wMT.
crcrerdy
rsodueiry
25,000
DMT
of
&lo ad
rupplylng
Flaebinoho
phut
wherc
zinc
maaj
ig
resycled-
I{*binobo
plasq
to*d
in
Aommi
pre&cture,
is
joLrdy
onrnod by
Mirsri (umin
podoa),
Srlnitomo,
Toho
and
Mltnrb{shi
I
8
fl
p"#ffi#'J-
rr.ge
Z
of
2
m,,,*-@iH_u.**i&ar
pt
proercing
rz.o*rrrr:,r,
a,
-
uual
c"slz
orjdeproduch'on
of
d,3,0oo
wnfl',.
rY{uI'.
--J.
r
rc"lstf,LuE
*j
ilffi*Y:q
l,z:wDr-,?a'()
t1#,'rtffin"rorum:$4{r)
;uruidir*ffi*:
ffiJ,Hmffi
ffif,
",;;
316
suppjvrne
;;::::*
i{rrima
pranr
and
and
Haa&in.he
Hac&inohc
ucc
steel
producen
I
iffi.T**
&6ct'rc1
srirh
eilrn'r
waere
oxjde
Z.
C*__Ji
*rU;;*
Eii,fT.
-q
rrcrcch'€)
srirh
eilrn't
waele
oxjde
,,^ : : :
d
;ffi
f
fliffiff
*,Tffi,imn
if.Bumn
ff"*eilffi:mff*,Tffiffi:fftr
uoa
ro
I
es&uc
i
ff:f,'?"p"#'Jl:3"
l.
pr*ririiffi#*K
'
ffiiryi"ffi{&ffi*j
F'rruahias
prerecr'rsv
wi,,
enruiar
,ncrz
2
ffi"tf:::i
to,o"{h*o'
oxide
tn
uecu*;;ffi
u6''
Ltd
rg
tiac,
Sey
are
dso
rc€ycjr4g
cadnripn
aao
iead-
Wt
hope
thisrnforme
r* _
lioo
proves
belpfut
to
you.
$
tr
!
I
I
EXHitsIT 1g
r
fi
E
H
fi
fi
n
t
n
7
;
ir
in
I
ir
il
li
lE
ln
ln
u,
tE
u,
.i
A'6
L<,
:JE
cO
u&t
oql
t/n
Ge
iS
fo
'rtJ
(..',>
eJ
6*
C-
ir
,c
t
c.
>t
[o
g
l)
5
c
o
v)
N
cl
i
l\
ol
rf
I
N
rf
<i
t-
R
0r?
,\
E
;)
.t1
a.)
,,y
o
-o
o
GI
c
(,)
()
c
o
o
o
N
'0
c
G:
N
q)
()
o
v)
it
'i\t
E
F
0a
I
e.l
I
,l
\q
I
+
\o
Y
.f.
Y
(n
.i
.N
vl
cr
q
ir
t\
!l
F
.t
r.r
?
"t
ct
A
-'
,<
Y
|-.
i
c.l
,\
r-
O
;
-r
/^
-r
(\l
r:
-f
;
9
,-i
oo
,\
Y
&
?
,^
?
.\
o.
c.r
I
N
T
aa
a'
Y
al
Y
a.l
,^
fi
rl
a
N
tr
.g
?.
o?
d
A
.}
-:
l}.
-|.
c)
al
-\
Ct
u)
{t
o\
E
o-
c{
o.
r-
t-
?-
b,
r-
,f,
r\
o.
ao
o.
c.
(!
a
t
t
r*
a
N
^
c;a
c?
C>
sl
;{
()
8
o
o
<i
<r
c)
N
<>
C)
I
o
h
o
.lf
-;
o
o
c?
5
6
0i
I
U
r.J
t\
.?
N
o\
FI
9
9
FI
f:
I
o
o\
Y
o
;
I
a
al
Y
q
et
;
Y
c)
I
a
E
;
o
c)
o
(\.
a.
r-
F-
E,
rt
I
c)
t+
A
c
p
G
L)
N
g!3
I
V)
rt
o
(n
o
c{
E F"
o
(J
,l'
U
z i; Q
O
B
E
(
a
(")
c!
ct
tr
U)
tL
ffi
H
ffi
ffi
$
ffi
H
ffi
m
ffi
H
H
$
$
$
$
n
$
il
EXHIBIT
T
T
E':'
E
E
g
a
a
&
ffi
H
ffi
g
E
tr
a
F
H
n
g
g
t:..:
'a
n
ffi
H
&
ffi
8
g
ILL,INOIS POLLU'ilON
CONTROL BOARD
April
15, 1999
lN Tl'lE
ir.,lAlTER
OF:
PETITION
OF tsIG RIVER ZINC
CORPORATION
FOR
Ar.,u ADjLISTED
STANDARD
UNDER 35 ILL, ADM.
CODE
720.131{c)
A-S 99-3
(Adjusted
Standard
-
RCRA)
LEE R.
CUNrtilNGHAlv{
AI{D RICHARD M. SAINES OF CARDNEI?. CARTON &
DOUGLAS
APPEARED
ON BEIIALF
Ol'PETITIONIR:
and
C!-iRISTOPHER
P.
PERZAN
APPEA-RED
ON
BEHALF OF TI.IE II-LIhIOIS
ENVIRONIvIENTAL
PROTECTiON
AGENCY.
OPINION AND
ORDER
OF
THE
ISOARD
('oy
K.lv{.
Hennessey):
Petilioner
Big River
Z.inc Corporation
(BRZ)
operates
an electrolytic zinc refinery in
Sauget,
St. Clair
Courrty, Illiaois.
BRZ uses various
zinc-containing
materials as feedstock
for
its refinery.
One of
ihe
zinc-containirrg
materials that
BRZ rvould
like
to use is recovered
frorn
dust emitted
from
electric arc furnaces
used to
produce
steei. This
secondary zinc oxide
material s'ould
ordinarily
be considered a
"solid
waste" and
a
"hazardous
v,'aste"
under the
Resource
Conserv'ation and
Recovery Act
(RCRA),42
U.S.C.
SS
6901 erseq.,
and
corresponding
Iliinois
hazardous rvaste laws
and regulations. BRZ
would like
to use this
secondary zinc
oxide material without
becoming
subject to lllinois'
hazardous rvasie
requirernents.
To that end,
BRZ has
filed a
peiition
for an
adjusted
standard
under 35 lll.
Adm.
Code
7ZA.l3l(c). Section
7Z0.l3l
(c)
allows the Board to determine
that
ceriain
materials
are nor
solid
rvastes,
and
therefore not hazardous wastes,
if they meet
certain
criteria. BRZ
asserts
lhat
zinc
oxide material
recovered from
electric arc furnace
dust
(EAF
dust)
by a high
temDeraiure metals
recovery
process
meetg these
criteria. BRZ
aiso
propcses
several
conditions
on the adjusted
standard. The lllinois
En'rironmentai
Prctection
Agency
(IEFA)
recommends
thai the
Board grant
ihe adjusted standard, suh-iect
to certain
conditiorrs.
The Board
finds
that BRZ
has established
that zinc
oxide materiai
recovered
from
EAF
dust by
a
high
temperature
metals
recovery
process
is not
a solid waste
. The Board
theretbre
grcnis BRZ's petition
for
an adjusred
standard,
subjeo to the conditions
set
forth in
the
order
that follorvs this opinion.
rBacEpugAl
HtsroRY
On
September 24,1998,
BRZ filed
a petition
for an
adjusted standard,
subject to
conditions. On
October 15,
1998, the Board
accepted
this matter for
hearing
and on
$
I
g
E
a
E
n
g
ffi
H
a
g
&
fl
w
I
ocrober
lrj, lggB,
IEpA
fiicct
a
rcsponse
to the
petition.
ln that
response,
IEPA
recommendccl
rhar
rhc
Board
Eant
BRZ's
rcquest
for
an
adjusted
starrdard
with
conditions,
subject
to certain
adclirional
conditiorrs.
On
October
27,
1998:
BRZ filed
a
reply
in
rvhich
ii
proposed
nerv and
rnodified
condirions
on
the
adjusted
standard,
including
rhe
ionditions
that
IEPA
requested.'
Hearing
Officer
John
Knirrle
held
a hearing
on the
adjusted
standard
petition
on
Decenber
17,
lgg8"
BRZ
presented
one
witness,
whom
the
hearing
offic_e,r
found
to
be
credible.
BRZ
also
inrroduced
four
exhibits,
each
of
which
the
heaiing
officer
admitted.?
At
hearing,
BRZ
proposed
to amend
one
of
the
conditions
it had
proposed_for
the
adjusted
srandard.
Tr.
at 5-O:
g*n.
4. Counsel
for
IEPA stated
at
hearing
that
IEFA
agreed
to all
of
rhe
conditions
rhar
BRZ
had
proposecl
both before
and
at
hearing.
Tr.
at24.
IEPA
offered
no
iestirncl'ly
or
exhibits.
The
parlies
chose
not
to fiie
posthearing
briefs.
I--LGJL
FBAMEWORK
The
status of
marerials
as
'solid
$/astes" !s significalrt
because
up-der
the
larvs and
i-eguiations
rhar
Congress
and
the United
Statcs
Enviionmental
Protectiotr
Agency
(USEPA)
hai,e
esrablished,
only
those
materials
that are
"solid
wastes" can
be
regulated
as
"hazardous
$.,astes- under
RCRA
and corresp,onding
lllinois
hazardous
waste
laws and
regulations'
Accordinglv.
materials
that are
not solid
wastes
are not
subject
to lllinois'
ha;zardcus
rvaste
reguiariois,
rvhich
impose
various requirements
on
persons
rvho
generate, treat,
store,
dlspose,
re[ycle.
or
transport
trazardous
\'.'aste. See ii5
Ill. Adm.
Code722-726,72E.
Generally,
a solid
rvaste
is any
discarded
material.
See
35 ill.
Adm. Code 721.102.
A
solid
s'aste is
a
hazardous
waste
if it
exhibits a
"characiel'istic"
of
hazardous
rvaste
(i.e',
it
is
roxic,
corrosive,
ignirable,
or
reactive) or
if it is
"listed"
as
hazardous
waste
(e.9..
it comes
from
a specific
type
of
process,
such as
electroplating).
See
35
lll. Adrn. Code
72l.lA3,7Zl'
Subparts
C and
D.
BRZ
would like
to
reclaim zinc
from
zinc oxide
material
that
has been
recovered
from
EAF
dust
wirhout
becomingsubject
to Illinois'
hazardouswaste
regulations.
Exh. 3 at2,2l.
BRz
asks
the
Board
to
determine
that zinc oxide
material
recovered
from EAF'
dust
with
a
hlgh
temperature
metals
recovery
process, rvhich
the
Board
will
refer ro as
"EAF
zinc oxide.'"
isiot
a solid
wasre.
BRZ
seeks
this determination
under
35
Ili. Adm. Code
720.131(c).
That
provision
establishes
standards
and
criteria
for the
Board to
use in
determining
rvhether certain
marerials
are
not solid
wastes.
See 35 Iil,
Adnr. CoCe
720.t30(c).
Section
720.131(c)
reads
as
foilows:
'
BRZ's
petition, which
was entered
into evidence
at hearing
as
an exhibit,
is cited as
"Exh.
3
at
_.'
The
parries rreat
BRZ's
reply
as
parr of the
petition
and
the
Board
will consider
it as if
it
\,-,as
entered
into
evidence
at
hearing with the
petition.
However,
for
clariry,
the
Board cites
BRZ-'s
reply
as
"Reph'at
-."
IEPA's response
is
cited
as
"Resp.
at
-."
?The
rianscript
of the
hearing
is
cited
as
"Tr.
at
-."
Hearing
exhibits
are cited as
"Exh.
H
E
I
Is
H;
In
In
ln
lE
la
F
F
f
E
H
g
The
Boarci
$'ill
deterrnine
thar
,l,or.
nru,o.rials
rhat
havc
been
reclaimerj
'r,ut
nci
be
initia!
reclaitned
ye(
reclamation,.
a
commerciai
frrrther
the
before
product,
resulting
rccovery
ancr
maicrial
has
is
tc
compleretl
be
is
iommodiq,-like
recraim.in,ni,ei).-
arc
not
solid
{even
rvastes
r-rrir-""
thoueh
if,
ir
aftermusi
is
"
dcrermination
r'ir!
be-based
on
rhe
folrorving
;rl;;;r,"
l)
The
further
degree
processing
of processing
ttrat
ii
required
the
materiai
:
has
undergone
and
the
degree
of
2)
The
'aiue
cf
thc
nrateriar
after
it
has
been
recraimed.
3)
The
ciegree
to
n'hich
tire
reciaimed
nraterlat
is
iike
an
analogous
raw
maleri3!:
4)
The
extent
rc
rvrrich
an
enc
market
for
the
reciaimed
material
is
guaranteed.
5i
and
The
extent
to
.'l'hich
the
reclaimed
marerial
is
handlect
to
rninirnize
loss:
6)
Orher
relei.anr
facrors.
35
Ili.
Adm.
Codc
7Z0.t3l(c).
FJINDINGS
OF FACT
proposed
zinc,
(2)
In
BRZ's
operations.
this
section
cunenr
of
operaiions,
rhe
opinion,
(3)
the
naf.dusr,
Board
sets
(4)
forth
Eniri".
its
findings
oxide,
of
and
fact
(5)
regarding
BRZ,s
(l)
Zing
m:lliontons'
In
lggT'
Zinccar!beusediogalvaniz_e,p'or.i'iilr"d"ucebrass;
thetotal
rvorldproductionanclconsumptionof
zincivasapproximarelyg.5
at
powder
2'4o/o
produce
3' Att'
from
for
such
B
alkaline
lg88
at
items
5'
to
Tt]:::.t1ge
as
l9-q7.
batterles
docr
nxn.
handleiand
and
annual
r,
zinc
Rri
growth.in.on**piion
oxide;
n,ut
ca,rburetor
to.ouir,**i,-unC
parts;
to
create
orzinc
for various
chemicals
in
the
toi..rruJioyrusedro
other
wesrern
such
rrses.
as
worid
zinc
Exh.
was
3
demand
on
rhe
London
Merals
Exchange
(LME).
l:..T8
gt,'.
priie
i.i'1.'
of
zlnc
is
established
by
suppry
and
BRZ's
Current
Operations
BRZ_g
Prgductg
BM
operates.an
electrolytic
zinc
refineq
i1sa1cer,
Si.
clair
counry,
Illinois.
Exh.
3
irrt
'
,
BRZ
currenrrv
producei
approximarery
105
,
ooo"i".,
"r
zinc
per yeir.
Exh.
3,
Arr.
J
.''...'.''
n
E
g
g
B
fr
a
H
E
4
pails,
and
produce
zinc oxide
(e.5.
Exh' 3,
Att'
-l
at
2'
BRZ
pound logs
for
large
galvanizing
lir.es.
BRZ produces
special.high
grade
quality.zinc
igg.ggSy6
zinc),
ghi.-h
is the
most
rvidely
recognized
standard
for
zinc.
Dcpcnding
on
iurto*.,
specihcations,
BRZ
also
debases
its
speciat
high
grade
zinc to
produce
alloys
that
Exh.
3
at 19.
BRZ
has long-term
end
markets
for all
of its
products.
Att.
N.
BIZ_IProce-sl
BRZ
recovers
zinc
from hvo
types
of
materials,
the
first
cf
ri'hich is zinc su!fide
concentrates
that
are
mined.
BRZ
alsorecovers
zinc
from
secondan'z-inc
oxide rnaterial.
Secondary
zinc
oxide
material
is a
by-product
of
other
industries
that
use
zinc,
including
steel
mills,
briss
rnills,
brass and
bronze
ingot
factories.
and
galvanizers.
The
nrined zinc
sulfide
concentrates
arrive
as
\\,et filter
cake:
ihe
secondary
zinc
oxide
material
atril'es
as
wet
fllter
cakeo;^asdrymaterial
in
"supersacks."
Exh.3
at2,4,
l0-11,
14,
l7'20'
Att
Jat2'
In the
first step
of
BRZ's
process,
BRZ
may
use
an acid
solution
to
remove
magnesium
from
rhe zinc
sulfide
c()ncentrates
to
prepare
them
for frrrther
processing.
Exh. 3
at
l0-ll.
Secondary
zinc
oxide
material
does
not
rer.;uire
this
initial
step.
Exh.
3 at
l0-ll, l7-18,
Ati.
H,
J
at
2-3.
BRZ
then
processes
zinc sulfide
concentrates
and
secondary
zinc oxide
material
in a
fluid
bed
roasrer.
The
roasting
step
removes
sulfur
from
the feed material.
Exh. 3 at
12,
19,
Att.
J
at 2.
BRZ
then
leaches the
roasled
material
io separate
zinc and
various other
metals'
From
the
slurl'that
results,
BRZ
filters tr,e
solids.
and
puts
the remaining
solution
through
four
purification
stages.
The
purification
process
yields a-purified
zinc
sulfate solution
from
rvhich
zinc
is recoveied
through
an electrolytic
process.
'fhe
electrolytic
process
yields zinc
carhodes
thar
are
of special
high
grade
que,ity
(99.995o/o
pure zinc).
BRZ then
melts
the
cathodes
into one
of six shapes
fo. delivery
to customers.
Exh.
3 at
l2-13' 19.
BRZ's
refining
process
produrs a number
of
by-products,
including
sulfuric
acid,
lead-silver
concenirate,
copper
cement,
copper-cobalt
concentrate,
cadmium
oxide,
and zinc
sirlfate
nonohydrate.
BRZ
has
long-term
end
rnarkets
for
lhese by'products.
Exh.
3 at l2'13,
l9-20.
fi
g
E
t
E
n
a
3ln
this
opinion,
when the
Board refers
lo a
percentage
of a constituent
in a maierial,
it does
so
by
weight.
:::;;:,.i:tt-
:,ii.
fr
n
n
E
H
fi
n
f;
E
H
fi
E
E
f;
E
E
fl
fi
5
EAF
Dusr
EAF
dust
is a
sourcc
of
secondary
zinc
oxide
materlal.
EAF
dust
is generated
in
electric
arc
flrnaces,
rvhich
produc<-'steel
b), heatingstee!
scrap.
l'hese
furnices
emit
gases
lhal
conlain
EAF
dust.
Air polluticn
control
equiprnent
in theie
furnaces
remcves
EAF dusr
flcni
t.hq
8ases.
l-hese
furnaces
generated
approxirately
900,000
rons
of EAF
dust
in
rhe
United
Srates
in
i997.
Exh.
3
ar
5. l3-14.
EAF
dust
is
composed
of approximarely
20Vo
rc
3096 iron
and l5;"4
to
30g6
zinc.
It
also includes
other
constituents
such
as lead,
c:dmium,
chloricle,
fluoride,
aiuminum,
calcium.
poiassium,
tnagnesium,
manganese,
sodium,
and
silica.
Because
of its
high
iron
content
and
other
impurities,
zinc
cannot
be
recovered
directly
from
EAF
dust
in rosi,
if not
all, zinc
smelting
and
refining
operations..
Exh.
3 at 5,
l3-14.
ln
lgg6'
nearly
4Ao/c
of
the
EAF
dust
generaterl
in
thc
United
Stares rvas
disposed
of
in
landiills.
E.',"h.
3 at
6.
Il
costs
approximately-S80
per
ron
to
clispose
of
EAF
dusr.
Exh.
3 at
l6
EAF
Zinc
Oxide
$ql"rlgrfpg1gr"
re
Merals
Recovery
\Vh:le
zinc
cannot
bc
recovered
directly
from
EAF
dusr
in
most
zinc
smeltem
and
refineries,
zinc
oxide
material
recovered
from
EAF
dust
can
be
processed
in
zinc
smelters
ancj
i-efineries'
Zinc
oxide
material
can
be recovered
fiom
EAF
dusi
when
the
dust
is
put
through
a
high
temperature
rnetals
recovery
(llTMR)
process.
HTMR
units
includ"
rotrry'kiinr,
.oinry
hearth furnaces,
plasma
furnaces,
and
eiectric
furnaces.
Exh.
3 at
6-2,
io,
Rtt.
F,
H.
HTh4R
processing
increases
the levels
of zinc,
lead,
and
cadmium
in
EAF
dust.
These
changes are
desirable
in
the zinc
refining
process.
HTMR
processlng
alsc
lorvers
the ler,els
of
constituents
iltat
are
considered
contaminanis
in the
zinc
refining
prciess
{e.g.,
iron,
calcium,
magnesium,
alumina),
except
forsodium,
chloride,
fluoride,
and'pctassiu*]
g*h.3
at
I0,
lg,
Att. H.
ln
1991,
approximately
l.z
million
tons
of EAF
dust per
year
was
processed
tvorldrvide,
mostly
to
produce
zinc
oxide
material.
Exh.
3 ri
rg,
An.
L.
'ERF
drst
processing
is done
in
a
varielr
of HTMR
units
and
the resulting
zinc
oxide
material
is sold
primariiy.
ro
produce
zinc,
but also
to produce
zinc
chemicals.
f*.
g
at
18, Art.
L.
Severaifacilities
in
the.United
States produce
er are
capable
of
producing
EAF
zinc
oxide.
Exh.
3 at
6, lg,
Arr.
l-' M.
Markets
ior
EAF zirrc
oxide
exist
in North
Airerica,
Asia,
and
Europe.
Exh.
3 at tg.
Once EAF
dust
has been
through
the
HTMR process,
the
value
of
the resulting
zini
oxiOe
malerial
approaches
the value
of mined
zinc
sulfide
concentrares
(currently
Sz}o
to
5300 per
ton)
.
Exh.
3 ar
8,
16- t7
.
Zt .
&
fr
*
g
*
g
ffi
ffi
ffi
ffi
g
B
I
$
E
g
f,
g
6
BRZ
u'ould
iikc
ro
purchase
EAF
zinc oxiCe.
Tr.
at
l3; Exh.
3
at
l-2, 6.
BP-Z
intends
to
use
rhe
material
as
fcedsiock
for
its
zinc
rcf ineiy.
Exh.
3 a{
1,8.
EAF
zinc
oxide
can
s.ubsrirure
for and
suppler.nent
mined
zinc
sulfide
concentrates.
Exh. 3
at2,l4.
After
lvashing
EAF zinc oxide
(described
belorv),
BRZ
pians to
use
the
materia!
iu the
same
manner
it uses
rhe
nrinecl zinc
sulfate
concentraies.
The products
and by-products
from
EAF
zinc
oxide
u'ould
be essentially
indisringuishable
from those of
the
mined
materials.
Exh.
3 at
i6, 19,
2t.
i.lot all
zinc
oxide
marerial
recovered
frorn
the
HTMR
processing
of
EnF
dust
would
be
suitable
feed for
BRZ's
refinery.
Exh 3. at
7.
To
be economical
for
BRZ, EAF
zinc
oxide
must
mecl the
follorving
spccif,ications
(on
average):
>
5Ao/o
zinc',
<ZQo/o
lead',
< 57o
iron:
14o/o
latal
Sangue
rnaterials
(silica
plus calcium
plus
magnesium):
and
<?a/c
chtoride
or
capablc
of
being
g'aier
washed
to
achieve
< 2% chicride.
Exh. 3:.7.
For BRZ
to be
able to
rvash EAF
zinc
oxide tc <2o/o
chloride,
the
feed
should arrive
at BRZ's
facilin,\','iili
<
13%
chioride.
Reply
at 5-6,
Att. O.
ln
addition,
BRZ
could accept
EAF zinc
oxide
produced
durir:g
the
three-rnonth
slart-up
period
of
an
HTMR
unit
rvith
up
to 796 iron.
Tr. a
5-6; Exh.
4.
AmetlSteel
-!
nL_s
HTMR
Ploc-ess
One
of the
cornpanies
that
processes
EAF
dust
with
an
HTMR
unit is AmeriSteel,
Inc.
(AmeriSteei).
AmeriSteel
is a steel
n,anufacturer
located
in
Jackson,
Tennessee. ArneriSteel's
H1't\4R unit
is a
rotary hearth
furnace.
Exh. 3 at B-9.
To
process
EAF
dust, Ameristeel
first mixes the
dust
with
a source of carbon
(commerciil
grade coal
or
coke
purchased on
the open
market)
to form briquettes.
The carbon
acts
as
a reducing
agent.
Ameristeel
places
the
briquettes
in the
rotary heanh
furrrace
to
recover
both zinc
oxide
material
and
an ii-on
material.
Materials
that
volatilize at lower
temperatures
vaporize and
leave the
furnace
in a
gas
stream.
These
materials then oxidize,
form a solid,
and
are collected
in an air
pollution control
device
called
a
baghouse.
This
marerial
collected
in the
baghouse
is EAF zinc
oxide.
Tr.
at l8-19:
Exh. 3
at
9-10:
Reply
at
3,
Att.
P. Once
A.meristeel
achieves
full
capaciry,
it is expected
to
produce
approximately
9,600
rons
per year
of
EAF
zinc oxide
frorn the
24,000 tons
of
EAF dust
that Arneristecl
generatcs annually.
Exh. 3 at
10.
'l
','
'
w
g
w
w
fl
t
ffi
im
ls
ln
ila
ArneriStecl's
EAF
-4!!-c
Oxide
Ameristeel's
FlTh4R
prccess
increases
the
zinc
content
sf
EAF
dust
frorn
20'25o/o
to
59.5olo.
increases
the
iead.on,un,
from
37o
foi'5Yo.
increases
the
cadmiurn
co;iient
from
0.05%
tc
0.1%,
and
decreases
ihe
iron
conten{
from
!
9-24o/o
tc
0'ioz'o'
Arneristeel's
HT}r4R
proce-ss
lorvers
,n.
fnn*o
oi
constituents
that
are
considered
contarninants
in
BRZ's
refining
;;;;';;;;pt'i"r
toJi"m,
chloride,
t-tuoride,
and
potassium'
Exh
3
at
l0'
i8'
Att'
l{'
K'
BRZ
hasdetermined
that,
excepr
fbr
the
chloride
level
o|the
material,
AmeriSteel's
EAF
zinc
cxicie
is
an
ideal
feed
for
its
zinc
refinery'
Exh'
3
at
S'
Anreristeel's
E'AF
z'inc
oxide
is chemicaily
sinrilar
to
niined
zinc
oxide
and
zinc
sulfide
concentrates:
Exh.3atl4,l?,Att.D,H,K.Withtheexception-ofc'hiorideandfluoride'AmeriSteel's
EAF
zinc
oxide
also
ni.,t'typi.uizinc
refiner
ipecifications
for
zinc
sulfide
concentrate
blends
and
falls
within,fr.
rr"g.
riJ.*trOuty
feed
speitfications
that
zinc
refiners
have
establishe4'
Exh.
3, Att.
F, H'
K.
EAFzincoxideproducedbyAmeristeelandothershaslevelsofzinccomparableio
rhat
of
mined
concentrates.
Exh.
iat
t7,
Att.
D,
t{,
K'
If
used
in
BRZ's
refining
process'
EAF
zinc
oxide
would
have
chenical
advantages
and
disadvantages
compared
trr.mined
concenirates.
l'he
prituty
uluantages
of EAF
zinc
oxide
are
that
it
is higher
in
leaC
than
mined
c.ncentrate,
und
to*u,
in suifur
than
mlned
zinc
surfide
ccncentrates
AmeriSteel's
EAF
zinc
oxide
has
the
additional
advantage
oi
being
lorver
in
iron
than
mined
collcentmtes'
Exh.3
at
14,
l'i-18,
Att'
Il' l{.
J
at3,
K
Constituent
I
N4ined
Concenirates
A:neristeei's
EAF
Zinc
Oxide
Zinc
Oxide
1
Zinc
-
Sullide
I
-
-
l,
a/o
zinc
54
59.
I
3v.3
-oz^
lr.ad
4.9
t.?
7.: cadmium
0.38
0.5
u.r
i
0.1
I
u.l
!
96 iron
/.
-J
o;b
coDDer
I
------;-::fr:-:--t
3l
,|
.t
<
0.02
i
G
"a-ejs.lgryf--
% silica
?4
I
U.Uf,
l4.B
0.8
0.02
o/o
maanesiurn
0.01
96 alumina
0.6
0.4
0.1
0.02
<
0.02
J
9t
sodium
"/"
chLcride
oy'o
fluoride
tvA
nn?
<
0.1
B
0.03
0.0s
0.15
;.::!,.,:i
!;':...:
*
*
ffi
ffi
fr
fi
E
a
E
8
EAF zinc oxidc
has t\vo
prirnary
rjisadvantagcs
\\'hen cotnpared
to
!nined
concentrales.
Firsr, EAF z.in:
oxide
has
highei
leveli
of sodium,
chloride,
fl,.roride,
and
potassium,
lvhich
are
presenl
as inorganic
saltsl
While
EAF
zinc
cxide
can
be
irrtroduced
directly
to
BRZ's
roasicr,
inorganic
ialts
ir, rhe
naterial
could
corrode
BRZ's
rr:fining
equipment
if
their
levels
are nor first
ieduced.
Hou,ever,
as discused
beiorv,
BRZ
plans
to
rvash
EAF zinc
oxide
to
reductt
its leveis
of incrqanic
salts.
Tr.
at
l3-17;
Exh.
3
at
ll, 14,
l7-18,
Att.
D,
H,
J
at 3'
l\.
The second
primary
disadvantage
of
EAF
zinc
oxide
is
that
it may
b-e in the
form
of
dry
dusr
rather rhanrvet
fiiter
cake.
Eih. 3
at
11.
The
dry
d_ust
is morc difficuit
to
handle.
fxir.
g
ar 14.
Afr.
J
ar 3.
As discussed
below,
hovrever,
BRZ's
u'ashing
proccss
will
tum this
dry
ciusr
into $'et
fllter
cake
rhat 13RZ
can then
put through
its refinery
equiprnent.
BRZ's
ProPosed
OPer
atjqns
EAF zinc
oxide
is expecred
to
arrive
at
BRZ's
Sauget
facility
in the
lornt of
dry dust.
BRZ
plans
ro keep the
dr1, EAF
zinc
oxide
totally
encloseC
from
unloading
urrtr!
rvashing.
BRZ
iras designeri
a
mareiiai handling/rvash
system
to
handle
that
material.
Exh' 3 at
14,
20,
Att.
J
at 3-4. On
Seprember
22,
1998,
IEPA
granted
l3RZ
an
air
pollution control
permit to
ccnstrdct
the system.
The ccnsrruction
permit
limits
emissions
of
paniculate
matter
from the
handling/u'ash
facilitY
ro
1.68 lons
n''
,
'rr.
Exh.
2: Exh.
3,
A:t'
J'
Dry
sccondar;'
zinc
oxiCe
material
is expected
to
arrive
ar BRZ's Sauget
faciliry
in bulk
or
in supeisacks.
Approximately
90%
of
this
rnaterial
is expected
to ai-rive
by rail.
BRZ
plans
ro unload
railcars
of
the bulk
material
through
ventllated air slides
to silos
equipped
tvith
ilign-nfnciency
Parriculaie
Air
(HEPA)
filters.
Ultimately,
BRZ
plarrs to add four
silos, each
rviiir a
capaciry of
1.5
ra!lcars.
BRZ
proposes
to
locate
the silos
on concrete
or
asphalt
pads
thar
BRZ could
wash into a sump.
BRZ
plans
to
pump ihe
sumP contents
into
the
rvashing
process.
Exh. 3
at 15,
A{1.
J
at
4.
Supersacks
of the
nraterial
are expected
to arrive
by
boxcar
or
truck.
[3RZ
plans to
leave
supersacks
in
boxcars
lor intermediate
storage.
The
boxcars
would be unloaded
at a
c,.rvered
loading
dock
rhat is
to
be attaclied
to
the
washing
olant.
Supersacks
that arrive b1'
tr,rck
rvould be stored
inside the
washing
plant. tsRZ
would
be
able
tc store
approximately
150
tons
of
that
rx3lerial inside
the
washing
plant.
Exh. 3
at 15,
Att.
J
at 4.
BRZ
plans
io
use a rruck lo
move
the
supersacks
to a supersack
discharge station to
ernpry
them.
BRZ
proposes
to
maintain
ihe
discharge
station under
negative
pressure
to avoid
fugitive
emissions.
BRZ
rvould vent
the
disclrarge
station
through a
baghouse to colicct any
secondary
zinc oxide
nraterial. ExI. 3
at
15, Att.
J
at 5.
BRZ
proposes ro
convey the secondary
zinc oxide
material
(from
tl'e silos
and
ihe
supersack
discharge
sration)
in
an
enclosed,
ventilated
conveyor
(or
by
pneumatic
conveyor) to
a iank
rvhere
BRZ
would mix the matcrial
with
water.
BRZ
proposes to
pumP the
resulting
slurry
into a
washing tank. BRZ
plans
to
add soda
ash
to the
rvashing tank
to raisc the
pH
to a
n
&
n
f;
fi
ft
n
';.;
.
w
&
#
fr
&
B
u
w
E
g
f;
a
B
&
a
g
g
E
g
I
levei thai \\'t)uld not dissoh'e zinc
and cthcr
hear1,
mci:rls bul
r','ould
dissolve
the inorganic salts
that could corrode BRZ's
refining equipnrent.
Exh.
3
at !1, l5-i6, Att.
J
at 5.
Alter s,ashing, BRZ proposcs to create
u'et filter
cake
by removing
water
from
the
slu:-ry
rr'ith
a
pressure
filter. BRZ
plans
to
transport the
filter' cake by encloseC
conveyor
belts
to the concen(rate
slorage
building. In the concentrate
s(crage
building, BRZ rvo'rld
blend the
rvashed
secondary zinc oxide material
\t'ith zinc sulfide
ccncentrates to create feed
fcr the
roaster,
afterrvhich
the material
rvould
go
through
thc
rehning
process
outlined
on
pagc
four
of
this
opinion.
Exh.
3
at
15-16,
Att.
J
at 5.
Some
producers
of EAF
zinc
oxide
may rvasli
the rnaterial
beforc delivering
it
to BRZ.
ln
that
case, the material
rvould
arrive at
BRZ's Sauget
facilitv as
wet filter
cake
,
which
BRZ
can harrdle in rhe same n'anner that it
currently
handles filter cake
feed rrraterial.
'i'r.
at
l4-15.
Exh. 3
at
14,20.
Typically,
the largest suppliers
of secondary
zinc oxicie matcria!
pither
rvash
thc
material at rheir f3cilities lo
produce
wet filter cake or
snip the
rnaterial
as dry
dust in
pncumatic
trailers.
Smalier
suppliers
q,picaliy packagc
the secondary zinc oxide material
in
supersacks. Exh.
3,
Att.
J
at 4.
B
RZ's Proposed
!or.ij!rac(
\\/ ith
Amerisjqg!
BRZ and AnreriSteei have reached agreenrenl
on contract terrns under which
BRZ
pl3n5
to
buy AmeriSteel's
full
production
of
EAF zinc clxide.
Tr.
at
l7-18;
Exh. 3 at
8,
Att.
G at l.
AnieriSteel's full monthly production is cstimated
to bc approximately
800 tons. Erh.
3,
Att.
C. Under
the
contract, ihe
price
of
EAF
zinc oxide
is
based on a
percentage
of its zinc
content and the LME
price
for zinc. Exh. 3, Att.
G at 2. Because EAF
zinc
oxide can
substitute
for and supplement BRZ's mined zinc
sulfide
concentmies, BRZ
would
pay
AmeriSteel
a high
pe
rcentage
of
what it rvould
normaliy
pay
for mineci zinc
sulfide
concentrates.
Exh. 3 at 8, 17.
BRZ
is rvilling to pay
a price for
EAF zinc
oxide that far
exceeds
its
co:t
of freight.
Tr. at l3; Exh. 3 at
17.
The
AmeriSteel
contract would
be
effective upoq execution and continue
until
l)ecember
3l of the year following
the
year in rvhich BRZ begins
conrnrercial
operation of its
washing
piant.
Thereafter, the
contract
rvould
continue
from
year
to
year
"rvith
annual
negotiation
of the terms to reflect
current
market
conditions."
Exh. 3,
Att.
G
at l-2. As
proposed, either
parry
could cancel the
contract
by
giving
the
other
parry
180
days notice
of
canceliation.
Exh 3, Att. G at 2. AmeriSteel has indicated that it rvill
not execule
the
contiacr
'until
all regulatory issues have been resolved, including this adjusted
standard proceeding.'
Tr. at
17-18:
Exh. 3 at 9.
DISCUSSION
In ihis section, the Board
first discusses
whether
EAF
zinc
oxide
is
a solid',vaste.
The
Board
then
dlscusses rvhether
lhe
provision
under
which BRZ seeks
this determination
is
available
in this
case.
Next,
the Board
evaluates each of
the
factc"rs upon which
this
;:i:
-a
:=-17':-
:-:'::="'7.-'-lr-:-:-'t:;';
-'
;
:
1
E
&
a
a
a
g
ffi
n
m
n
E
fr
H
fi
8
H
F
F
ffi
f;
r0
dcrerrninaricn
is based.
Lastl'y,,
the
Board
discusscs
the
conditions
thaf
appll'to
this
cr'ienninalion.
Status
of
EAF Zinc
Oxide
Section
720.131(c)
allorvs the
Bolrri
lo
deterrnine
tha'certain
rnateriais that
rvould
othenvise
be soiid
\v3stes
are not solid
wastes
if certain
conditu-rns
are
rnet.
'fherefore,
the
Board irririaJly
musr deterrnine
that
EAF
zinc oxide
is a solid
waste:
if it is
not, RRZ
has no
need
for
an
adjusted
standard.
A
"solid
tvaste"
is any discarded
matcrial
not otherrvise
excluded
in the
regulations.
See
35 lii.
Adm. Cocle
72t.t02(a)(l).
One
of the
scveralrvays
that
a
material
rnay
be
cgnsidered
"discarded"
is by being
"recyclcd"
in a manner
specified
in
Section
721.102(c)
of
rhe regularions.
See
35 lll.
Adm. Code
721.102(a\(2).
Section
721.102(c)(3) specifies,
irt
parr,
t-hai if a
"lisred
siudge"
is recycled
by
being
"reclairned,"
it
is a solid
rvaste.
See
35
lll.
Adni.
Code
72l.l0Z(c)(3)
and
T2l.Appendix
Z.'
l'hc Boarri
finds
rhat EAF
zinc oxide
fits
\.,'ithin this ca(egory.
First,
EAF zinc
oxide
is
considered
a
"lisred
sludge."
A
"sludge"
includes
a
"solid
. .
. waste
generated from
lanl
. .
.
airpollution
conlrol
faciliry
35
lll.
Adm. Code
72l.l0l(c)(2): 35
lll.
Adm'
Cocte
720.110.
EAF dusr,
from
rvhich EAF zinc
oxide
is
recovered,
is
Senerateci
frotn
an
air
poilurion control
faciliry
aqd is
thereiore
a sludge.
Furtherntore,
EAF
dust
is
"listed"
bccause
it is listed
as a
h;rzardous
waste
from
a
specific
scurce
under 35
lll. Adrn.
Code
721.13?
(listilrg
emission
control
dust/sludge
lrom the
primary
production
of steel in electric
furnaces
as
hazardous
waste
f.061).
While rhis listing
applies
to EAF
dust
rather than
EAF
zinc
oxidr, Sections
?21.103(c)(?)(A)
and
(d)(2)
further
provide
that
a
material derived
ironr
the
trcatment of
a
listed
hazarcious
waste is itself
the
listed
hazat'dous
waste. See
35 Ili. Adm.
Code
721.103(c)(2)(A)
and
(d)(2).
USEPA,
which
promulgated
the
federal
regulations upon which
these
regulatlons
are bascd,
explains
that
"all
of
the resiriues
frotn treating the original
listed
viastes are likewise
considered
to be
the listed
waste
54
Fed. Reg. 1056, 1003
Uan.
il,
1989).
Therefore,
EAF
zinc oxide
is also
considered
a listed sludge.5
Second,
the
Boarrj
finds
that EAF dust
and
the resulting
EAF zinc
oxide
are
being
recycled
by reciamation.
A
material
is
"reclaimed"
if it is:
,
For
a detailed
discussion of
hovr materials
become
solid
wastes,
please
refer to
PetitioQ_ol
Chemeico,
Inc.
for Adiusted Standard
Ftgry35-Ul.
Adrn,--Cqdg-4!J!.-llel-a8g--Gj
(lr4arch
lg,
1998),
AS
97-?, slip
op. at I l- 12.
5
Compare
!eij!-or'.-9t
E.gcJgle-Tqc!19!9g!9t,
Inc.
fgl-q1 :\t!1t5!9El!a"dad-!l1del
35 lll.
Adnr..Codq
720. l3l(g)
(September
3,
i99S), AS 97-9,
siip op.
at 7-8
{if
used antifreeze
(spent
material
that
is
not a listed hazardous
waste) is a characteristic
hazardous
wasle,
the
initially
bui
yet to be complslsly
reclaimed
materlal derived
from
that used
antifrecze is
a
hzuardous
waste
only
if
ir
exhibits a characteristic
of
hazaidous
waste),
ffi
g
*
&
recov(p|ocessed
to
reco.",er
a
usabi."
Drnrf,,^r
3
s,
il :
?#
tl:l,t
f',ffi
lx
;'
ii
ii
l";
::g::::l
;
i,l;
.,T,i,
J:i[:,1t:,*
\\,aen
USEP/
if,:^r,Fgt
i'l'
T'ffi
j::'i:#
i:fr.|lf
H:;:'
n''n'l
h
i
s-regu,
a,
i
o
n
i
s
ba
se
d
i,
#tHr*{fi
rvashed
lo
rernr
ffi
#***$],r+fttrfi
*f[+i,ffi
'lli
j::inf#;:ffi:?rilx,::Ti,T:::+t*f
*al;ilitf..u
j_#r,f
itt,#.
:11
gila!'ii!rv
oi
seqrion
zzo.
.l
lllg)
:i:#j.Ln#*y,:;,i#:t;:',i:il'f
;:T,l:,,i;:il,.r:rr,
rec,ama,ion
is
con,p,c,ed
'..ruin,i.s'i;i;.t:.::,1;?jfiffiIr
,r'0,
i'
ini,i;ri,:ili;.,g51Jil.1ili?flT"r,,.;#
i,li,i#f;,fi,nr$:l{.fi:rl
ffil:'ri:jjjilitriilJri;i{,fi;.ii;ij#rr*:;t:,,y-
'no
p'o'r"i';"j'u#1',:lt[:::"if'lx.f'fu?'*i:'*
l;:l
,i;ili
,,u..uun
rrio,ghlo
,HrHd;illilf#Lt]rp;
r#
#M",*,ffi
:
il;
#rTs :ft
ff'-"'#l
ffgg*,5u,gf1i,gl*rffi'
*l
il:
n*f
,11n
l#-
*titg*iiu;
r,u,
u.oJ!?,?:llrtl,:-rhar
se.ion
72a
13,.,
,,;;;;;:,:'t'grade
quarirv
zinc
co
mp
r e
r
e
r!'
;ffi
.';
"
ilih;R
il','
#.::i,:",
i
iT,,iltJ;,[
:ii
f,ltl.Til,
m*
A
F
d
u
s
r
Ieqtru,?A..L31O&gqs
se*ioni5
i:il:,Ti;'J"l:,;J;,;ff;'j".lg.r,f;j:f,;f1il,::,?ii,g":',
fi,#,,il
,,i,-
&
a
a
,g
g
f;ff
o*oois
cornn;
-l,o
AoarO
addresses
ili,e.se
fact,rs
in
:;',ii:,!'i,H,dff
:"triififiil,;:il;1;:lf
ffi
.-
8
*ffi*qffil
coJ
F
,fJ
,
and
it
#
ntnodiry.tike.
T"r'
I
a
l3
Tire
\/a!uc
of the l.laterial Aftcr
lt Has Been
Reclaimed
tjSEPA
stares rhat
"the
inore
valuable
a
material is after
initial
proccssing,
the
more
likely it is
to be commodity-like."
50 Fed.
Reg.6l4,655
(ian.
4,
1985).
As noted
above,
once
EAF
dust
has ixen
throi:gh
the HTMR
process,
the
value
of the
resuiting
secondary
zinc
oxide rnaterial
aporoaches
the
value
of
nrined zinc
sulfide
concentrates.
BRZ and AmeriSteel
have
reached agre'ernent
on contract terns
and
the
price
of
EAF zinc
oxide
is to be
based on
a
certain
percentage
of
the zinc
content
of the
material
and
the LMIE price
for zinc. BRZ
u'ould
pay
A-meriSteel
a
high
percentage
of rvhat
BRZ rvould normally
pay
for mined zinc su}fide
concentrales. BRZ is
prepared
to
pay
a
price
for
EAF zinc oxide that
far exceeds
its cost of
freight.
Thc Board
finds
that
EAF zinc oxide has significant
value.
The Degree To Vihich
the
Rrglaimcd Material is l-ike an Analogous Rarv
rylqlejial
Acccrding
to USEPA,
"lilf
thc
initially-reclainred material
can
sulrstitute
for a
virgin
materiai, for instance
as a feedstock
to a primary process, it is more
likely
to
be comnrodirT-
like."
50 Fed. Reg.
614,655
fian.4,
1985). EAF zinc oxide can
substitute
for
z.inc
sulfide
concenti"ates from mines. V/hiie not identical, the
nvo
materiais are
chemically
srrnilar.
Both
mateiials
rypically
s'ould require
sorne
form of contarninant renroval before BRZ-
'r.t'ould
introdr:ce
them to
ils roaster
(r.e..,
BRZ
processes
mined
concenlrates
with
an acid solution
to
remove magnesium: BRZ proposes
to rvash EAF
zinc
oxide with a nrixture of water and soda
ash to
reduce
levels
of
inorganic salts).
Alter the
wash, BRZ plans to use
EAF zinc oxide
filter cake irr
the same manner
it
uses
the
fiiter cake of mined
concentrates.
The
products
and
by-products frorn EAF
zinc oxide
would
be
nearly identical
to those cf the mined materials.
Aside
frorn
its
chloriCe and fluoricie
levels,
AmeriSteel's EAF zinc
oxide
meets
the
specifications of
a
rlpical zinc refiner for zinc sulfide
concentrate
blends.
The
Board
finds
that EAF
zinc
oxide is
very
sinrilar to nrined zinc
sulfide concentrates
and
can be substituted for
the
mined
concentrates.
The Extent To Which an End Market for the Reclaimed
Material is
Cuaranteed
ln ciiscussing this factor,
USEPA states:
If the
[petitionerl
can show
that
there is
an existing and
guaranteed
end market
tor the initially-reciaimed
materia!
(for
instance, value,
traditional
usage or
ccntractual airangements),
the
materlal is r:rore likely
to be commodity-like.
50
Fed. Rcg. 614,
655
(lan,
4, lg85)
ln
this
case, lhe evidence
established
that EAF zinc oxidc is sold
primarily
to produce
zinc, but
also
to
produce zinc
chernicals. Several facilities in
the United States
produce
or are
capable
of producing EAF
zinc
oxide.
'fhere
are niarl<ets
for EAF zinc oxide in North
America,
Asia, and
llurope.
ffi
g
il
n
t(l
B
n
E
i**rf$$;'d.1;i;sggffi#gg
"
lil:i:,T:tfliL
ti'lJ'.
ttl'iouotut"'nut
,
---,ro,
rnr EAF
zinc
oxide.
;-.
il;;;.ntt
there
is
an
end
market
ror
EAF
zinc
oxide'
,.I:imedVAg4'@
lilll;
.-',
3.l,i.l
i.H?:
l
lillHiJTli::T't#''ri""'"pprt'rs
rypicallv
pacKa*v
r"u
s--
lr;ilil"
^.,^orrorr
,
io
io
arri\
arfive
***,gffi
:l',i,it,-**ffi
i#H
f
-
ll''"lffiin:;;"ff
T:'t
;
**
[ $ ft i ;"i
$Uti-{fi'l''dt1,ffi
$f
*:t*iii;**.'"'-*vl'"ff
il"r*shracry
;:t'l;'':::
[::T:"
^
-
r r
-r-r
nzid€
and
BR.Z
have
financiai
rheBoarda']siil"[:Ei:{:l.i1f,",:,jfftifr
h'r.$':d;;1;;;'losel*o
Hi?l',",?f;i':'::ffi""i'i'
i'
t'"
t
TheBoardfindstlratEAFz'incoxi<lewillbehandledtomrnimizeloss,
:j*''r-ll!ii):f iriTlP-*:in,:iii[:'-;ff
j":t{i:t.;'''.f
i',:llhd'i*d
Tffif*ft'ilff{ff[*f*U.g",,''i*'
**'r+*t'i#','"
that
question
'
g
r
I
g
I
Psardasaturalq!
'E
o""'oilJi,:ff:'.::li
ll'J,-ffiiffi'ffl,:,.i.Til,:ix;,i:
:il,,,.;::l"rmodiry
like
nditions
on
.
The
Board
r,.,iri A..,
,usted
sran4i!.d
srandarc,
ilil;
lij,.Lr:::'lnh
:t
rorrh
,r',-
a"rrjir-,ill*l'::
the
condiriorrs
rhar
rher
BRZ
*=
pr
BBZ's pronosed
condirionr
ndings
on'rtotlton;;;:::*
on
the
acljusted
rcriect
'f;i:"ilfiH:',i::,?fi'T:rt
::H:,o",
on
rhe
adjusred
srandard,
which
;r
amendecr
a.
The
m
to
b.
The
0",
ma
rn'JllT:Tfirj;i'ilH;xf
zinc
oxide
recraimed
fronr
EA.F
,uor.r*lifiul
accepred
shall
rneer
rhe
forou,ing
specificaiions
as
monrhiy
{l)
>
SCo/o
zinc:
(2)
<
20o/o
lead:
(3)
<
5%
jron;
(4)
t"fi'.ilJilr:an8ue
nraterials
(silica
plus
carcium
prus
(5)
,rluSjlfride;
providr
c.
BRZ
shall
i,;illff
mainr,;^
tJtjf
--
.
iii{.i,ff;rr:iff
il
;#
;:::i:.,
,..""vuril9
tne
materials;
,':'ffi
;
$
l}}'ffi..:i,rffi
ffi
i, { ilH:c
a
i
m
e
d
d'
RRZ
shall
maintain
rhp,o^^-,-
-
'v""
ttt
u('rflcitiofl
t''
uuouu;
uni
I'rours
ff#iii!'i;rfiffi
upon
rrqr.ii
iyll;i;*il
li',i1"J1fi
;;;onaure
ili'il.:.'J#:litff
,:;boverora
rime
ourinfnorrrr
busi'es
Tr.
ar
5-6;
Exh.
4;
Reply
ar
6.
-"
Tr,Jj::'
:
n\B!€rro'
"ilorJ**.dffi
,,nc\ear
h?l"iii|rh::l'"i,o".1'5
lris
**,ffi**.
a\su
""
qirsr,
ii
Seco
,n;l;$il!:'j}i'[-
"*Yilt:::#
si'ix
l'oces
t
R
;
,
E
,?
I a nr *'l:i'i'"*'l$$liiflj*i""'
I
,tfl*;,iT,iT}:i{,I,:;,W
m*s*ffi
er'r
EAF
*':ffT$ffi$*mfft*ffi
**,**,,,;**ffi
''ffifrit:ful't''t'iffi
",.*..'d',*ft5d,;i;I****$*$*{*"}ffi
:ll;'ilt-li
iin"
ilil-*..],;;..l*;..
ln
at
','.,-'..:,,i...i
,tt,$t*',ryry+,u.:,,{u'
-#'*::rulll-'''l*i*'t'ttt"g'"tt'"lti-rf
t':"'$:lu
(nay
aPl
choose
-..';;i',*,*
ffi+lt*W::
;
n
Hffi,:,*?$iq$ff}}.Hliil'-!ffi[|*;*;:;;;;';,,"
,,:::*s"*,u:*q#ilin},fn'iikilu*fi"lii'":uu'd
zlr
g
a
E
r
T
t
T
E
E
ORDER
I .
Thc Board
finds that zinc
oxide material produced
by subjecting electric
arc
furnace
(EAF)
dust
from the
prinrary
production
of steel
(K061
under
35 lll.
Adnr.
Code
721.132) to a high temperature
metals recover.y
(HTMR)
process
is
not
a solid rvaste
and grants Big
Riter Zinc
Corporarion
(BRZ)
an
adjusted
standard
under
35 lll. Adm. Cotie 720.131(c).
?.
The adjusted
standard
is subject ro
rhe follorving
conditions:
a.
The
determination
describu'd in paragraph
one of this order applies
oniy
to zinc
oxide material:
(l)
that is to be
processeci
through
BRZ's
electrolyric zinc refinery in
Sauget,
St. Clair
County, Illinois:
(2)
that is in lllinois;
(3)
that has
arrived
at BRZ's
Sauget,
St.
Clair Counry, Illinois
facility
or thar is
under
a legally binding
conrraci for
sale
to BRZ:
and
(4)
that meets the
follorving
specifications
by
rveight:
(a)
> 507o zinc;
(b)
<20o/o
lead:
(c)
< 5%
iron
(or
<7%o iron in material
produced
by
an
HTMR
unit
during
the first three
months
rhar
rhe
HTMR
unit produces
zinc
oxide n:aterial
from
EAF
dust
from
the
primary
production
of steel
(K06i
under
35 lll.
Adnr.
Code 721 .
132)):
(d)
<
4%
rotal gangue
marerials
(silica
plus
calciunr plus
magnesium):
and
(e)
1l3o/o
chloride:
I
I
T
n
t
':..4.t':
b
C
t9
BRZ
nrust nraintain
records that document
the
sources
of all zinc
oxide
material
that BRZ
accepts under
this
adjusted
standard;
tsRZ
must rnaintain
records
that
demonstrate
that
each
shipment of zinc
oxide
material
that
BRZ accepts
under
rhis adjusted
standard meets
the
specifications
set forth in paragraph
2(a)(a)
of
this order;
for this
demonstraticn,
representative
samples
of each
shipment
oi zinc oxide
rnaterial
nrust
be
collected, composited,
and
tested !n
accordance
rvith
genemlly
accepted
practices,
such
as
those
specified
in
"Test
N,lethods
for Evaluating
SoliC
Waste,
PhysicaVChemical
Methods,"
EpA
Publication
No.
SW-846
(Third
Edition);
anci
13RZ
must
mainrain
the
records
required
under paragraphs
Z(b) and
2(c)
of
this
order
for
a period
of three years
and rnust
mike
such
records
available
for
inspection
and
copying at
any reasonable
time during
normal
businers
hours
upon
the Iliinois
Environmental
protection
Agency's
request.
IT
IS
SC ORDERED.
Secticn
4i of the
Environmental
Protection
Act
(4l5lLCS
5/41
(lg96))
provicles
for
the
appeal
of final
Board
orders
to the
Illinois
Appellate
Court rvithin
3S
days
of service
of this
?td9l._
Iliinois
Supreme
Court Ruie
335
establishes
such
filing
requirementi.
See lZ2
Iil.
Zd
R.335:
see also
35 lli.
Adm.
code
101.246,
Motions
for
Reconsiderarlon.
l, Dorothy
M.
Gunn,
Clerk
of
the Illinois Pollution
Controi
Board,
herebv
certifv
lhar
the
above opinion
and
order rvas
adopted
on the lSrh
day
of April
tggg
by
u
"ot.
of Z-0.
Dorothy M.
Cunn,
Clerk
Illinois Poilution
Control
Board
::'.,:rl--.
i'.:
:
J
'
d.
!
v
fi
fr
fi
t
g
n
E
E
g
E
il
T
E
t
E
l
E
g
E
E
E
fl
il
fl
n
E
I
ILLINOIS
POLI-UTION
CONTROL
BOARD
May
6, i999
lN THE li4AfiER
OF:
PETITION OF
BIG RIVER
ZINC
CORPOP-ATION
FOR
AN
ADJUSTED
STANDARD
LTNDER 35 ILL.
ADM. CODE
720.
l3l
(c)
AS 99-3
(Adjusted
Slandard
-
RCRA)
ORDER
OF TllE
BOARD
(by
K.Na.
Hennessey):
On
April !5, 1999,
the Boarrj
granted petitioner
Big
River
Zinc Corporation
(BRZ)
an
adjusted
standard, subject
tc certain
conditions.
On
April
28, 1999,
BI?Z
moved
the
Board to
reconsider its decision.
BRZ
also moved
the
Board
to decide
the rnotion
to reconsider
at
the
Board's Ma1,6,
1999 meeting.
On
May
5,
1999, the
Illinois
Environmental
Protection
Agency
(IEPA)
filed a
response to the
molion
to
recorrsidcr.
The Board
granrs
BRZ's motion
to decide
this
matter
today.
The Board
also
grants
BRZ's nrotion to
reconsider
and sets
forth
in this
order
the modified
terms
of
BRZ's adjusted
standard.
ulKGRqL)
The
Board's findings of
fact
and conclusions
of
larv are set
forth in
its opinion of
April
15,
l99g
and are
incorporated
here
by reference.
Below,
the
Board highlights
the facts
and
proceedings
relevant
to
BRZ's
motions.
BRZ operates an
electrolytic
zinc refinery
in Sauget,
St. Clair
Counry,
Illinois.
BRZ
uses
various zinc-containing
materials
as
feedstock
for its
refinery.
BRZ sought an adjusted
standard
because it
wants to use
a
zinc-containing
material
recovered from
dust ernitted from
electric arc
furnaces
used to
produce steel.
This secondary
zinc oxide
materiaiwould
ordinarily
be considered
a
"solid
waste'
and a
'hazardous
waste"
under
the
Resource
Conservation
and
Recovery
Act
(RCRA),
42 U.S.C.
SS
6901
ef
seg.,
and corresponding
Illinois
laws and regulations,
BRZ
rvants to
use this secondary
zinc
oxide
material without
becoming subject
to
Illinois'
hazardous
waste requirements.
Tc
that end, BRZ filed
a
pctition for an adjusted
standard
under 35 lll. Adm. Code
72A.Bl
(c).
Section
720.131(c)
allows
the
Board
to determine that
certain
rnaterials are nor
solid
wastes
if they meet certain
criieiia.
The status
of materials as
"solid
wastes" is
significanr
because
under the
laws
and
regulations that Congress
and
the Llnited States
Environmenta!
Protection
Agency have
established, onll'those
materials that are
"solid
wastes"
can
be regulated
as
"hazardous
wastes- under
RCRA, and corresponding
llhnois lals
and
regulations.
Those
laws and
regulations
impose
various requirements on
persons
who
generate,
treat, store,
dispose, iecycle, or transport
hazardous
waste. See 35
Ili. Adm.
Code
E
E
n
a
s
$
I
7
22-726,
,.ogutr,,onl.28'
Alatcriais
rhar
e
2
an:.::o:*"
"n.
:-,;re
not
solid
11'351"'
are
nct
sub;e*
ro
tlinois,
hazardous
rvasre
$rof{'5ffi,:*'n-filpfii,H'i'ifrg,*,---*,{
-'--,--ffi,
;r*H;;ri;:lil*rl.:trHf*',*i*i*[l--,*rf
i,i#.,3i,;,*.,,,.
ru
*E_:i,i#nf
{or.
Exp.
ar
*j,HJ.,f
,,fr
1,fi
Ji:,,;**X.*:i"j*,[Tfi
,,;[?ffi
ff
il.:
,*ni*m***
,Hru*ira
#ff#[-#fiffi##fi{#luf|lf$
z.
The
adjusted
srandard
is
subject
ro rho r^,,
'-"':
.,rr
r...
norn.
code
a
'
The
r,eternrinrr,*",
"'*tt
to
the
foliorving
condjtions;
*ol, li'Jfr'
ff
':il.r;fi
:rfl
i:iy.1**ph
o
n
e
or
rh
is
o
rde
r
b.
d.
T
il
E
n
e
n
3
E
t
T
T
I
r
t
I
t
n
t
I
J
(l)
rhat is to be
processed
lhrough BRZ's
electrolyic
zinc
refinery in
Sauget,
St. Clair Counry, Illinois;
(?)
that is in lllinois;
(3)
that has arrived
at BRZ's
Sauget, St.
Clair Counry,
Illinois faciliry
or rhat is ilnder
a legaily
binding contract
for sale to BRZ:
and
(4)
that meets
the follorving specifications
by
rveight:
(a)
)SAo/o
zinc',
(b)
<
20% lead:
(c)
< 5% iron
(or
<79'o iron
in nrateria! produced
by
an HTMR
unir
during the first
three
months
that
the HTlvlR
unit
produces
zinc
oxide material
from
EAF dust from
rhe
primary
production
of steel
(K061
under
35 lll. Adm.
Code 72t.132)):
(d)
<
4%
total
gangue
materials
(silica
plus
calcium
plus
magnesir.lm):
and
(e)
< 13% chloride;
BRZ
must maintain
records
that document
the
sources
of all
zinc
oxide material
that BRZ
accepts
under this
adjusted
standard;
BRZ
must maintain records
that
demonstrate
that each
shipment
of zinc
oxide material that
BRZ
accepts
under
this adjusted
standard rneets
the specifications
ser
forth
in
paragraph
Z{a)(a)
of
this
order; for this
demonstration,
representative
samples
of each
shipment
of
zinc
oxide material
must
be collected,
composited,
and tested in
accordance
with generally
accepted
practices,
such
as
those specified
in
"Test
Methods
for
Evaluating
Solid
Wasre,
PhysicaVChemical
Methods,"
EPA
Publicarion
No.
SW-646
(Third
Edition):
and
BRZ
must
maintain
the records
required
under
paragraphs
2(b)
and 2(c)
of this order
for a
period
of three
years
and must
make
such records
available
for
inspection
and
copying
at any
reasonable
time
during normal
business
hours
upcin the
Illinois
Environmental
Protection
Agency's
request.
,g
*
fi
f;
g
g
n
H
4
frf
nePe11!gnpf
Bis
R.rrglz4qlg1pglelon
(April
15, 1999),
AS
99_3,
stip
op. ar
lB-tg.
BRZ
takes
exception
to
the sampling
requirements
of paragraph
2(c)
of
rhe
adjusred
srandarrl.
ln
particular,
BRZ
asks
the tsoard
tJ
amlna
rhis
provrsion"roihr,
each
shipmenr
of EAF
zinc
oxide
need
nor
rneer
the
specificarions
of
oaragraph
Z(a)
(a).
Rather,
AnZ'frofoses
to
determine
samples.
lr4ot.
compliance
Rec.
at
tvith
4,
15.
the
specificarions
uasea
on
a'monthly
cornposit!
iJip*.n,
The Board
notes
that
t3RZ
previols]y
qloposerl
sampling
basecl
on monrhly
aveiEges.
sgg
glg-8lYerz.!r9'L:
?9-3,slipop..at
1i.
'However,
asitre"noarc
noied
iri
iti'elr1
rs,
BRZ
i999
faile-d
opinion,
to
explain
BRZ
failed
how
to
it
a'lequaiely
u'ouid
compoiite
explain
samples
horv.
irs
ant]
proposal
whether
would
samples
*ori,.
rrr,1i
ip.iin.rrry,
ain
runr
producers
rvorlld
be
composited
to-getheior
separarely.
In
addirion,
BRZ
proposed
blending
shiprnents
that
exceeded
the
specifications
n'ith
otheimaterials
"such
that
the
blended
rnaterials
meet
ihe specifications,
"
but failed
to
explain
horv
it
rvould
determlne
whether
the
bierrded
rnaterlals
meet
the
specifications.
/d.
at
16.
BRZ
norv
explains
lhat.it
proposes
to
sample
each
truckload,
barge,
railcar,
or
supersack
of
EAF
zinc
oxide
thaiariives
at
its faciriry.
rr.{or.
Rec.
ar
z,
s-T;Affidavit
ar z-3.
to
BRZ
delermine
states
that
compliarce
it rvould
test,a
rvitlt
thaipecifiiations.
supplier-specific
composite
Id.
BRzstares
on
a
monthiy
that
ii
uses
basis
this
for
s.ampling
each
supplier
and
testing
approach
for
its
mined
zinc
sulfide
concentrates.
ir{or.
Rec.
at
2,6-7:
AffiJavit
at z.
BRZ
asserts
that
the requirement
that
each
shipment
of
EAF
zinc
oxide
meet
the
specifications
is
ccst-prohibitive.
Mot.
Rec.
ar 8:
Afiidavit
at
3. BRZ
states
that
Arnerisreel,
Inc'
(Ameristeel)'
rvhich
is
expected
to
be
a
primary
supplier
to
BRZ,
and
orhers
like
it
would
ltty:
,:
send samples
off-site
for
testing.
Accorcing
to
bnz,
these
suppliers
would
have
ro
hold
the
shipments
for.several
days
to
iwait
test results,
resulting
in
demurrag*
r..r.
gnz
states
(?0-4ao/o)
that
of
the
the
off-site
value
testing
of the
fees
EAF
and
zinc
demurrage-fees
oxide.
would
repiesent
a significant
portion
-lr4ot.
Rec.
at
g-io;
Affidavit
ar
3.
For
these
reasons,
BRZ
concludes
that
the requirement
th-at each
shiprnent
meet
the
specifications
rvill
Ptt-uelt
3,
5,
8;
BRZ
Affidavit
from
at
purchasing
3.
EAF
zinc
oxide
from
its prospective
suppliers.
Mor.
Rec.
at
2-
supplier
ensure
that
BRZ
conlinues
all
states
EAF
to
that
zinc
provide
it
oxide
can process
inferior
received
an
product,
is
occasional
processed.
"BRZ
shipment
wili
Aflidavit
terminate
cf
at
inferior
3.
its
BRZ
contracr
product
stares
vrith
anrj
that
that
its
if
a
it
*,ill
supplier
and process
v/hatever
produci
remains.
"
Id.
In
its response,
IEPA
n0tes
that
rvhile
the Board's
conditions
were
more
strict
rhan
tT:tltt
BRZ
propos:d'
IEPA
iQuaiity
Response
Assurance/Qualiry
(Resp.)
at
Td
3.
contrcrll
IEPA
]!!n.asreed
believes,
standards
to,
honever,
are
the
met
Board's.onoirion,
and
that
consistently
if
"proce$s
\,\,ere
follorved,
and
nor
[supplierl
without
rha:
should
eAieC
basis.
f;
t
H
fi
3
$
g
T
r
E
il
&
w
B
5
ensure
a
consisteni
Product
anci
less
frequent
sarnpling
of
actual
content
rvould
be
acceptabie'"
Id.
at
^..
lEpA
atso
sugSesrs
rhai
lt.BtSld
definl
shJpment
as
a
production
cycle'
or
Qn
a
roiting
a\.erage.
,.,n.r:il.n
an
individual
;;;;;iliar'
Id
.IEPA
also
proposes
that
the
Board
permir
BRZ
ro
blenc
only
rv*hin,il;;;rn'frt.1
ta.
tgp,q
furiher
suggests
that
the
Board
altorv
..a
,.Ou.,io*'iniiie'sa,r'pting
;fi;;;;t
!*:.0-"i'lte
senerator's
ability
to
use
eA/eC
proceciures;';il;;;.*ris.;ily
ni-rpuiin.,tion
rnareiial''
Io''
at
5'
It
is
not
clear
*,hcrher
lEpA
belierl;"thr
;;rial
should
u."tt"iJ
utfore
or
after
it
is
shipped'
TheBoardno.,esthatBRZproposedthespecificationsasaconditionofthcadjusted
standard.
Horvever,
as
nored
above,
rh";;;l
ilurta,ittt
BRZ's
proposed
conditions'
as
interprcted
by
BRZ,
were
potenti.ly
uneiic"rctaure'
Accordingly'
the
Board
crafted
enfoiceablc
ccnditions
,o
ddr*r,
,p-..in*ion,
unO
"*pf
ing'
iUtrite
BRZ
norv
has
clarified
its
prcposal,
gnZ'r',nturp*r",ion
,if
its
ptffiJ
conditions
remains
problematic'
TheseprobiemsarisebecauseB|Zcontinuestoproposethatthespecificationsbea
condirion
of
the
adjusted
standard'
But
nRZwill
not
know'
*'ii
tf't
end
of
the
tesdng
period'
,
t
. *u,.rt"i-;t
as
already
r"..iJuJ
**ets
the
required
specifications
on
an
average
basls.
lf
the
rnateriai
fails
io
lneet
,ftt
'ittin*iioni'
'1tt'otusted
standard
rvould
not
apply
to
the
nrareriai
and
the
marerial
rvould
b.;;;;;;;tl
a
hazardouu*ut"'
in
that
situation'
BRZ
v,ould
have
violaiec
illinois
hazardous
*;;;;;
and
regulations'
For
these
reasons'
BRZ's
p.potua
condilion
is
not
lvorkabie'
Accordingly,theBoardrvilltakeadifferentanrlmorevrorkableapproach'TheBoard
already
has
found
in.ii*-iis,eel's
EAF
zinc
oxide
meets-specifications
necessary
for
BRZ
to
process
tt.
rnu,*riri';;;;;[tllv
see^lf
i!iefd,^,l:'3n
3'
slip
oP'
at
l4'-
other
HTMR
processes
;;;;;bl*
of
;roduci'G;
siTilar
qualiry
material'
liearing
Exhibit
3
at
l0'
Atrachment
n.
rn.'dirJi;ti;J;
in.u
ifrut
BRZ
plans
to
Process
all
E'AF
zinc
oxide
that
!t
receives
and
thar
ii
a
supptier
ton'i'ttnity;i;"tid:lT.t::tt
product'
BRZ
rvould
ierminate
irs
conrracl
rvitir
thrai
#;il;
"lrnJruiiri
3.
Li*i,ing
the
scope
of
the
acjusted
standard
to
EAF
dust
,r,.,
i,ur'i*;"Jr;;;rr;J
;t
TiMR
tt'O
ir.,.,
i!
to
be
piocessed
through
BRZ's
eiectrotyric
r,^.
,^u[Tud';;;iltd';',d';;ilAfril
r5'
teg'g
o'Jer'
is an
adequate
prory
for
the
monrhly
."*;;;#.ti.r,r*.-
lJffiilty'
irtl
q"tlarvill
delete
the
con<iition
regarding
specification,
t
oH',ffi"r-g0.1;ry.J:'i#6"-rd
also
rvirl
rnodify
the
adjusted
standard
to
clariFy
that
ir
applies
onlyto,EAF
zincixid;,n.,*i*
undergo
BRZ';
electrolytic
zinc
refining
Drocess.
the
goard
also
will
make
other
minor
changes
to
fire
terms
of
the
adjusted
standard
ior
clarification'
TheBoardtookasimilarapproachinllre-PetilionoiBecvclg.E!|P.15'9s
(Septemberl,rs98jl.eigi-sl,iiil;;';,ffiGiedstandardunder
Section
7Z0.13i(c)
to
a
petiticner
tn'ip'ot"'edused.au*tomotive
antifteezt'
The
Board
did
not
impose
a
condirion
regarding
,p..t"lt*i","1but
oia
limit
the
scope
of
the
adjusted
standard
to
used
autorrotive
antifre-eze
that
the
feiitloner
had
prnr.ttttl
in
a
specific
m.anner
and
rvould
fuither
process
in
a
specific
manner.
!..'il.E.i.ii.lui"*it:,
As97-g'
slip
op'
at
l2'
B
t
E
*** ,,** *ru
lf*= ffi
EE[Effi*-ffi:
&
n
fr
n
E
6
l-lou'ever,
the Board
does believe it nccessary,
as
IEPA
suggests, that BRZ
sample and
test
the malerials
it rece ives. BRZ
lras already proposed
that
the adjusted
standard
require it
to
do
so, and this
infoimation rvoukj
allorv IEPA
to asse.ss rvhether BRZ
is indeed
processing
ntalerlal
that rs
EAF dust that
has undergone
HTMR proce.ssing.
Accordingl.v,
the
Board
rvill
require
BRZ
each month
to take representaiive
sanrples ol
the material
it
receives
from
each
supplier
anri
ccmposite the
samples on
a supplier-specific
basis.
BRZ mr.rst
test each composite
sample
on a montirly
basis, and
maintain records
of sampling
and
test results
for
three
years
and
make those
records available for
IEPA
to inspect.
The Board
grants
BRZ's
moticn
to reconsider
and
grants
BRZ the follorving
amende<l
adjusted
standard:
L
The
Board finds
that zinc
oxide
mate!-ial produced
by subjecting electric
arc
furnace
(EAF)
dust from
the primary
production
of steel
(K0Gl
unCer 35 lli.
Adm.
Code 721.132)
to a high
temperature metals
recovery
(I{TMR)
process
is
not a
solid rvaste and grants
Big
River
Zinc Corporation
(BRZ)
an
adjusted
standard
under
35
lll.
Adm.
Code
720.l3l
(c).
2.
The
adjusted
srandard is
subject
to the following
conditions:
a.
The
Cetermination
described irr paragraph
olre
of this
crder applies
onll'
to zinc
oxide malerial:
(l)
that rvill
undergo
ISRZ's
electrolytic
zinc
refining process
at irs
faciiiry in
Sarrget,
St. Clair
Counry, Illinois:
(2)
thar is
in lllinois;
and
(3)
that has
arrived
ar BRZ's
Sauger,
St.
Clair
Coirnry.
Illinois
facility
or rhar
is
under a legally
binding
contract
for
saie to
BRZ;
b. BRZ must
maintaln
records
identlfying
the suppliers
of
all zinc
oxide
material
that BRZ
accepts
under
this adjusted
stanriard;
c. Each month,
BRZ
must
take
representative
samples
of the
zinc
oxide
materiai
that it accepts
from
each supplier
and
ccmposite
the
samples
on a
supplier-specific
basis.
BRZ
must test
each
composite
sample
on
a
monthiy
basis to
determine.
the prrcentage
by
weighr
of
zinc,
lead,
iron,
total
garrgue
materials
(silica
plus
calciurn
plus
magnesium),
and
chloride
in
the
sainple.
Each
sarnple
must be
collected
and
tested in
accordance
with
generally
accepted
practices,
such as
those
specified
in
"Test
Methods
for
Evaluating
solid waste,
Physicavchenrical
Merhods,"
EPA
publication
No.
SW-Ba6
(Third
Edirion);
and
fi
E
fi
ffi
E
E
n
fi
E
fl
u
t
tE
I
t
7
d.BRZrnustmainrainrecordsoftheinformationrequiredinparagraphs2(b)
and2(c)oft}risorderforaperiodofthreeyearsindrnustmakethem
available
for
the
lllinois
nniiion*.ntai
Protection
Agency
(IEPA)
to
inspect
anicopyatanyreasonableiineduringnormalbusinessltoursuponiEPA's
request
'
IT
IS
SC}
ORDERED.
Secrionz'iloftheEnvironmentalPr'otectionAct(4l5lLCS5/41(i996))providesfor
rhe
appeal
of
fir,at
go;rd
oiO.o
to
the
llliniii
npftilutt
C3yrt
within
35
days
of
sen'ice
of
this
order.
lllinois
:;uprem"
court
Rule
gls
,riulrrit'r,i,
su.t
fiiing
requirementi'
See
172
ill'
2d
R. 335:
see
alsc
ss
iii.-ndnr.
code
101.246,
i\'{orions
for
Reconsideration'
I,
Dorothy
LI.
Gunn,
clcrk
of
the
lllinois
Pollution
control
Board'
hereby
certify
that
rhe
abo'e
order
rvas
,;p*;
on
,n.
6th
day
of
May
lggg
by
a
voie
of
?-0'
Dorothv
M.
Gunn,
Clerk
Illinois
Pollution
Conlrol
Board
<@ffi-lEryffiBl
EXHIBIT
12
:
::".i:.!.::.'-
j
=-.';iar-r
:{-:::
.
_
'
STATE
gf,lEHItItrstr
.
OEFaRTHEI{T
Ef, E}ItIFOfigEHT&ffB
€H:}€RVAT}SI{
P,irtrtas
d
g€ed
Wt* Hgqr*atr
BffiFkrL& GTsee
1S&n:t8tsd
I(#bTrutT?4t't-*l
6E3*{ll4T8E
Sgtecnbcr
l,l, l99E
Mr.
?ftru
J. Sack,
Yret€cs ldsasgs
A.ncsi$ited
Dust
ftocs:ing
Dividos
U.5. 45 NortL
P.0.k3fi0
ts&sq
TN
38303
SIJBJEf;i:
i{€{ucd fcr Varistr*
Fmm
fiexiEcrim
ar a
Haradar
WrgP
fu
Cruds
Effi
Clrido
R"e,l^eirard
S,@
X061
gl€ccrtc
Arc
fur!{€o
lhrst'
Ilar&{r.
Sscb
Afu
g@prbEc
nstice
of o'rrr+rrrca to
gr&d
rmd@d$sm
d$dscdtimas
a
Pfid
wanq'ad
tffisa fron dasaifiasdsra E!
a
hrardcqx
"ta$q
["+
Aroei$tn'd
I}:e
Poc*irtagDividss't
ziDs
erids
cooetrrds
(€nte
tha
sxi&
cr €OI tb DhiSa
brc
rwsivsd
ao cmm"c
qc
$e
Fropor€d
aeiisd fu@e€
30dsy
€@{ils
Bcssd-
Bared
@
@r
pietar
sevicpe
ofawiSred's
ryaf
rrptlo
knt
8ke&ld
$Eri6t
pupty-rrcurged EErt€ wi[
p@
a
thrdit
to
tb€
F'6lis
beals
qr
ths
envtsrssg,
esd
iry*
&
k€k
Apr!ff€
Ed@L4
tle
d€isio'D
!c
gr@ r
rnrtens fs
tbic
tnslcrfutl b now
&&t
Tbis
wiareq
e/ruc,b
it
ryficslrtc
to mlda
tirc
axido
pmdured r &o
ry{
frtrF/ty
rrrfa@sdafi
onCOgf
Edti" Are
FurrraneEs$,
gnd
dcdscd
for sb
to
llorehcad
&csquruo
DseElrpctc!4
atd ts
?fu€
Nscisnsl
fbr
f,s$cr
ptct4sg itto Hgbcffr*{€
dre
qdda,
b
E
ffirduod{r
6,:
&0srdag €osdHoos:
l)
Tbt
the
ffta,ist
h/il1
ccdjrulo
to b6
blrdlcil
ecd
nryca iu
a
tneascr
miarzn
whh
a
ercrnmo*tifflike rua4
i,s"
trqnsfur
B
ecbsd
d6$$rt
syg'€ss
fraar 6e
nust
e<ffry
to
tho
tecdvlngfiiilry
l::1-;:;::l
n
n
n
n
g
n
g
a
fr
;
u
E
a
:
E
fi
I
fi
H
':-.,1'.':t':,,,'r:i,;J:',
Sielrdt,
4{.
@
u
g
a
n
H
t
a
T
t
,1,:
f;
n
E
I
g
r
E
g
E
l{r.
Tbmr
t
Srek
Srpt@bs ll, 1998
tsagoTwa
2)
ThEt tbd D,ryuuar
sdl
bs
idru$d in
nruting
pdorto
sle
to
(ecr
qlstDEcrsr
ddng,
tbs 6od-u*
d
preese*
tc
bc
wlcrf'd
by
tld
r€cxfufos facillry.
Thir
vsisnce
qill
rmris b
cfst ftr
a
pctiod
of
fivo
(5)
telr-r
&@
&i5 ddr of irssnmr
crrrfiilSeEt*robs
Il,2W3.
Tbis
asticris anbsi#Fns:sd to twsaet'gRulo
Cl+{€ I2@l-11-.01(4Xs}3"
rcd
h
e*nrdaucr
siib
fu c&dbio&r
Estcxl
tder Rds
12oo-1-1r".01({xbJ.
Shouid
far
b*re
qucdom
rags{ag
ffi
Exrioq
yiru
rest
cqdrd
E$abd
A taym
sf
my se.ffril
(615)
53?'0834,
TosT1sls. Dke€tor
Dtvicioo
of
So&l
Wnrts
t'temgwcu
cG
J. Alfep
CddEU
A$trTsy,
Barg,
B,art & Sbs
FI"C
EEa&d
A lqm,Fidd
Opraiau
Sngpccg
DSgl{ Nrsl,rilb
&Edt
Esii, DSWM,
tackm
&rvircmcd
Ardrbc€
C€raet
Drr€Dorphq
ftse
AcdvityAsdfl
DSWA,f,
Nulntrc
Eq*er:
Dcscobo,
Wgrts
A*iry Adh, DSffiA4 NEdffllls
gabby
&doffl$oq
wa.ere
A€rhrbt dtudq
PWf{ No^ebrdls
".'
.;.
EXI{IBIT 13
*
a
a
x
a
t
g
n
!
!
E
B
n
I
T
g
I
t
t'.;::':-'.
:
.:'
I
r
t
n
n
E
a
t
'l:t::'
::_.rr.:
:
i'-::;
,
r:-."",::
:
i';
:'li'=t1f:;.i:5i-:-i:r,'::l'
I.
:i:.=
:,:.
E
E
SReeyeling
Zine.
Products
made from
zint or
coated
with zinc
ate very
durable.
Thus
the interval
between
the use
of zinc
for
manufacturin!
a
procluct
and
i
its return
into
the recycling circuit
as
scrap
may
be longer'lhah
a
centuly.
1
-zinc.is
conrpletely
recyclable
without any
loss
of its
physicalor
chemical
Propenres.
2
-
88
oti
of the
zine
available
for recycling is
currently recyeled.
3
-
36%
'rf
the world's
-zinc
supply
-
nearly
2,8 miltion mt
-
comes
from
recycled
zinc.
The remalning
64%
origlnated
lr6m
zinc 6res.
4
-
Brass
recycling
alone
recovers
over
600,000 mt of
zlnc each
year.
5
:
T19
su5tply
of-zlne-coated
steel
scrap ls
expected to increase
by
more
than 50%
over tne
comtng
ten
yeans.
6
'
Pge
to
tho
long_llfo
opan
of rnost
zinc
products,
whlch tn some
casos
may
last
malntonancs-fFeo
for
ovst'!00
yearr,
mufh
of the
zlnc
produced
In the
past
[s
sUll ln
use,
eonttltutlng
a
valuable
antl
eus-talnable
resource
of
zinc
for
futuro'generailond.
7
-Zine
ls
recycled
o
from
manufasturing
and
proeesslng
opera$ons
{'process
scrap"
or
'new
scrap")
such
as zinc
sheet
and
qalvanlzed
sieel
offcuts
aird
trimmlngs,
galvanlzers
resldues,
dte cisilng foundry
returni,
Urass
machlnlng
scrap,
steel
recycllng.
o
from
dlscarded
products
("post
consumer
waste*
or'old
scrap')
auch
as
automoblles^,
gles,
househo!d
appllances,
electronlcs
componants,
etroet
furnlturp,
galvanlzod-darts
froft
bulldlngs,
dismsntled zlnc roofa
and
guttering,
etc.
F
r
I
n
E
E
B
8
Shd
hclusrry
Filbr
Dssf
Ene
6Ei
Slg/$enris
6ts
-
;
De
C-ostiru
Saoe
Product
Typicat
Life
Uses
S'emi6t
hdu:.fry
&
'O*ta
lB
Gohcnizing
F;j*dr:z7Ts
Cycles
for
Zinc-Containing
products
Llfe
cycle
qyears)
E
T
Znc
sheet
Brass
products
Die
casUngs
Galvanized
coatings
Fabrieated
products
Zinc
cornpounds
Source:
fZA
-
Europe
Roofing
Cladding
Vast
range
Gars
-
appliances
-
hardware
-
tools
-
etc
Cars
-
roofing
and
ctadding
for
buildings
Wde
range
sf
structr
Power
instarlations
ures
:
in<lustry
-
road
-
rait
and
Tyres
100
+
200
+
10+
10-15+
10-50+
25+
1.5
i
I
I
I
PrS
&
Nbxt
#
ar,V".,?€cfc,
tu"-
(€o
FoREv€'R
-s*'*Womobiiesiredsi'ss
shorvn
in
this
picture
yietd
bp.Euatity
new
,At"y""Atr",."r*A?"
j,r.*_
www,*.J;;#_
's
r
*IflTffi4a**#
,
*aJli?ff
r
n
H
t
a
E
B
fi
F
E
a
il
fi
g
E
E
a
E
shredders.
About 40
poun_ds
of
zine are in
a
Uplcal
Norttr
American automobile -
19
pounds
in
zinc castings,
l8
pounds
forzlnc
coatings
and 3
pounds
in brass,
zinc
oxide in tires'and
solder.
In
the
next
decade,
the
amount of zinc
recovered
from scrapped
automobiles
will
ine
rease significantly
as
a resuii of
growing
use of
zinc-coated
stebi
and
rust
protection.
Intricate zinc
die-cast parts
are
easily
recycled into
new
parts
vrith no
loss-of
quality.
hriillions
of
products
that
contain
zinc are
discarded each
year
in
the United
States.
These
include:
After
Discarding
c
appliances
e
etectronics
components
e
automobiles
o
ehildren's
toy6
a
highway
guardrails
and
signs
o
HVAG ductwo*
e
other
galvanlzed
parts
from razed
buildings, bridges, and
tires.
ltJhile
zinc-containlng prcducts
have a
long life
-
from about five
years
for
tires
to
more
than
200
years
for ztnc
sh-edrctaddlng:
thJysii"evinttjitti
fi;-ui:zirnc
rs removec-rrom
these
Products'
known
as'old
scrap,'-and
fut
back
into the marketplace
at
a currerrt
rate of 1.2
million
tons annually
in
the
Western
World.
E-rass, found in
buildings,
lt.mpq,
doorknobs,
and
bric-a-brae,
is a
major
source
of recycled
zinc, accounting
lor
32%
al
total zinc recovery.
Recycling
Steel And
Zinc
c
A
growlng
source
of reeyeled
zlnc
is
the steel Int{ustqy's
elcetrlc arc
fumaces,
where
zine
is recovered
from-ths
llua dust
produced
when scrap
steel
-
much
of whlch
is
zlne-
coated
ls mefted
for
recycllng"
'
o
Zinc
partlcles,
whlch
can
make
up
to
40%
sf the dust, are
collected
and
pui
back
lnto
production
rather
than
expelled
Into
tfre atmosphere, benefiting
lndustry
and
tho
envlrgnmenL
Recycled Zinc
t$arkeB
Zinc flrcycllnq
b fuelled
by a
thrlvlng and
diverse market
for reusabte
Zinc.
Mors than
t
10,000
metfie ronE
ot
Etab
ztnc
ars
pmduced
annually
In
the u.s" from recycled
Zlne.
Felost-lngots
.
or
slat)s
-
of
zlnc are melted
and
used to coat steet,
protectlnq
tt
from
rusL
Slab
zttg!3.1E9
Tgltqd,
tolled
end flatiened
Into
oir.eets.
Nlnety-eighf
fercent
ofttre
U.S.
penny
ts
macls
from zlnc
shoet
wqh_a
gQppet
platlng.
These sheets aldo
eird up as
countertoirs
anb
building
down
spouts
and
rinlstilhgs.
Over 35,000
m.etric.tons
of recycled rlnc
oxides are
produced
annually
In the U.S.. They
are the
healing Ingredlent
In
dlaper-ra"sh
olntments,
soaps, bharnpoas,
and
oiher skln creams.
Zlnc
oxido ls
also a
fortfiing
mlneral
In cereals
and fertilizers.'And,
slnce it ls
required for
curing
rubber,
h'e found
In'ev6ry
tlra.
RecycleC-zlne
ls ueed
to make
zlnc dusf
an Ingredient
providlrrg
corroslon
protection
in
manv
pov/der
Plf lF-._{-ls_
ls a
a-l!o
cornponent
founcl
lrr
In
chemlcals
dry-cell
batterles.
and
lubricants
Alloyed
and
with
ie
othi:r
employed
firetals,iiko
in
gold
copper
iecovery.
or
And
alurninum
ztnl
Yfi'::',:':;:;.:
"=
:'
''i'
::
;.,
r.-l:r:j\.i:
n
.:
.
.li::r.,.
r
recycled
zine
ie
cast into
prerlision
parts
for
appliances,
hardware,
electronies and toys.
How is
Zine
Used?
r
Zine's
dominant use ig
as
a coating
on steel
to
protect
it
frorn
rust,
extending
the life
of
automobiles,
bridges
and other steei
structures.
o
Because
zinc
has
a
low
melting
point
and
is
light
weight,
it is easiiy
die
east into
ccmponents
for
appiiances, automobiles
and
childrerr's toys.
And zinc
combines with
copper to make brass.
r
Zinc
oxide
is
also a
necessary
ingredient
in rubber
products
and ls used
in
pharmaceuticals,
including dietary
supplements
and healing
ereams.
@Back
*
n
&
*
fr
a
u
fi
t
r
n
n
I
il
g
n
*
I
i
I
I
I
I
I
BEFORE
TF{E
ILLINOIS
POLLUTION
CCNTR.OL
FOARD
*.F,F,.T1Y,RE'
AUG
2
7
1999
^
?IArE
oF
tLUNors
ln
the
lr.latter
of
rouutlon
Control
Boar,
Petitian
of
Horsehead
Resource
Developn.lent
Company,
Inc.
for
an
Adjusted
Standard
ljnder
35
ill.
Adm.
Code
720.131(c)
)
)
I
\
NOTTCE_OI
Frl,tNg
To:
Dorcrlry
h.i.
Gunn.
Clerk
Illinois
pollution
Control
Board
100
W.
Randolph
Street
I i'h
Floor
Chicago,
IL
6060
t
Mr.
paul
E.
Gutermann
Akin,
Gump,
Strauss,
Hauer
&
Feld
1333
Nerv
Hampshire
Avenue,
N.W.
Suite
400
Washingron,
DC
20036
ii,|*::;ffi,T::,:Lji*jl:"::oday
nred
with
rhe
oftice
of
the
cierk
of
the
rriinoi
LlIf
':-"-
g:
T*l
B
o
ard
ure
r
uinoi
s
il;;#;;#ilj:,;
AS
00-02
(Adjusted
Standard-RCRA)
N{r.
John
N.
Moore
200
I'"lorth
LaSaIe
Street
Suite
2200
Chicago,
IL
6060
t
lvlr.
Roben
Lawley
t-liinojs
Depa.rrment
of
Natural
Re:ources
524
Soilth
Second
Srreet
Springfield,
Lt_
627Al
irno!s
u,hich
is
attached
hereto
and
serv_ed
ufon
you.
ron
Agency's
RESFONSE,
a
copy
of.
Date;
AUovST
aZtqq?
Illinois
Environmental
protection
Agency
Division
of Legal
Counsel
l70l
S.
F'irst
Avenue,
Suite
600
Maywood,
IL
60153
708/338-7890
PEO:pgb:nor
TIII.S
FTLINC
IS
SUBI\{I?TED
ON
RECI'CLED
PAPER
PLA,INTIFF'S
I
EXHIBIT
E
n
6"n:',.
'.'i4!p,
{.2
'
'l
"',
BEFORE
Ti{E
ILLINOIS
POLLUTION
CONTROL
BOARD
STATE
Poll'ttion
F[ECETVE[}
r':
E.nr|q
t^r:rt-E
AUG
2
?
1999
C)F
TLLINOI:i
Control
Board
t1.\'
Ti{E
MATTtrR
OF:
PETITTON
OF
HORSEI-{EAD
RESOTIRCE
DEVELOPN,IENT
COMPANY,
nic'
FOR
Al'i
ADJUSTED
STANDARD
I.JNDER.35
ILL.
/.DM.
CODE
720.131(c)
AS
00-02
(Arijusted
Standard-RCRA)
RESPONSE-OF
T}TE
IL.LSOI.S
EPA
Tg
P ETITI
QN
ESBJU)-JIISTED
S
T4JNDA'RD
pursuant
to
35 lll.
Adm.
Code
10f.414,
the
lllinois
Envirorunental
Pro+.ection
Agenry
("Illinois
EpA"),
through
its
attorne-vs,
hereby
submits
this
Response
to
the
Petition
of-Horselx:ad
Resour-c-e
ran
("Petition"),
f,f"a
U"ior.
rhe
lllinoiiPollution
ControlBoard
("Board"),
and states
as
foliows:
l.
BACKGROLTND
ln
its
petition,
Horsehead
Ressurce
Development
Company,
fulc.
("HRD.)-
a recycler
of inorganic
hazardous
wastes,
requests
a tietermination
by
the Board
that
the
crude
zinc
oxide
("CZO")
which
it
produces
at
its Chicago
faeility
is not
a
solid
waste.
HI{D
receives
electric
arc
furnaee
("EAp';
dust
from
stee!
mills.
ire
EAF
dust
and small
quarrtities
of other
metai
b,xring feedstocks
along
wit6
a
carbon
source
are
introdueed
into
a
high
temperature
metal
recovery
("I{TMR")
process.
Drrring
the
HTlv{R
process
the
nonferrous
metals
are
concentrated
to form CZO.
The CZO is then
shipped
oFsite
bY
railcar.
II
APPLiCABILITY
OF SECTION
720'l3l(c)
The
terms
of
the
acijusted
standard
require
that
a
hazardous
waste
be
panially
reclaimed,
and
suffrciently
,,commodity-like"
after
injtial
reclamation.
A
material
must be
a RCRA
hazardous
waste
to
be
eliglble
for
this
sotid
rvaste
determination.
The
materiais
in'rolved
in this transaetion
appear
ro
be
withiri
the category
eiigible
for the
requested
adjusted
standard.
EAF
dust is
a listed haanrdous
waste
under
35
lll.
,La*.
coor
7zl.l3z, carrying
the
code
K06l.
The Petitiorr
describes
the
czo
production
process
as
an
HTMR
system
which
through
the
application
of heat
in
a
rotary
heartlt
furn"r*,
substantially
raises
the
percentage of
zinc
in
the
EAF
dust.
This appears
to meet
the
definition
of
"reclamation"
of
3
5 lli.
Adm.
code
721
.
l0
I
(e)(4), since
it
is being
processed
to
recover
usable
product,
zinc.
For
the
same
reason,
it is
also
consistent
with
the
United States
Envirclnmental
protection,{gency's
explanatian
sf the
term "rec,lamation"
in
the
Federal
Register.
See
50
Fed.
Reg.
614,633
{}a1aa4,5,
l985).
Baseci
on
the
infornration
presented
in
the
Petiticr,,
the materials
fbr
whiel.i
the
acljusred
standard
is
soughr
appear
to
be
eligible
for
consideration
under
Section
i2A.ftr{c).
lt
EV.AluATloN
oF
FACTORS
UI.IDER
SECTION
720 i31(c)
After
a
determination
that
the
material
are
generaliy eligible
for
the
adjusted
standard,
the
lllinois
EpA
rvill evaluare
rhe
proposal
in the
Petition
using
the
factors
set
forth
in Section
720- l3 I
(c):
I
)
The
degree
oiprocessing the
nnaterials
has undergone
anC
tire
degree
of fi'rrther
processing
that
is
required:
2)
The
value
of the
maleriat after
it
has
been
reclairneei;
3)
The
degrec
to
which the
reclaimed
materiai
is
iike an
analogous
rarv
material:
4) The
extent
t.:
w.hich
an errd
market
fcr the
reciaimed
material
is
guararileed;
5)
The
extent
to
rvhich the'
reclaimed
rnaterial
is handled
to
minimize
loss;
6)
Other
relevarrt
factors.
A.
Degree
oiFrocessing
See
ron
7ZO.l3l
(c)(
I
)
rcquires
consideration
cf the
degrei:
i.f
prc,cessing
initially
perforrred and that
furr-her
requirecl.
Th"
ror.
sr,bstantial
rhe
initial
processing,
the
morc
completely
reclaimed
the
rnatenai
ii,
and,
hence
the
more
comrrrodity-tike
the
rnaterial.
[n this
instance,
the H]MR
proeessing,
which cyposes
the
material
to
high
tetnperatures
in
a
rotary hearth
furnace,
subxantially
th*g",
tle
contr.:ni
of
the
material.
The
Petition
states
that
the
zinc
is concentrated
from
upprl*i*ut
ely
15,%
in
the
feedstock
to
approximate\y
6014
in
the
resultant
CZo.
The Peiition
a.lso
,iuio
thet
thJ
process re6uces
the
mass
of
the
feedstock
by
approximately
two
thirds.
Afterthe CZO
is
produced,
only
a relatively
small
amount
of
additional
processing
is necessary
to
prod'lce
zinc
products.
B.
Value
after
Recla-rnation
Under
Section
i2o.lil(c)(z\
rhe
value of
the material
after
reclamation
is
a factor
in the
decision
as
to
whether
to
grant
an
adjusred
standard.
The
higher
the
value
of
the
material,
and
the closer
lhat
value
is
t'
the
value
of
the
raw
material
it
s:rpplements
or
replaces,
the
more likely
it
is to
be
considered
comrnunitY-like.
Thepetition
inelude,r
confidenrial
data
which
compares
the
ipproxinate
transacticn
priees
of
czo
sold
by
HI{D
,.*rith
the
appropriare
transacticn
price of
zinc
concentrate
produced
from mined
zir*c
clre.
since
the
infcrmation
is
confidential,
it
will
nor
be
cited
irr this
response.
[t is
sufficier't,
horvever.
to
sra',e
that
the
Minois
EpA
believes
that
the
sales
pnce
for cZo
is
in the
sffo€
$€Fr€ffil
range
as
that
ofthe
sales
price
for
zinc
concentrate'
C.
Comparisolt
with
Ra*'N{aterial
Section
7?a.fiI(cx3)
requires
consideration
of
the
comparison
of
the materiars
to be recrassified
by
rhis
aCjusted
standard
ivirh
the
rarv
malerial.
The
closer
the
material
is
to
the
rarv
material
that
ii
supplemerrts
or
replacr;s
the
more
likely
it
is to be
commodity'iike'
The
marerials
to be
compared
are
zinc
c.oncentrates
from
minecl
ore
and
czo
which
is
partially
reclaimed
EAF
dust.
The
percentages
of
zinc,
iron,
and
lead
in
zinc
concentrates
and cZo
Bre
very
similar.
I{or.rrever,
the
percentage
of
chlorine
present
in
C.Zo
is
considerabiy
higtrer
than
that
occurirrg
in
zinc
,on"*nirrr.s.
The
lllinois
EPA
iequests
that
IIRD
comment
on
wltether
the
higher
chlorine
content
poses
anv
pollt.rtion
control
problems
not
inherent
in the
processing
of mined
zinc'
D.
Errd
lrtarket
The
extcnr.
tc
which
an
end
market
is
guaranteed
is
a
factor
for
consici-'ration
tnder
seaion
72O.l3l
(c)(a).
The
sironger
the
market,
tile
more
likely
the
material
is to
be
r.
'rmmodity*like'
The
regulations
do
rrot
specifically
state
r,,hether
the
market
to
be
considered
i:
the
rnarket
for
the
material
after
initiai
rectamarion
oi
for the
products
produced
after
reclamation
s compiete'
USEPA
commentary
inoicaies
that
the
principle
ron".rn
is
with
the
market
ater
initial
reclamation'
50 Fed'
Reg.
614,655
(JuruarY
4, 1985)'
Historicail,y,
most
E/rF
clu..st
rYas
disposed
of
as
a
waste.
However'
markets
ar
e
now
developing
as
altenatives
to
oisp'sat
are
being
exprored.
HRD
rnakes
a
point that
since
it
began
producing,czc
at irs
Ctiicago
facility,
it
has
never
had to
stockpile
any
ofihe
CZo
because
it
ha:
contracts
lbr all
that
it
produces.
mtb
Aso
srates
that
all
of
its
buyers
process
tha
CZO
immediatel)'upon
receipt'
Given
rrrose
facts,
the
Lilinois
EpA
berieves
that
end
markets
for cZo
appear
to
be
guuanteed.
E.
Minirnization
of
Loss
Section
72Afil(cx5)
t'equires
consideration
of
ttre
metltods
employed
to
minimize
loss
of the
nlaterial
during
harrdling.
Tiris
factor
goes
towa'l
t"vo
considerations.
Fi'st.
vrhen a materiai
is
handled
carefirlly
so
as
to
prevent
!oss, ihis
tends
t(]
indicate
that
the
material
has
value.
second'
the
methocls
impiemented
to
prevent
loss
csn
reduce
the
potential
negative
environmental
consequences
crue
to
possible
rereases
oithe
materiar
rvhich
wourd
have
been
considered
a
hazardous
waste
absent
the
rteljusted
standard'
tff{D
mirrjmizes
loss
by
the
&llorving,neansi
f
.
,4Jl
u
r#T:;ffgr:*.::"vevins
operarions
are
encrosed
and
con.rc
2'
?'lre
HTL{R
processing
produce*
n^.r.-
'---
q"Lr
controlled
by
lflinois
E'PA
3
After
rh,. *---
-'-
'rsrrc-s
arid
no
water
di-sch"-^^
o,",,,.iliooii.,,l,j
,,
,",;:*"r,
ffi::''
no
water
discha''ses
4.
Theoff
-ru
!s
rransferred
to
railcars
in
an
encr*sed,
rrnu,uriri,l'rt
transponarj
on
of
czornusr
compl.v
r
The
iri'ois
ar^
,";.p..
rL _ , __
'--"'
;.rtnPl'v
I'rith
u
s'
Depanmeni
ofTransportarion
ffi
:::::"f-f,'"'n::ffi:m,m'""'*.Jr:il:H"#J1.ff-.,f
F
orher
Facrors
ft:ffi':,,::s,.However.
qLcto€tltii
tpittt,
rrpt,,rij
::lli,Ji1"l,!,,,J!12,i!,,"H:':
'*,ou,',.,"";u",v,,,J*;ffii##::tff:',Tr"lil:il1lil:ffffi,.*lt.rr-J:i[i]i,,t,i?
The
lllinois
Ep
BRZ
#ffi1
is
vinuallv
weighr
to
its
pri,
G'
The
lllinois
Epa
"l^^
"gr
trre
t
-
".,6iivs
ttlurr;lt
ri:.'"*il{?i#*"".:*ilff:'ffi
,:ff;#d*,n:u:d,u,',i?r.,*Tn'
r:onservado;
IV
ITECOA4N4ENDATION
Iir:,
upon
the
inf
"'''
?
-1r
"
"
*"'
ffi
tfir*r#ffiffrumffi
standard
pursuant
r
ffi,i:,:ffi#J;
ore;
rhat
there
is
a
n
4
_...4v
russ.
AdrJitionaJly,
By
L{RD
has
exprained
why ihe
refief
it
is seeking
is
similar
ro
the
rerief
granted
by
the
Board
to
BRZ'
The
illinois
EpA
has
posed
some
questions
ro
HRD
in
this
Recommendation
Assuming
that
t{RD
answers
those
qu"rfion,
to ihe
illirrois
EPA's
satislaction
and
supports
the
facts
assened
in
its
petition
vrith
adequate
evidence
during
*"
r,*rring,
the
lliinois
EPA
recomrnencs
that
the
Eloard
grant the
adjusted
standard'
Respectfu
llY
submitte,d,
ILLINOIS
ENVIRGI-IMENTAL
PROTECTION
AGENCY
lllinois
Environmental
Protection
Agency
l?01
South
First
Avenue
Suite
600
ldayrvood,
IL
60153
7081338-',7894
PEO:pgb:hrd.Pct
CERTIFICATE
OF
SERVICE
l, the
undersigned,
certily that I ha.ie sen'ed
the
attached
NOTICE
OF
FILINC and RESPONSE
upon
the
persons specified
in
the
NOTiCE
OF
FILING by
First
Ciass Mail
on iilis
&?""
day
ofAugust,
1999.
PEO:pgb:c'os.2
ffitF-
(ffi
ot
i4tr:;t:"'
a''):.::.
R[:{)EEWE,EF
CLERK'S
OFFICF.
EEFCRE TffE
TI-ITNCIS PGLI,IITION
COflF,ROL
BOARil
sEP
1
0
1999
A'{ TEE
MATTER,
OF:
PETTTION
OT HORSEHEAD
R.ESOURCE
DE}IELOPMENT COMPANY,
INC. FGR AN
AD.il.ISTED STANDARD
UNDER
35ILL.
AD&{.
CODE
720.131(c}
STAIE
OF
ILUNOIS
Pollutlon
Cantro!
Board
AS
00-2
(Adjusted
Standard
-
RCRA,)
NOllcE
-on-FILINq
TO:
Dororhy
M,
Gunn, Clerk
Feter Orlinsky
ILLINOIS POLLLTIION
Assistant
Counsel
CONTROL
BOARD
Division
of
Legal
Counsel
100 West Randolph
Street
-
l
ie Fioor
ff-LiNOIS
ENVIRONI"4ENTAL,
Chicago,
I[. 60601
PROTECTION AGENCY
l70l
S.
First Avenue.
Suite
600
\{ayrvood, tL 60153
Robert
Larvley
Chief
Legal
Counsei
TLI,INOIS
DEPARTMENT OF
NATURAI" RESOURCES
524
S. Second Street
Springfield,IL
62701
Please taire
rrotice
that
on Friday, Septernber
10,
1999, we hand
delivered
to
lhe
Cierk
of the Pollution ControlBoard
I{ORSEHEAD
RESOURCE DEVEI-OI'MENT
COMPA}.IY,
INC.'S REPLY
TO Ti{E
RESFONSE OF
TItr TLL,TNOIS
EbMRO\rh,fENTAL
PROTECTIO}-I
AGENCY, a copy
of
rvhich
is
attached
hereto
and
served
upon
you.
Respectfu
ll
y
submitted,
HORSEHEAD
RESOLTRCE
DEVELOPMENT.
COMPANY,
TNC.
'tu_*_
John
N
Moore
LAW OI.-FICES
OF JOHN
N
200
North LaSalle Street
Suite
2200
Chicago,
lL
60601-1095
(3r2)
782-esl3
MOORE
By:
PLAIhITIFF'S
6
FXHIBIT
a
n
111:'c'ci.-(
/'
One of its Atlorneys
THIS I.'ILING {S
SUBMII-IED
ON
RECYCLED
I}APEtt
*5,=?'5-"Y'Ff
sEFoRE
TEE
$',L1Ho$
PoLLuTloil
coNTRoI'
BOARF
sEP
1
0
1399
-qTATE
oF
tLLlNOls
1
F"ii;;;""
controi
Board
fi.{
TITE
MATIER
OF:
ff.il,rl-u
sta'dard
-
p
Er
xrron
qI
g"l3u#11i,
S'.11$tJ*
I
RCRA)
ffi[:BT'$#"',{3ttr^?t:*"c
fff
no
I
flxl'rqr64P'
ij*
ne
n
3
s
ILL
)
)
lil"*i.
"ooE'
720'131(c)
s0RsEBtttgl*1"-""?:13"tH;;$yffi
.xmn$.*#Fii#"f"ag:l
:
)evelopment
c.ornpany'
lne'
(L1RL,)'
rruvu'"
rna\ Rcqoors€
to
petition
ior
Adiustcd
Environmeirla!
Protection
Agenc't's
(lllinois
EPA)
Responr
Standard
filed
on
Ausust
2't
'
rses(Rasponse)
-*r:::::::
ffitJ::-":-
GsD
petition)
requests
an
adiusted
standard
for
crude
""
;;:'",
"*.,
r,"
trie
recycring
oi.
electnc
arc
furnace
dust
and
sna*er
quantities
of
other
zinc-bearrrrg
haz-ardous
and
non-hazardous
wasre
feecrstocks.
In
its
resrronse'
Illi.ois
EPA
agreed
tltat
t{RD,sFetitionsatisfiestlreappiicableregulatc'rycriteriairrS5lll.Adm.Code
rz0.t31(c)
and
recomnended
that
the
tsoard
grant
the
adjusted
standard'
assunring
that
F{RD
satisfactor*y
responds
to
two
questions
and
presents
supporting
evidence
at
gI
TFE
II*FNQIb
s'5's'='-
*
'
-
. r r,L\ neririoner
Horsehead
Resourcc
Pursuant
to
35
lll'
Adm
Code
106
4i4(b)'
t"-t:::rt;;;
,n-
I)evelopment
c'ornpanv'
lne ${nn,'
th:oush
t:',"::::":",'T
il:.,':":
^""::
hearin''*o
agrees
witlr
the
anarvses
aud
recontm*nout'on'.::]j|],,,uff's
Response'
and
answers
I'inois
Eprr,s
two
questions
in
this
Reply
ln
particular'
t{RI)
concurs
with
lllinois
EPA
that
the
Board's
recently-granted
adjr'rsted
standarcl
ibr
Big
River
Zinc
corporation,s
(BRz,)
zinc
oxide
nrateria!
rras
"strong
precedential
value"'
and
that
the
relief
obtaine.d
by
BM
is
,.virtuaty
the
same
as
the
rerief
souglrt
by
HRD'"
Response
ar
ia!r:;
l::a-r.!
:,'):1;
.;4
4
(citing
In re
Petition
of Big
River Zinc
CorporatiQn
for
an
Adiusted
$tgodaldlJaderlS
IIJ
Sdrr:-!odp--?2gJlll!)
(April
15,
1999), At
99-3
(BRZ
Opinion
and
Order),
A!1p1ded
May
6, 1999). FIftD's
CZO
and
BM's
zino oxide
ntatcrial are
virtually
iderrtical in source, composition, and
process
suitability,
and
F{RD has demonstratcd
conrpliance
with
the
regulatory
criteria
in 35
Il!. Adm. Code
7T0.l3l(c) in
a
ntan;ier
similar
to BRZ.
The rernainder
of this
Reply
addresses
Illinois EPA's two
questions.
1.
@hlatsrial
FIITD agrees
vrith
lllinois EPA's
statement
that
the
percentages
of
zinc, iron,
and
lead in zinc ooncentrates
produced
from mined ore
and
in
CZQ are
very
similar.
Response
at
,. Illinois
EPA
asked,
however,
vrhether the
"highcr
chlorine content
lin
C.ZO)
poses
any
polluti<ln
control
problems
not
inherent
in
the
processing
of mined
z,inc."l
Id. [n short, as explairred belorv,
the
presence
of
chlorides does not
pose
anv
pollution
control
problems
or othenxise
inrpede the
processing of
CZO
Direct
Fegllstock
fo.f Zinc- Produqtioq
One
ot'CZO's uses
is as a direct
feedstock
in zinc
production
at ZCA's
zinc
production
facility
in Monaca, Pennsylvania. See
HRD
Petition at
l3-14.
No
separate
salts
removal
step
is necessary
for
this use.
CZO
is blended
rvith
cther feedstocks
(roasted
mined
zinc concentrate
and
other
k:rv-sulfur
zinc oxides) and
the comtrined
feedstock
is sintered.
which
results
in
two
materials:
zinc
sinter and
lead
concentrate.
The salts
partition
primalilv
to the
lead
concentrate,
which
serves
as
a
feedstock
irr
another
processing
circuit at the
facility. incidental salts
in water
froro
that
processing
1
while FIRD
reported
the
elemental
presence in
terms of
"chlorine,"
this
element is
present
in CZQ
as
"chlorides"
or
"salts."
In
the
remainder of
this Reply,
HRD
uses
the
terms chlorides
or salts
to
respond to
illinois
EPA's
questions.
circuit
are
dischargel
to an
NPDES-permitted
outfall.
'fhe
zinc
sinter
is charged
to the
electrothermic
furnace
for the
final step
in
the
production
of
zinc
metal.
D
i
rgct-ry-e
ed st
q
cB
fo s-eglc
i
n in
g
CZO also
is used as
a direct
feedstock
for calcining
at
tAD's
facility
in
Palmerton,
Perrnsylvania.
See
HRD
Petition
at
i5-i?.
HRD
caleines
a
portion
of the
CZO
in
part
for
the
same
reasons
that
BRZ washes
its
zinc oxide
matarial.
egStpaIg
HRD
Petition
ar
16
(calcining
of CZA
increases
the effrc.iency
and
longevity of
ZCA's
sintering
product
collectrrrs)
with
FPZ
Opinion
and Order
at
12
(salts
could
corrode
BRZ's
refir.ery
equipmenl).
Calcining
results
in
zinc
caleine
and
lead concentrate,
and
further
purifies
the CZO by
iemoving
the
lead
and salts,
whicii
partition
to
the lead
concentrate
Again.
no
additional
polluticn
control
measures are
required.
Tlte lead
concentrate
is
processed at an
HllD
affiliate
for metals
recovery and
the
salts are
6isposed
of
in
a
permitted nonhazardous
underground
injection
well. The
ziric
calcine is
ssnt
to
ZCA's
Monaca,
Pennsylvania
facility,
where it
is
sintered
in
combination
with
CZO
and
other
zinc concentrates.
Z.
M_ininnlaatio_a_al-togg
Illinois
EPA agrees
that
HRD satisfactorily
minimizes
the
potentiai
for CZO loss
during
handling
by severa!
means,
including.
l.
All unioading
and
conveying
operations
are enclosed
and
controlled by
Illinois
EPA
permitted baghouses;
Z. The
HTMR
process
produces
nc
wastes and
no wastewatet'discharges;
3. After
procluction,
CZO
is
transferred
to
raiicars
in an enclosed,
pressurized
system;
and
4. Off-site
transportation
of
CZO
must
cornply
with
U.S.
Department of
Transportation
reguIations.
Response
at
4.
While
agreeing
that HRD minimizes
the
potential
lcss
of
CZO,Illinois
EPA
suggested
in its
Response
that IlIl-D
"address
its
procedures
fcr
dealing urith
accidental
spills.
ruptured
baghouses,
or other
environnrental
coneerns."
Response
at
4
Fundanlentally,
HRD's process
design
minimizes
the
potentia!
for loss
of
CZO
As
explained in
HRD's
Petiticn (psges
7-9\,
CZQ
is
managed
enrirely
in
enclosed
buitdings,
containers"
and
conveyances,
and therefore
is
protected
from
exposure
to
the outsidc
environment.
Nevertheless,
in
ihe highly
unlikely
event that
a
spi!l
of CZO
occurred,
HRD is
fully
prepared
to
respond
quickly
and effectively
to
prevent
exposure
and recover
the
CZO.
Employees
trained
to responC
to
emergencies
staff
HItD's
Chicago
facility
24
hours,
seven
days
a
rvcek
HRD
implements
a multi-pronged
prevention
and responsc
strategy, irtcluding:
e
Employee
training
programs;
o
Inspection
and rnonitoring programs;
e
Preventative
maintenance;
o
Comprehensive
housekeeping programs:
o
Emergency
equipment;
and
o
Arrangements
with
appropriate
authorities.
These
programs
and
procedures
greatly
minimize
the
possibility
of
a
spill
of
CZO
6r
other
material.
If
a spill
of CZO were
to
occur, however,
trained
FIitD
personnel
rvould
immediately respond
to
the spill.
The
entire
area where
CZO
is
managed
is
pavecl
with
asphalt or concrete, which
rvould
contain
any
CZO
spill
and tlcilitates
removal
of
the
material
by
industrial
'racuunt
trucks,
road
srveepers,
and othcr
apDropriate
containment
equipment.
The
recovered
czo
would
be
returned
to the recycling
process.
I
I
I
I
I
I
I
I
An ascidental
release
due
to
product
collectol
bag
failure
also
is unlikely
to occur'
HRD
operates
two
tllinois
EPA-permitted
product
collectors,
each
witlt
12
compartments
A
24-lrour
opacity
ntoni'ror
continuously
rneasures
exit
gases
from
the
product
collecrors,
and
an alarm
connected
to
the
monitor
alens
the
kiln
operator
if
opacity
levels
increase.
Giventhe
large
number
of
bags
in each
compartment,
indi'ridttai
failures
are uniikely
to affect
the
recycling
opelation
or
otherwise
require
an
emergency
response.
Never-tlreless,
if a
procluct
coltectcr
bag
or
compartment
is tiie suspected
causc
of
a
failure,
FiRD
imn-re<iiately
rernoves
the
affecied
unit
fronr service
without
internrption
to
the
renraining
compartments,
initiates
repairs,
and
returns the
unit
to
sen'ice
Thus.
I-1RD
has
*,ell-designed
and
effective
procedures
lbr
addressing
irnmediately
any
conringencies,
including
those
posited by
lllinois
EPA'
denronstrating
that
CZO
is handled
carefullv
to
minirrtize
loss'
trNELUS]ON
WHF.REFOI{E,
for
the
reasons
set
forth
above
and
in [{RD's
Petition,
whiclt
demonstrate
thai
HRD
satisfies
all the
criteria
for an adju;ted
standard,
HRD
respectfully
requests
that
the
Board
grant
HRD
an
adjusted
standard
for
CZO
produced from the
recycling
of
electric
arc
filrnace
dust,
and
smaller
quantities of
zinc-bearing
hazardous
and
norr-hazardous
waste leedstocks.
Respectfully
submitted,
HORSEHEAD
RESOURCE
DEVELOPT\IENT
COMPAhIY,
lNC
svS
FaulE.
Gutennann
AKD.I,
GUMP,
STRAUSS,
HAUER
&
FELD, LLP
1333 New Hampshire
Avenue, i-{.'W.. Suite 400
Waslrington,
DC
20036
(202)
887-4000
John
N.
Moore
LAW
OFFICE,S OF
JO}{N N. MOORE
200
North
LaSalle
Street.
Sui'.e
2200
Chieago,LL
60645
(3t2)
782-eso3
Date:
September
10, 1999