| | - Dorevitch IPCB Testimony rev_7-25-08.pdf
- Dorevitch CV 7-24-08.pdf
- July 1999-June 2001
- Resident, Occupational Medicine, UIC Medical Center
- Aug 1999-May 2001
- Environmental and Occupational Health Sciences, UIC School of Public Health; Degree awarded: Masters in Public Health
- July 1990-June 1993
- Resident, Emergency Medicine, Cook County Hospital
- July 1989-June 1990
- Intern, Internal Medicine, Northwestern University-Evanston Hospital
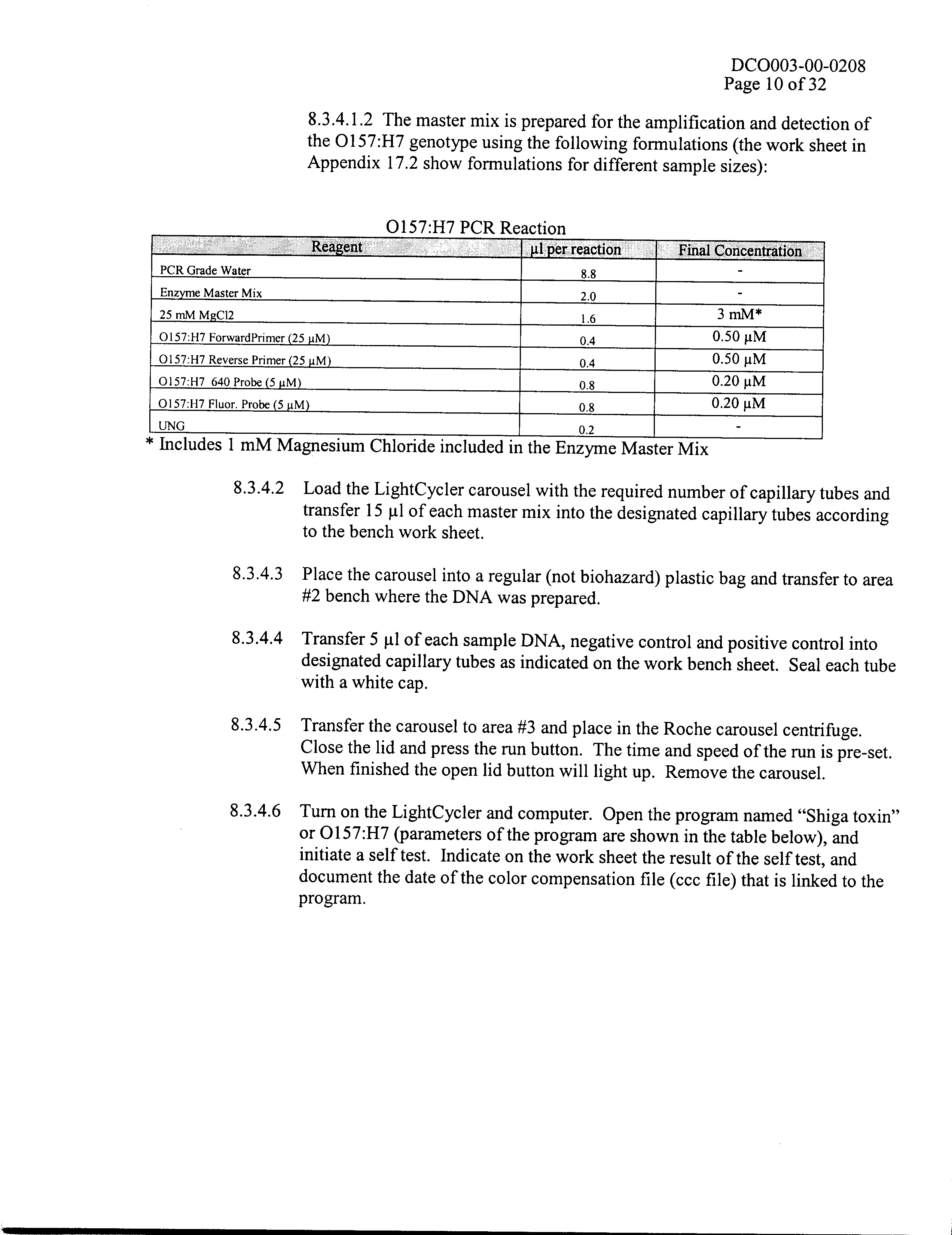
- Sept 1985-June 1989
- University of Chicago, Pritzer School of Medicine;
- Degree Awarded: MD
- Sept 1981-June 1985
- University of Illinois at Chicago
- Employment
- Research Funding
- Scientist Reviewer, CDC/ASTPM/ASPH/AAMC Special Emphasis Panel, Atlanta, Georgia June 7-8, 2004
- Testimony at public hearings: US EPA proposed PM2.5 standard, March, 2006
- Licensure and Certification
- CHEERS recruitment to date.pdf
- CAWS Use survey summary.pdf
- Complete QAPP.pdf
- QAPP_Overview Jul 2008 FINAL.pdf
- QMP.pdf
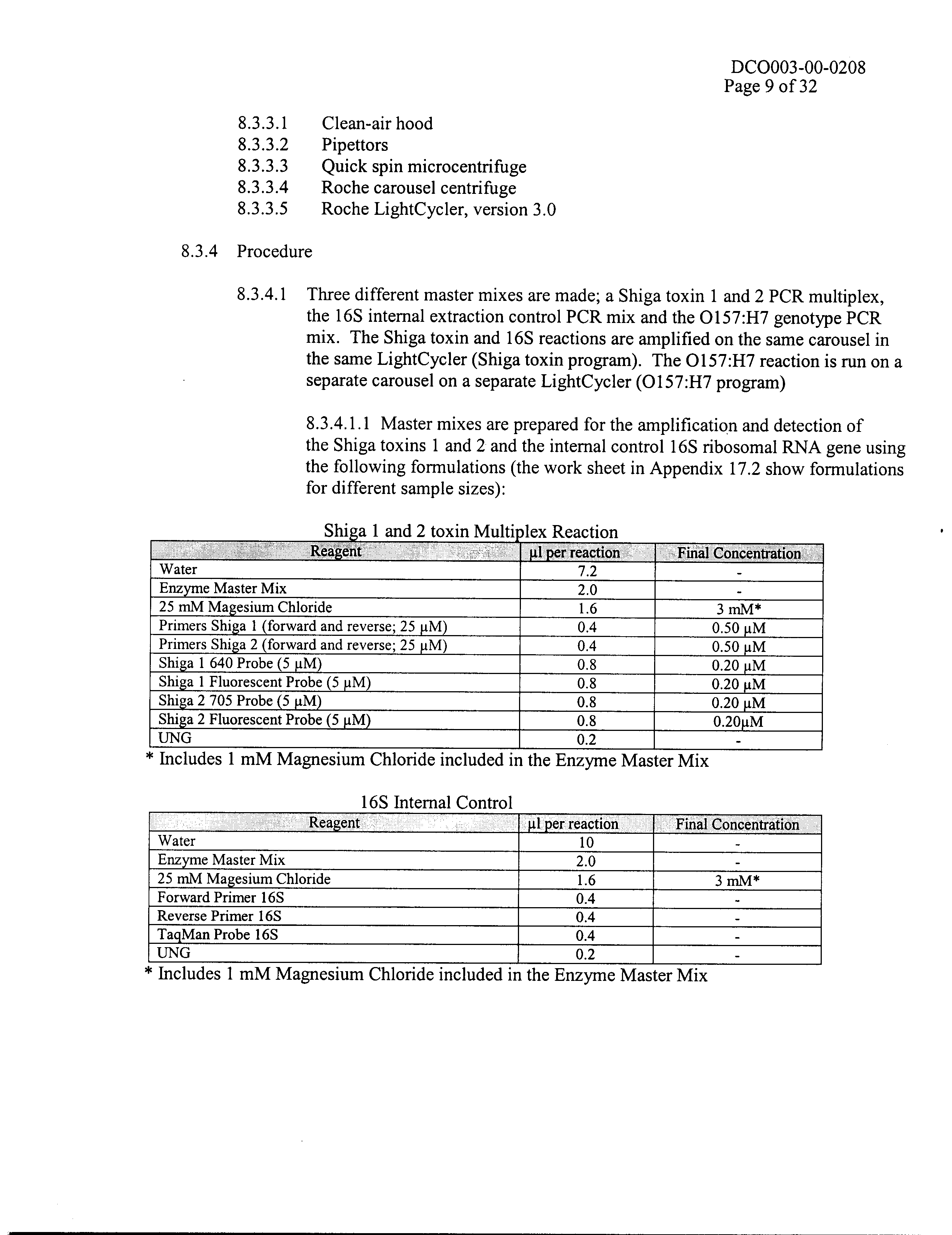
- 2.2.2. Performance Audits
- QAPP_1_Water.pdf
- QAPP 2 FINAL.pdf
- QAPP_2_APPENDICES 1-19.pdf
- QAPP 3 Jul 2008 FINAL.pdf
- Statistical Analyses.pdf
- References
|
BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
IN THE MATTER OF:
)
)
WATER QUALITY STANDARDS AND
)
EFFLUENT LIMITATIONS FOR THE
)
R08-9
CHICAGO AREA WATERWAY SYSTEM
)
(Rulemaking - Water)
AND THE LOWER DES PLAINES RIVER:
)
PROPOSED AMENDMENTS TO 35 Ill.
)
Adm. Code Parts 301, 302, 303 and 304
)
PRE-FILED TESTIMONY OF SAMUEL DOREVITCH
My name is Samuel Dorevitch and I am an environmental health researcher at the
University of Illinois at Chicago School of Public Health. I am a medical doctor, with training
and board certification in Emergency Medicine and also in Preventive Medicine, with
specialization in Occupational Medicine. Over the last six years, I’ve conducted research on
local environmental health issues, such as the effects of public housing demolition and the
reconstruction of the Dan Ryan expressway on air quality. In addition to being a scientist, I have
been an advocate for reducing pollution and improving the environment. Over the years, I have
testified at U.S. EPA hearings in favor of setting more stringent regulatory standards for ozone,
particulate matter air pollution, and off-road diesel emissions. I have also spoken out in the
media about the impact of coal-fired power plants on local air quality. I have added my name to
the National Resources Defense Council’s list of those opposed to the U.S. EPA’s recent effort
to stop regulating lead as an air pollutant.
I have advocated for tighter regulations in the above contexts because there is an
overwhelming body of public health research that demonstrates negative consequences of air
pollution. For ozone, particulate matter, lead and other air pollutants, a solid scientific
foundation exists for setting a regulatory standard. Just as I support improvements in air quality
as a means of promoting public health, I recognize the critical role that improvements in drinking
water quality have played in promoting the health of the public. The scientific basis for
improving air quality and drinking water quality are well-established, strong, and based on
thousands of scientific studies. However, in the case of water recreation, and limited contact
recreation in particular, we are just beginning to develop the scientific data that will help define
what regulatory measures are appropriate for protecting the health of public.
In contrast with the thousands of scientific papers that have addressed the health effects
of air pollution, less than 20 observational epidemiologic studies of primary contact recreation in
the US have been published. For limited contact recreation, no studies have been done in the
US, less than 5 have been done in Europe, and those looked primarily at whitewater canoeing, an
activity that does not take place on the Chicago Area Waterway System, or CAWS. No research
has ever characterized the health risks of activities observed on the CAWS, namely boating,
paddling, rowing and fishing. We do not know if people who engage in limited contact
recreational activities develop illnesses, such as gastroenteritis or eye infections or skin
infections or respiratory problems at higher risk than the general population.
Because the scientific literature does not provide guidance for establishing health-based
regulations for CAWS recreation, one would want to know the following in developing efforts to
improve water quality on the CAWS:
•
Are rates of illness higher among CAWS recreators compared to recreators doing the
same activities on waters that do not receive treated wastewater?
•
If so, how frequently do such cases of illnesses occur above background rates?
•
Are the pathogens responsible for illness bacteria, viruses or parasites, which may require
different water quality treatment strategies?
2
•
Are people who engage in specific recreational activities at increased risk while those
who engage in other activities are not?
•
Are there differences in risk on different CAWS reaches?
•
How does the contribution of water reclamation plants to microbial measures of water
quality compare to the contributions of runoff and sewer overflows?
•
If the Pollution Control Board were to establish a disinfection requirement rather than a
microbial water quality standard, how would risk to the public be determined along
various CAWS reaches?
•
Following rainfall and other events that are unrelated to wastewater treatment, what
microbes should be measured in the water to evaluate and communicate risk to the
public?
•
If the Pollution Control Board were to establish a water quality standard, rather than a
disinfection requirement, is there a microbial water quality level above which risk is
unacceptable and below which risk is acceptable?
If there were known outbreaks of disease linked to CAWS recreation, I would suggest
public health action now, rather than research. However, I am not aware of epidemics attributed
to CAWS recreation. Since 1978, the U.S. Centers for Disease Control and Prevention has
monitored disease outbreaks linked to water recreation. Using “WBDOSS,” the Waterborne
Disease Outbreak Surveillance System, the CDC compiles information about outbreaks due to
treated and untreated recreational waters. Hundreds of outbreaks and thousands of cases of
illness have been identified, described, and in varying degrees, investigated over the years.
Outbreaks from Illinois – including a recent outbreak of
Cryptosporidiosis
in Tazewell County –
have been reported. To the best of my knowledge, local health departments, the Illinois
3
Department of Public Health, and the CDC have not identified outbreaks of disease attributed to
CAWS recreation.
This does not mean that people haven’t gotten sick after using the CAWS. It is possible
that such cases fly beneath the radar of the public health monitoring system. That is why it is
important to identify such cases, to determine the microbes responsible for illness, to evaluate the
locations where water contact took place, to characterize the water quality at that location, and to
estimate the frequency with which such illness occurs. The fact that outbreaks linked to CAWS
recreation have not been identified does suggest that we have the opportunity to define the scope
and specifics of the problem before developing a potential solution. This lack of known
outbreaks of disease is consistent with the finding of the recent quantitative microbial risk
assessment. That study used hundreds of measurements of water quality on the CAWS and
estimated that rates of illness among limited contact recreators are about 1-2 cases per 1,000
uses.
Although risk assessment can be very useful in comparing various risk scenarios, such
analyses do not involve direct measurement of risk in populations. That type of research – the
study of the distribution and determinants of states of health and disease in population – is
epidemiology. Because epidemiologic studies involve the direct measurement, rather than the
statistical modeling of risk, they are of great importance in developing plans to protect the health
of the public. I am directing the epidemiologic study of CAWS recreation known as CHEERS,
which stands for the Chicago Health, Environmental Exposure, and Recreation Study. This is
the first epidemiologic study of the health risks of fishing, boating, rowing and paddling. This
research uses the gold standard of observational epidemiologic studies, the prospective cohort
design, and has been developed by a multi-disciplinary team of experienced researchers, with
4
backgrounds in infectious disease medicine, environmental medicine, epidemiology,
biostatistics, industrial hygiene and environmental science. A panel of recognized leaders in the
fields of water microbiology and health from the U.S. Centers for Disease Control and
Prevention, the U.S. Environmental Protection Agency, and other universities has reviewed and
endorsed the design and protocols of the research, and continues to monitor the quality of data
collected. A copy of the review panel’s endorsement has been submitted by Mr. Daniel
Woltering of the Water Environment Research Foundation and is Public Comment Number 63 in
the docket for this rulemaking.
I would like to give you a broad brushstroke view of the CHEERS research. A copy of
the epidemiologic study protocol has been submitted as an attachment to my written testimony
for anyone who wishes to see the details of this research. We recruit people into one of three
study groups. The CAWS Group is composed of people who row, paddle, fish or go boating on
the CAWS. The General Use Waters Group consists of people who do these same activities on a
number of area lakes, rivers and lagoons not including the CAWS. The Unexposed Group
includes people who do outdoor activities that do not involve water (such as jogging or biking) at
about the same time and about the same place as the recruitment of participants into the other
two groups. Individuals in all three groups undergo interviews on the day of recreation, and
then are contacted for three telephone interviews over the following three weeks. All interviews
are conducted using computer assisted methods, which ensure that participants are asked the
same questions in a neutral fashion. Field interviews address current health, and for those who
engage in water recreation, the extent of their contact with the water. Telephone interviews
address changes in health status and additional water exposure since recruitment. While the
participants are on the water, samples of water are collected and sent for analyses of bacteria,
5
viruses and parasites. If a participant develops illness, clinical specimens are collected so that
the pathogen responsible for illness may be identified. The study uses state-of-the-art methods,
which in several respects, surpass the U.S. EPA’s ongoing research about primary contact
recreation known as the National Epidemiological and Environmental Assessment of
Recreational Water (NEEAR) study.
Additionally, a module of CHEERS known as the exposure study seeks to answer
important questions regarding water contact among recreators. Rowers, paddlers, boaters and
fishers may be exposed to water microbes via several routes: ingestion, inhalation, and skin
contact. Ingestion may result from getting water on ones hands and then touching ones mouth, it
could result from a splash to the mouth, or it could occur in the unlikely event of capsizing or
falling into the water. The exposure study will allow us to describe for the first time how much
water exposure occurs by each route for specific recreational activities. These results may be
useful in establishing whether some activities pose lower levels of risk (due to lower exposure)
than others. We will also have the opportunity to evaluate the assumptions of risk assessments
regarding exposure, dose, and risk. Preliminary analyses of 2007 data show that assumptions
regarding the duration of various recreational activities were quite accurate. The conduct of an
epidemiologic and a risk assessment in tandem is unusual and this opportunity to evaluate the
strengths and limitations of risk assessment methods is one reason that there is considerable
national interest in applying the final results of this research to the development of water quality
regulation.
Epidemiologic studies provide an opportunity to directly measure, rather than model,
risk. For this reason the U.S. EPA places considerable weight on epidemiologic studies when
establishing environmental standards. A well-designed epidemiologic study seeks to minimize
6
the possibility that the research will fail to identify a real risk that may exist (a “false negative
result”) and to minimize the possibility that a risk will be identified when none exists (a “false
positive result”). Early in the development of CHEERS, the research team evaluated numerous
approaches for minimizing the possibility of a false positive or a false negative result. In
calculating our necessary number of study participants, we used typical values of a 1 in 20
chance of a false positive result and a 1 in 5 chance of a false negative result. We made
numerous conservative assumptions in that sample size calculation, and it is becoming apparent
that we will have more statistical power than originally anticipated because the rate of drop out
by study participants is less than a third of the 15% we had projected. Thus, the chances of
failing to identify a real risk are likely less than one in five.
We calculated that a total of 9,330 people should be enrolled in the three recreational
categories (i.e. approximately 3,110 people per recreational category as described above). Last
summer and fall – the first year of the study – over the first 800 participants signed up for the
study. CHEERS has been scaled up substantially this summer, and for the months of May, June
and July, an average of more than 1,000 participants were enrolled per month. A breakdown of
recruitment by group, by month is included as an appendix to this testimony. By the date of this
hearing, we project that 5,500 participants will have been enrolled in CHEERS. We collected
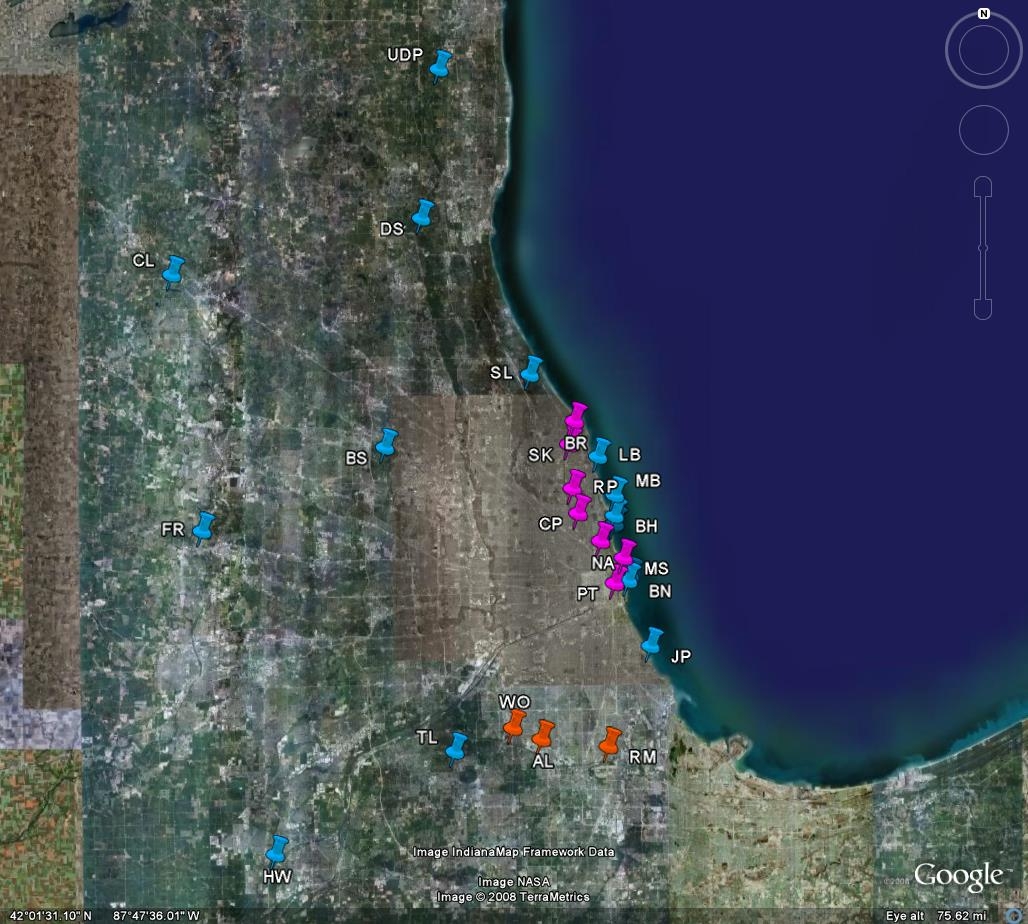
data about use of the CAWS, for specific activities at specific locations. A summary of the
findings of CAWS recreational use survey in 2007 has been submitted as an appendix to this
testimony. Highlights of that summary include the observation that the dominant uses on the
North Branch and North Shore Channel are rowing and paddling while the dominant use on the
Cal-Sag Channel is motor boating. Fishing from shore is relatively uncommon, and jet skiing is
rarer still. Swimming and water skiing were never observed. Data obtained from field
7
interviews of study participants demonstrates that several dozen individuals on rowing team each
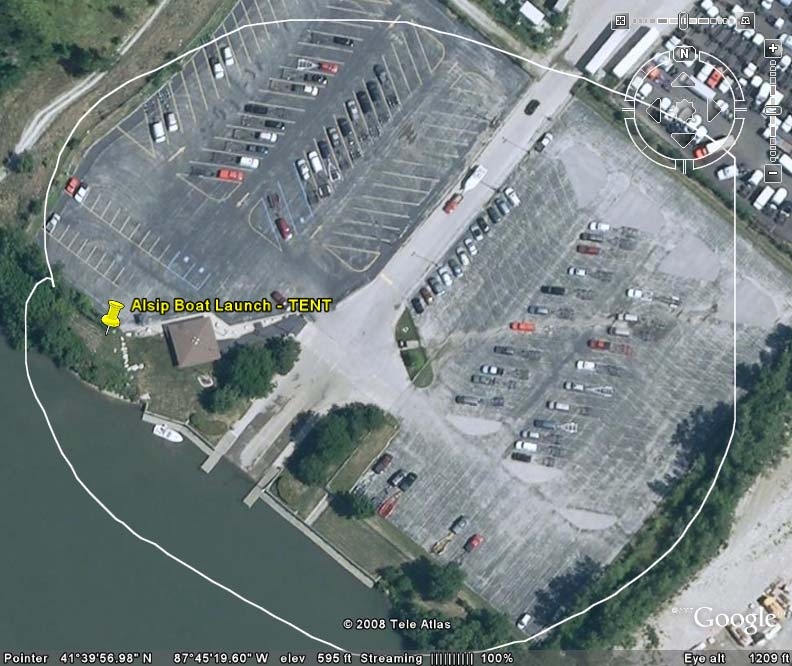
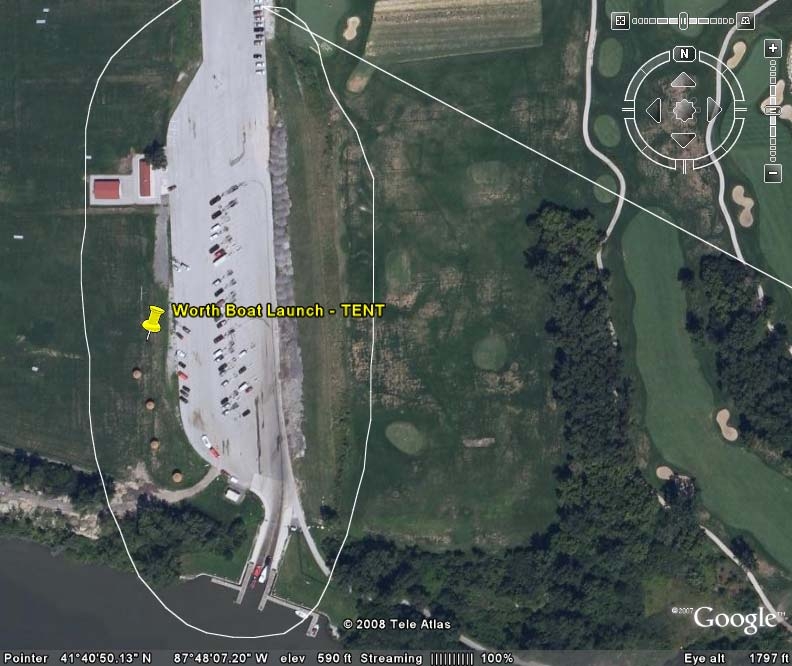
use the CAWS more 100 times per year. Similarly, some boaters at the Worth and Alsip
launches use the Cal-Sag Channel dozens of times per season. Thus, a small number of users
account for a large proportion of uses. These observations add detail to the picture sketched out
by the assessment of current uses reported in the UAA. Inconsistencies between our
observations and those of the UAA regarding the frequency of specific recreational activities and
the distinction between uses and users are likely due to difference in methodologies.
Over 5,000 water samples have been analyzed and more than 150 stool samples have
been obtained for analysis by the UIC laboratory and the Illinois Department of Public Health.
We are well on our way to completing data collection and moving on to data analyses. The
results of those analyses will provide answers to the critical questions about risk, the
determinants of risk, exposure, sources of microbes, and causes of illness. The final report will
serve as the basis for establishing standards to protect limited contact uses. Preliminary analysis
of the 2007 data identifies no difference in rates of gastrointestinal symptoms among recreators
in the three study groups. Because that analysis involved less than 10% of the total number of
participants who will have been enrolled at the completion of this research, firm conclusions are
premature. However, consistent with the lack of reports by public health departments of
outbreaks of disease linked to CAWS recreation, our preliminary observations suggest no danger
to the health of the population of limited contact recreators on the CAWS.
I favor strong, science-based environmental regulation as a means of protecting public
health. Reducing the potential risks of limited contact recreation on the CAWS is an important
and complex public health goal. From a policy perspective, one would want to know what the
benefits and risks are of current wastewater management and recreation practices, and what the
8
Testimony Attachments
1. Curriculum vitae
2. Recruitment by month, by group
3. Use survey summary
4. CHEERS Protocol (Quality Assurance Project Plan documents)
10
CURRICULUM VITAE
Samuel Dorevitch, M.D., M.P.H.
University of Illinois at Chicago - School of Public Health
Division of Epidemiology and Biostatistics
Division of Environmental and Occupational Health Science
1603 W. Taylor, M/C 923
(312) 355-3629
sdorevit@uic.edu
Education and Training
July 1999-June 2001 Resident, Occupational Medicine, UIC Medical Center
Aug 1999-May 2001 Environmental and Occupational Health Sciences, UIC School of
Public Health; Degree awarded: Masters in Public Health
July 1990-June 1993 Resident, Emergency Medicine, Cook County Hospital
July 1989-June 1990 Intern, Internal Medicine, Northwestern University-Evanston Hospital
Sept 1985-June 1989 University of Chicago, Pritzer School of Medicine;
Degree Awarded: MD
Sept 1981-June 1985 University of Illinois at Chicago
Degrees awarded: B.S. with honors, Biological Sciences, B.A.,
Psychology, with honors
2
Employment
2001-present
Assistant Professor, Research, Division of Environmental and
Occupational Health Sciences, University of Illinois at Chicago
Assistant Professor, Research, Division of Epidemiology and
Biostatistics, School of Public Health, University of Illinois at Chicago
Faculty Occupational Medicine Residency Program
2007-present
Clinical Physician, Department of Emergency Medicine, UIC O’Hare
Clinic, University of Illinois at Chicago Medical Center
1995-present
Staff physician, Emergency Medicine and Occupational Health Services,
Lake Forest Hospital, Lake Forest, IL
1993-2002
Clinical Instructor, Emergency Medicine, Cook County Hospital,
Chicago, Illinois
1993-1995
Emergency Medicine and Occupational Medicine, Westlake Community
Hospital, Melrose Park, Illinois
Academic Honors
Edmund C. James Scholar, University of Illinois, Chicago, 1983-1985
Phi Beta Kappa
Chief Resident, Emergency Medicine, Cook County Hospital, Chicago, IL, 1992-1993
NIOSH Trainee in Occupational and Environmental Medicine, 1999-2001
Central States Occupational Medicine Association, Scientific Session 2000. Award for
presentation by a resident.
Publications
(*refereed journal)
3
1. Westfall MD, Price KR, Lambert M, Himmelman R, Kacey D, DOREVITCH S,
Mathews J. "Intravenous access in the critically injured trauma patient: a multicentered,
prospective randomized trial of saphenous cutdown and percutaneous femoral access."
Annals of Emergency Medicine, 23:541-545, 1994.*
2. DOREVITCH S, Forst L. "Occupational hazards of emergency physicians" American
Journal of Emergency Medicine, 18:300-311, 2000*
3. DOREVITCH S, Marder D. "Occupational hazards of municipal solid waste workers".
State of the Art Reviews in Occupational Medicine, 16:125-133, 2001.
4. DOREVITCH S, Babbin A. "Ceramics: Hazards, health effects, and prevention". State
of the Art Reviews in Occupational Medicine, Oct-Dec;16(4):563-75.2001.
5. Geller RJ, DOREVITCH S, Gummin DD. "Air and Water Pollution" in Toxicology
Secrets, Ling LJ, Clark RF, Erickson TB and Trestrail JH (Eds). Hanley & Belfus,
Philadelphia, PA. 2001.
6. DOREVITCH S, Forst L, Conroy L, Levy P. "Toxic inhalation fatalities of US
construction workers, 1990-1999." Journal of Occupational and Environmental
Medicine, 44(7):657-62, 2002.*
7. Krantz A, DOREVITCH S. “Metal Exposures and Common Chronic Diseases: A
Guide for the Clinician”.
Disease-a-Month,50(5):220-62, 2004.
8. DOREVITCH S, Demirtas H, Persky VW, Erdal S, Conroy L, Schoonover T, Scheff P:
“Demolition of high-rise public housing increases particulate matter air pollution in
communities of high-risk asthmatics.” J Air Waste Management Assoc. 2006
Jul;56(7):1022-32.*
4
9. Martinez O,Gangi E, Mordi D, Gupta S, DOREVITCH S, Lefranc MP, Prabhakar BS:
Diversity in the complementarity determining region 3 (CDR3) of antibodies from mice
with evolving anti-TSHR antibody responses.” Endocrinology, Endocrinology. 2007
Feb;148(2):752-61.*
10. DOREVITCH S, Demirtas H, Scheff P, Persky VW: “Bias and confounding in
longitudinal measures of exhaled monoxides.” Journal of Exposure Science and
Environmental Epidemiology, Sep;17(6):583-90 *
11. DOREVITCH S, Tharenos L, Demirtas H, Persky VW, Artwohl J, Fortman J: “Inverse
association between rural environment in infancy and sensitization to rodents in
adulthood.” Annals of Allergy Asthma and Immunology. 2007 May;98(5):440-6.*
12. Patel M, DOREVITCH S, Williamson R, Buchanan S: Pilot study investigating the
effect of the static magnetic field from a 9.4 Tesla MRI on the vestibular system.
Journal of Occupational and Environmental Medicine. 2008 May 50(5):576-583.*
13. DOREVITCH S, Karandikar A, Washing GF, Walton GP, Anderson R, Nickels S:
Efficacy of an air pollution education program in a community at risk for asthma
morbidity. In press, J Asthma.
14. Wei H, Turyk M, Cali S, DOREVITCH S, Erdal S, Li A: Polybrominated Diphenyl
Ethers in Dust: Particle size fractionation, evidence of debromination and human
exposure. In revision, Environmental Science and Technology.
Research Funding
5
Principal Investigator: NIOSH Pilot Project Research Training Award “Immunologic Risk
Factors for Laboratory Animal Allergy,” 2001-2002. Total award $15,844.
Principal Investigator: NIOSH Pilot Project Research Training Award “Immunologic Risk
Factors for Laboratory Animal Allergy.” Renewed, 2002-2003
PI: NIEHS, NIH Research Career Award ES-K08ES011302 “Asthma and Demolition in
Chicago Public Housing” Total award $641,527 2002-2007.
Co-investigator: Laboratory Animal Allergen Production and Transport in a Working
Animal Research Facility, PI: J. Artwohl,
Co-investigator: NIOSH/CDC, ERC Training Grant, T42/CCT522954-01, 07/01/03-
06/30/08, $5,552,668. (Conroy PI)
Co-investigator (UIC PI): Grand Boulevard Federation: Reducing air pollution impacts of
Dan Ryan Expressway Reconstruction. USEPA NE96586801. Total award, $49,515.
Principal Investigator: Asthma, Obesity and Airway Oxidative Stress. American Lung
Association/Respiratory Health Association of Metropolitan Chicago. $80,000. Funding
period July 2007-June 2009.
Principal Investigator: Epidemiologic Study of Chicago Area Waterways. Metropolitan
Water Reclamation District of Greater Chicago. Funding period May 2007-December
2009.
Teaching
Course director, Occupational Medicine Weekly Conference
Lecturer, Environmental and Occupational Health Sciences Course, “Air Quality”
6
Presentations at National Meetings
2003 American Public Health Association Annual Meeting, San Francisco, CA. Housing
Demolition and Air Pollution: Working with a local public housing environmental task
force to minimize exposure. Poster Presentation.
May, 2005, American Thoracic Society International Conference, San Diego, CA.
Particulate matter exposure adjacent to demolition of public housing.
May, 2006, American Thoracic Society International Conference, San Diego, CA.
Elemental and organic carbon in PM2.5 are associated with exhaled nitric oxide and
exhaled carbon monoxide in inner-city asthmatics
May, 2006, American Thoracic Society International Conference, San Diego, CA. Exhaled
carbon monoxide in inner-city asthmatics is associated with ambient ozone concentrations
two days earlier.
November, 2006, American Public Health Association, Boston, MA. Science, Politics and
Air Quality Policy.
May, 2007, American Thoracic Society International Conference, San Diego, CA
Elemental Carbon and Organic Carbon in PM2.5 Are Associated with Exhaled Nitric
Oxide and Exhaled Carbon Monoxide in Inner-City Asthmatics
May, 2007, American Thoracic Society International Conference, San Diego, CA Exhaled
Carbon Monoxide in Inner-City Asthmatics Increases 1-2 Days after Ambient Ozone
Exposure
7
Presentation at State and Local Meetings
May, 2004, Illinois Public Health Association “Beat Asthma in Illinois”, Springfield, IL.
Community health educators as key personnel in inner city asthma research.
November, 2006, Chicago Asthma Consortium Data Conference, Chicago, IL. Air
pollution and lung inflammation among public housing residents.
February, 2007, UIC Medical Center Pulmonary Medicine, Air Pollution and Health: Epidemiologic
Methods
Other
Reviewer,
International Journal of Occupational and Environmental Health
, 2000
Reviewer,
Journal of Occupational and Environmental Medicine
, 2002
Reviewer,
American Journal of Public Health
, 2004
Reviewer,
Archives of Occupational and Environmental Health
, 2006-7.
Scientist Reviewer, CDC/ASTPM/ASPH/AAMC Special Emphasis Panel, Atlanta,
Georgia June 7-8, 2004
Testimony at public hearings: OSHA proposed ergonomics standard hearings, 2000.
Testimony at public hearings: US EPA proposed offroad diesel hearings, June, 2004.
Testimony at public hearings: US EPA proposed PM2.5 standard, March, 2006
Testimony at public hearing: US EPA proposed ozone standard, September, 2007
Member, Illinois Department of Transportation “Dan Ryan Health and Environmental
Focus Group”, 2004-present.
Testimony before Chicago City Council Transportation Committee regarding the Dan Ryan
Expressway reconstruction, May 15, 2006.
Invited member, Governor’s Blagojevich’s Illinois Climate Change Advisory Group,
January-October, 2007.
8
Licensure and Certification
Medical license, State of Illinois, 1990-present
Board Certified, Emergency Medicine, 1994-present
Board Certified, Preventive Medicine/Occupational Medicine, 2002-present
Instructor, Advanced Cardiac Life Support, 1994-2006
CHEERS
monthly
enrollment
of
4,402
participants,
by
group,
through
July,
2008
0
200
400
600
800
1000
1200
1400
Aug
07
Sep
07
Oct
07
Nov
07
Mar
08
Apr
08
May
08
Jun
08
Jul
08
GUW
Unexposed
CAWS
This figure displays monthly recruitment of participants, by group, by month who
complete the second field interview. CAWS: Chicago Area Waterways System group;
GUW: General Use Waters group.
U N I V E R S I T Y O F I L L I N O I S
A T C H I C A G O
Division of Environmental and Occupational Health Sciences (MC 922)
School of Public Health
2121 West Taylor Street
Chicago, IL 60612
Phone (312) 996-8855 . Fax (312) 413-9898
Dr. Tom Granato
Assistant Director, Research and Development Department
Metropolitan Water Reclamation District of Great Chicago
6001 W. Pershing Road
Stickney, IL
Dear Dr. Granato,
An element of the Epidemiologic Study of Recreational Use of the Chicago Area
Waterways, also known as CHEERS, has been a characterization of usage of the waterway. This
letter reports our work, to date.
Methods of Characterizing Water Usage
Over the course of the 2007 data collection season, UIC staff has recorded rates of recreational
use of the waterways at various locations. The methodology for the use survey involved
counting the number of new users on given day, at a given location, for a specific activity. Thus,
a boat carrying three people would be counted as three users rather than one event. An
individual who boated and then fished would be counted twice, once for each recreational
activity. In order to prevent counting the same user twice for the same activity on a given day,
and to estimate the number of new users per unit of time, we did not count people passing by a
launch point. Thus, users who were observed passing or exiting a launch point were not
counted, to ensure that individuals were not counted both at launch and again while they were
engaged in (or finishing) their water recreational activity.
Results of Observations
Data were collected on a total of twenty-two days of observation. Generally, study
personnel conducted the usage survey at one to two locations per day. This generally occurred
on days and at times that participants were being recruited into the CHEERS study. The average
duration of observation per site per day was 4.7 hours. This data includes participants at the
Chicago River Flatwater Classic, which were recorded as using the waterways at Clark Park.
Table 1 summarizes the amount of time spent collecting use data, by site.
Location
Days of
observation
Total
hours of
obs.
Avg.
hrs/day
of obs.
Avg.
start
time
Avg.
end
time
Alsip
3
15.5
5.2
8:00 AM
1:00 PM
Worth
3
19
6.3
7:00 AM
1:00 PM
North
3
13
4.3
1:00 PM
5:00 PM
Clark
4
18
4.5
9:00 AM
1:30 PM
River Park
2
10
5.0
7:30 AM
12:30 PM
Skokie Rowing Ctr.
7
28
4.0
1:00 PM
5:00 PM
TOTAL
22
103.5
4.7
Table 1: Summary of amount of time spent collecting use data, by site
Tables 2 and 3 present usage by category, by location, and Table 4 summarizes overall usage by
location. Figure 1 presents the overall distribution of recreational users.
Boaters
Canoeists
Rowers
Kayakers
Location
Total
(per hour) Total (per hour) Total
(per hour)
Total
(per hour)
Alsip
215
(13.9)
0
(0.0)
0
(0.0)
0
(0.0)
Worth
108
(5.7)
0
(0.0)
1
(0.1)
0
(0.0)
North
6
(0.5)
26
(2.0)
86
(6.6)
0
(0.0)
Clark
0
(0.0)
428
(23.8)
0
(0.0)
222
(12.3)
River Park
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
Skokie Row. Ctr.
35
(1.3)
7
(0.3)
520
(18.6)
25
(0.9)
TOTAL
364
(3.5)
461
(4.5)
607
(5.9)
247
(2.4)
Table 2: Observed uses and hourly usage rates for boaters, rowers and paddlers, by category and
location. Clark Park data includes the Flatwater Classic.
Fishers
Waders
Jet skiers
Water skiers
Swimmers
Location
Total
(per
hour)
Total
(per
hour)
Total
(per
hour)
Total
(per
hour)
Total
(per
hour)
Alsip
0
(0.0)
0
(0.0)
2
(0.1)
0
(0.0)
0
(0.0)
Worth
2
(0.1)
0
(0.0)
2
(0.1)
0
(0.0)
0
(0.0)
North
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
Clark
2
(0.1)
6
(0.3)
0
(0.0)
0
(0.0)
0
(0.0)
River Park
7
(0.7)
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
Skokie Row. Ctr.
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
0
(0.0)
TOTAL
11
(0.1)
6
(0.1)
4
(0.0)
0
(0.0)
0
(0.0)
Table 3: Observed uses and hourly usage rates, for fishers, waders, jet skiers, water skiers and
swimmers.
All uses
Location
Total
(per hour)
Alsip
217
(14.0)
Worth
113
(5.9)
North
118
(9.1)
Clark
658
(36.6)
River Park
7
(0.7)
Skokie Rowing Center
587
(21.0)
TOTAL
1700
(16.4)
Table 4: Observed uses and hourly usage rate, all recreational categories
z
Page 2
Figure 1: Overall use, by recreational category
In addition to the uses recorded on the twenty-two days that the use survey was conducted,
study personnel surveyed numerous other potential access points in two ways. One was by land-
based site visits. The other was on a survey of the North Branch of the Chicago River and lower
North Shore Channel by boat. Paddling, rowing and boating were observed on these surveys;
however, swimming, jet skiing, and water skiing were not.
Interpretation of Data
Several factors should be taken into consideration when interpreting the above data. First, the
dates and times of observation were not randomly selected. Surveys were generally conducted
when usage was expected to be relatively heavy--on weekends, when rowing teams and clubs
used the waterways, and during a major rowing/paddling event. Thus, multiplying hourly usage
rates by the number of hours in a recreation season would grossly overestimate actual usage.
Second, recreational users of the waterways were counted regardless of their eligibility to enroll
in CHEERS. For example, rowers who would enroll in the CHEERS study on Monday would
not be eligible to enroll on Tuesday (there is a 21 day period of follow-up during which current
participants are not eligible to re-enroll). Nevertheless, they would have been counted on both
days of the use survey. Members of rowing teams typically practice or compete 4-7 days per
week on the waterways for as many as 8 months per year. Thus, the number of uses greatly
exceeds the number of users and the number of users eligible to enroll in CHEERS.
The data summarized in the above tables contrasts with data summarized in the Use
Attainability Analysis (UAA), conducted for the Illinois Environmental Protection Agency.
Notably, fishing accounted for 73% of the activity observed on the North Shore Channel, 25% of
the activity on the North Branch of the Chicago River, 34% of the activity on the Cal-Sag
Channel, and 64% of the activity of the Little Calumet River. By contrast, fishing accounted for
less than 1% of all activity noted in our use survey. Power boating accounted for 32% of activity
on the North Branch in the UAA while it is observed infrequently in our study at North Brach
locations. Differences in the findings of the two studies are likely due to differences in
z
Page 3
Table of Contents
Organization of this protocol
1
Background
2
1. Chicago Area Waterways
2
2. Water Quality Regulation
2
3. Scientific Background
7
4. Limitations
10
5. Epidemiologic Study
11
Study Objectives
13
Field study overview and design considerations
14
1. Field study summary
14
2. Approach to choice of study methods
14
3. Rationale for prospective cohort design
15
4. Study groups
16
5. Estimating background rates for sample size calculations
17
6. Study name
18
7. Participant recruitment strategies
18
8. Study enrollment and locations
23
9. Survey data
30
10. Clinical microbiology
32
11. Human research subject protections
33
12. Overview of water sampling
35
13. Field team organization
37
14. Project management
38
15. Communications plan
44
References
45
List of Tables
iii
List of Figures
iii
List of Appendices
iv
List of Acronyms
iv
CHEERS Overview
ii
July 2008
List of Tables
Table
Description
Page
Table 1
Terms used to describe water recreational activities based
on degree of contact
5
Table 2
Summary of 2007 recruitment efforts, projected 2008
recruitment efforts
29
Table 3
Study participant recruitment achieved during 2007,
targets for 2008
30
Table 4
Pathogens to be detected in stool samples
33
Table 5
Primary purposes of water sampling, by location
36
Table 6
Locations for water sampling on the CAWS other than
recruitment sites
36
Table 7
Water sampling locations, by site of participation
enrollment
37
List of Figures
Figure
Description
Page
Figure 1
Chicago Area Waterways (CAWS) map
6
Figure 2
Overview of study components
15
Figure 3
Recruitment locations, 2007 and 2008 seasons
25
Figure 4
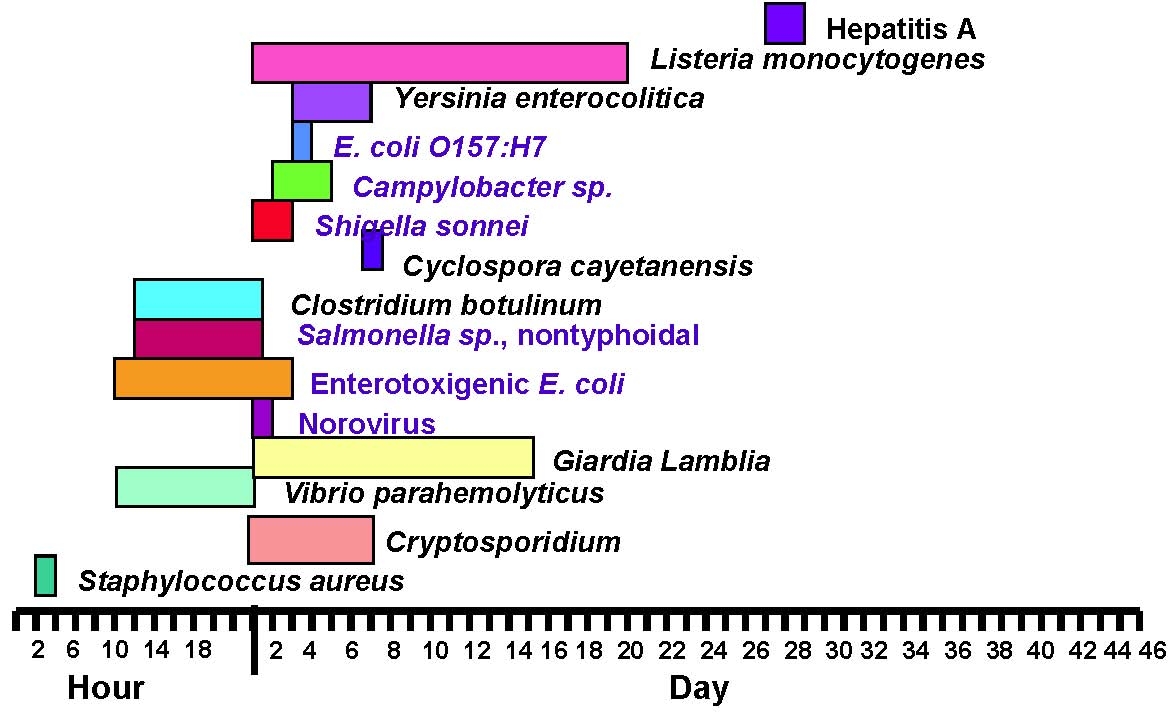
Usual Incubation Period ranges for selected etiologic agents
34
Figure 5
Shedding period and optimal collection period for select agents
34
Figure 6
Project management
43
CHEERS Overview
iii
July 2008
List of Appendices
Appendix
Description
Overview 1
CHEERS Logo
Overview 2
Publicity Flyer
Overview 3
Information for clubs, teams organizers and vendors
Overview 4
Water Quality FAQ Sheet
Overview 5
Recruitment sites – CAWS
Overview 6
Recruitment sties – GUW
Overview 7
2007 recruitment schedule
Overview 8
2008 recruitment schedule
List of Acronyms
AGI
Acute Gastrointestinal Illness
A-SPM
Assistant Survey Project Manager
CAI
Computer Assisted Interview
CAPI
Computer Assisted Personal Interviewing
CATI
Computer Assisted Telephone Interviewing
CAWS
Chicago Area Waterways System
CDC
Centers for Disease Control and Prevention
CFC
Continuous Flow Centrifugation
CFU
Colony Forming Units
CHEERS
Chicago Health, Environmental Exposure, and Recreation Study
COC
Chain of Custody
CPM
Clinical Project Manager
CSOs
Combined Sewer Overflows
DQO
Data Quality Objective
EPA
Environmental Protection Agency
FCR
Friends of the Chicago River
FDS
Field Data Sheets
GUW
General Use Waters
IDPH
Illinois Department of Public Health
CHEERS Overview
iv
July 2008
IEPA
Illinois Environmental Protection Agency
IPCB
Illinois Pollution Control Board
IPR
Initial Precision and Recovery
IRB
Institutional Review Board
MB
Method Blank
MS
Matrix Spike
MSD
Matrix Spike Duplicate
MWRDGC Metropolitan Water Reclamation District of Greater Chicago
NBCR
North Branch Chicago River
NEEAR
National Epidemiological and Environmental Assessment of
Recreational waters study
NGI
Non-gastrointestinal Illness
NSC
North Shore Channel
OPR
Ongoing Precision and Recovery
OSS
Office of Survey Systems
PFU
Plaque Forming Unit
QA
Quality assurance
QAPP
Quality Assurance Project Plan
QC
Quality Control
QMP
Quality Management Plan
QRC
Questionnaire Review Committee
RR
Relative Risk
SAS
Statistical Analysis Software
SMI
Scientific Methods Incorporated
SPH
School of Public Health
SPM
Survey Project Manager
SRL
Survey Research Laboratory
TNTC
Colonies Too Numerous to Count
UAA
Use attainability analysis
UIC
University of Illinois at Chicago
UIH
University of Illinois Hospital
USEPA
United States Environmental Protection Agency
WERF
Water Environment Research Foundation
WRP
Water Reclamation Plant
CHEERS Overview
v
July 2008
CHEERS Overview
1
July 2008
ORGANIZATION OF THIS PROTOCOL
The Chicago Health, Environmental Exposure, and Recreation Study, or “CHEERS,” is a multi-
year, multi-site, interdisciplinary epidemiologic study being conducted by a research team at the
University of Illinois at Chicago (UIC) for the Metropolitan Water Reclamation District of
Greater Chicago (MWRDGC).
When initially proposed, this study was called the
“Epidemiologic Study of Recreational Use of the Chicago Area Waterways.” It consists of three
distinct but interrelated data-collecting activities, which are organized as separate projects within
the larger study. Each project has its own Quality Assurance Project Plan (QAPP). The study
protocol is organized into six major sections:
•
Study Overview (this document)
•
Organization-wide Quality Management Plan (QMP)
•
QAPP #1: Water Sampling and Analysis
•
QAPP #2: Survey Methods
•
QAPP #3: Clinical microbiology
•
Statistical Analyses
The Study Overview and Organization-wide QMP are referred to throughout the individual
QAPPs and should be read first. Key analyses will involve data generated by two or more
individual projects within the larger study, therefore, the approach to statistical analyses are
presented in a separate document. Numbering of pages, tables, figures, and appendices is
specific to each of the six documents (in other words, numbering begins at 1 for each of these
major components of the overall study protocol).
CHEERS Overview
2
July 2008
BACKGROUND
1. The Chicago Area Waterways System (CAWS)
The Chicago Area Waterways System (CAWS) is a 78-mile-long, primarily man-made series of
channels and rivers. It is partly natural but irreversibly modified. The CAWS includes the North
Shore Channel, the North and South Branches of the Chicago River, the Chicago River, the
South Fork of the Chicago River (Bubbly Creek), the Chicago Sanitary and Ship Canal, the
Calumet-Sag Channel, the Calumet River, the Little Calumet River, the Grand Calumet River,
and Lake Calumet (Figure 1). The primary purpose of the system is to provide an outlet for urban
drainage in order to protect Lake Michigan, the source of drinking water for Chicago and many
nearby communities. Other purposes include transportation, commerce, and recreation. The
waterways also provide aquatic wildlife habitat. Four water reclamation plants (WRPs) of the
Metropolitan Water Reclamation District of Greater Chicago (MWRDGC) release secondary-
treated effluent (i.e., non-disinfected sewage) into the CAWS. It has been estimated that 70% of
the annual flow in the system is effluent from the WRPs (UAA). Storm runoff and combined
sewer overflows (CSOs) during and immediately after significant rainfall introduce water and
contaminants into the CAWS.
2. CAWS water quality regulation
2.1. Current CAWS use designations
The Illinois Pollution Control Board (IPCB) establishes use designations for bodies of water
in Illinois. Most of the CAWS is designated Secondary Contact and Indigenous Aquatic Life.
This designation allows recreational activities during which water contact is incidental or
accidental and for which the probability of ingesting appreciable quantities of water is
minimal, including canoeing, kayaking, and fishing, but not jet skiing or swimming. Three
relatively small portions of the system (the upper North Shore Channel, the Chicago River,
and Calumet River) are designated for general use. These use designation are not associated
with a microbial water quality standard.
2.2. Proposed changes to CAWS use designations
Because of water quality improvements in recent years, the Illinois Environmental Protection
Agency (IEPA) has recommended a use upgrade for parts of the CAWS that are now
designated Secondary Contact Recreation and Limited Aquatic Life. These improvements
CHEERS Overview
3
July 2008
stem from efforts by the State of Illinois to meet the goal of the Clean Water Act to make all
bodies of water “fishable and swimmable.” A change in use designation generally requires a
Use Attainability Analysis (UAA), thus, the IEPA had a UAA for the CAWS performed by a
contractor. The UAA included a review of current water quality, biodiversity, and uses of the
CAWS. After convening a stakeholder advisory committee and summarizing CAWS water
quality, current uses, and other data, the CAWS UAA recommended the creation of two
CAWS use designation subcategories, which differentiate recreational uses from aquatic life
uses.
Two recreational uses were proposed in draft form and posted on the UAA website in 2004
1
,
1) Recreational Navigation, which would apply to the Chicago Sanitary and Ship Canal, and
2) Limited Contact Recreation, which would apply to the other reaches of the CAWS that are
currently designated Secondary Contact and Indigenous Aquatic Life. Under the Limited
Contact Recreation use designation, canoeing, kayaking, fishing, jet skiing, and wading
would have been permitted. This designation would have applied from March 1 to November
30 and required the attainment of a water quality standard intended to limit excess illness to
10 cases per thousand contacts (a 30-day geometric mean of 1,030
E. coli
colony-forming
units (cfu) per 100 mL). The Recreational Navigation microbial standard would have
required the attainment of a standard meant to limit excess illness to 14 cases per thousand
contacts (a 30-day geometric mean of 2,740
E. coli
cfu per 100mL). Revisions to the Illinois
Pollution Control Board regulations were proposed in draft form on January 18, 2007. In that
document, the proposed recreational use designations were called “Incidental Contact
Recreation” and “Non-contact Recreation,” and had the same bacterial water quality
requirements, 1,030 and 2,740 geometric mean
E. coli
cfu per 100mL, as the “Limited
Contact Recreation” and “Recreational Navigation,” respectively. Ultimately, the IEPA
proposed one of three use designations for each segment, or reach, of the CAWS. These are
non-recreational use, non-contact recreation, and incidental contact recreation. Microbial
water quality standards to protect these use designations were not proposed; rather the IEPA
recommended the disinfection of effluent discharged into the reaches of the CAWS
designated for incidental contact and non-contact recreation.
A variety of terms have been used to categorize the degree of water contact expected to occur
during water recreation activities (Table 1). In order to simplify the terminology used in this
CHEERS Overview
4
July 2008
protocol, and to be consistent with other publications, we use the term “secondary,” rather
than “limited” or “incidental” contact recreation. For the purposes of this study, “secondary
contact recreation” is defined as any recreational water activity in which water contact is
limited to accidental or incidental contact, and precludes activities in which head immersion
is likely to occur. Secondary contact recreational activities include non-motorized boating
(paddling canoes or kayaks; rowing,) motor boating, and fishing (from a boat or from shore).
Because head immersion is expected in activities such as water skiing, jet skiing, and boogie
boarding, these activities are not considered part of our definition of secondary contact
recreation. Kayaking or canoeing on the low-flow waters of the CAWS are not expected to
result in head immersion. Again, the distinction between secondary contact recreational
activities and primary contact recreational activities such as swimming and water skiing is
that head immersion is not expected to not typically occur among secondary contact
recreators, while it is expect to occur among primary contact recreators.
CHEERS Overview
5
July 2008
Name
Source
Key elements of definition
Incidental contact
recreation
IEPA
2
Human contact with water is incidental and
the probability of ingesting appreciable
quantities of water is minimal, such as
fishing, commercial boating, small craft
recreational boating, shoreline activities.
Non-contact recreation
IEPA
2
Human contact with water is unlikely such
as
pass
through
commercial
and
recreational navigation
Limited contact
recreation
UAA
3
Incidental or accidental body contact,
during which the ingestion of appreciable
quantities of water is minimal, such as
recreational boating (hand powered boating
activity, canoeing, jet skiing) and any
limited contact incident to shoreline
activity, such as wading and fishing.
Recreational navigation
UAA
3
Non-contact activities including, but not
limited to, pleasure boating and commercial
boating traffic operations.
Table 1. Terms used to describe water recreational activities based on degree of contact
CHEERS Overview
6
July 2008
Figure 1. The Chicago Area Waterways (CAWS). Map produced by the MWRDGC
CHEERS Overview
7
July 2008
3. Scientific background for a CAWS microbial water quality standard
Four sources of information are useful for estimating health risks attributable to secondary
contact recreation on the CAWS. These are: 1) prior epidemiologic studies of secondary contact
recreation in other settings, 2) prior primary contact recreation studies, 3) the MWRDGC Expert
Panel Report, and 4) the CAWS risk assessment produced for the MWRDGC.
3.1.Prior epidemiologic studies of secondary contact recreation
Three studies have characterized rates or relative odds of illness among secondary contact
recreators in other settings.
3.1.1. Fewtrell 1992
This investigation was conducted at two freshwater canoeing courses in the United
Kingdom, one of which was downstream of several sewage treatment facilities, and the
other of which was on a “pristine” waterway.
4
Demographic information and risk factors
for acute gastrointestinal symptoms were recorded at baseline. Water exposure while
canoeing was assessed immediately following the event. Five to seven days later,
symptoms were recorded by telephone survey and recorded again by self-administered
postal survey 28 days after the event. Unexposed individuals were enrolled at each site as
a reference group. Fecal coliforms, fecal streptococci, total staphylococci, and enterovirus
concentrations were measured in water samples taken during the event. A total of 561
participants, 90% of those initially enrolled, completed the 5-7 day follow-up. The risk of
developing gastrointestinal symptoms at 5-7 day follow-up was 4.25 times greater among
those who canoed downstream of the sewage treatment facility than among those in the
unexposed reference group. In other words, the relative risk (RR) of GI symptoms was
4.25, with a 95% confidence interval [CI] of 2.60, 6.94. Among recreators downstream of
the sewage treatment facilities, the incidence of respiratory symptoms was also higher
than among those without exposure (RR 2.42; 95%CI 1.55, 3.79), as were “flu”
symptoms (RR 2.41; 95%CI 1.68, 3.45). Event participants on the cleaner body of water
were more likely to develop respiratory symptoms than the reference group (RR 1.61; CI
1.01, 2.55), but not other symptoms. Between the 5-7 day follow-up and the 28-day
postal questionnaire, 40 of 109 (37%) participants on the contaminated course reported
the development of gastrointestinal symptoms. This rate was more than 50% higher than
CHEERS Overview
8
July 2008
that observed among those who canoed on the other body of water. The authors speculate
that the development of symptoms between the first and third week post-event may have
been due to infection by
Giardia
or
Cryptosporidium
. Because stool sample collection
and analysis was not part of the study protocol, it was not possible to identify the
pathogens responsible for acute illness.
3.1.2. Fewtrell 1994
Following up on the previous work, a prospective cohort study was conducted in which
study subjects were enrolled at two rowing regattas and two canoe marathons in the
United Kingdom.
5
A total of 591 event participants and unexposed spectators were
enrolled in the study. A variety of demographic and behavioral characteristics were
recorded prior to recreation. Five to seven days after the event, subjects were contacted
by phone and postal survey, and asked about water exposure during the event; the
subsequent development of symptoms was recorded. Water quality measures included
fecal coliforms, fecal streptococci, staphylococci,
Pseudomonas auruginosa, Salmonella
spp.
, and
Cryptosporidium spp.
, as well as enteroviruses. Those who ingested water were
more likely to develop gastrointestinal symptoms (RR 2.20; CI confidence interval 1.35,
3.58) or any symptom (RR 1.35; CI 1.32, 2.32) compared to combined group of
spectators and participants who did not ingest water. Actual rates of illness were not
reported, nor were associations between rates of illness and measures of water quality.
Another limitation of this study is that participants were asked five to seven days after the
event about water exposure during the event. Recall bias may have occurred. For
example, having become ill, those who developed symptoms may have been more likely
to remember or report swallowing water during the event than those who did not become
ill.
3.1.3. Lee 1997
A third study of 473 paddlers was conducted in the United Kingdom on a whitewater
course
6
. Participants in whitewater events were given questionnaires and asked to mail in
their responses one week after the event. Water quality measures included
E. coli
,
enterococci, and F-specific RNA bacteriophages. The authors found that specific
exposure variables predicted the development of symptoms. For example, the risk of
illness was increased among those who reported swallowing water two or more times
CHEERS Overview
9
July 2008
compared to those who did not (RR 1.9; CI 1.0, 3.6). Accidentally falling in the course
and swimming (RR 2.3; CI 1.2, 4.3), and eating or drinking before changing out of
clothing worn during the event (RR 2.1; CI 1.1-4.0) were also risk factors for the
subsequent development of symptoms. The risk was lower among those who used the
slalom course seven or more times in the past year compared to less frequent users (RR
0.3; CI 0.1, 0.7) . The absence of a reference (i.e., unexposed) group precludes estimating
the risks of illness attributable to paddling in this setting.
3.1.4. Other prior studies of secondary contact recreation
Several other papers have been published on related topics, though they do not help
characterize the risks of secondary contact recreation. These include a characterization of
the RNA of a norovirus-like pathogen that was isolated from stool samples of several
canoeists, but did not include measures of water quality.
7
Illness rates among canoeists in
South Africa have been described,
8, 9
though the pathogen of interest in those studies,
Schistosoma hematobium
, is not a pathogen of concern in North America.
3.2. Prior primary contact recreation studies
Recreational water quality standards have been based on epidemiologic studies of swimmers
and bathers. The literature has been summarized in a review article
10
and a meta-analysis.
11
Many of the relevant primary contact research studies included in those reviews,
12-15
as well
as three published since,
16-18
were prospective cohort studies, while several were randomized
controlled trials.
19-22
Additionally, a case-control design was used in one study.
23
Furthermore, outbreaks of illness linked to recreational water exposure, generally involving
primary contact recreation, have been reported through the ongoing Waterborne Disease and
Outbreak Surveillance System of the US Centers for Disease Control and Prevention
(CDC).
24, 25
The above studies generally address marine water or freshwater lakes, with very
little known about health risks of primary contact in rivers. The above studies are fairly
consistent in documenting an increased risk of illness with increasing indicator organism
concentrations in recreational waters.
3.3. The MWRDGC Expert Panel Report
The MWRDGC convened an expert panel to evaluate the scientific basis for establishing a
microbial water quality standard for the CAWS.
26
After reviewing the literature and relevant
CHEERS Overview
10
July 2008
proposed and established regulatory standards, the expert panel noted that “there are virtually
no data available on which to rationally base criteria for secondary contact recreational
exposure, either in freshwater or marine situations.” Given the paucity of knowledge about
the risks of limited contact recreation, the Expert Panel recommended that “…studies to
ascertain risk from secondary contact in freshwater and the relationship between any such
risk and indicator levels need to be conducted. These may be epidemiological studies
designed with sufficient statistical power to detect risks at levels deemed to be acceptable for
regulatory purposes. Alternatively, a formal microbial risk assessment can be conducted…”
3.4. The CAWS risk assessment produced for the MWRDGC.
A CAWS recreation risk assessment was conducted for the MWRDGC by GeoSyntech
Consultants to compare the estimated health consequences of the current practice of not
disinfecting WRP effluent to a scenario of disinfection.
27
That study involved sampling water
at locations upstream and downstream on three CAWS WRPs. Samples were analyzed for a
variety of bacteria, viruses and protozoa. Rates of illness were then modeled using risk
established quantitative microbial risk assessment methods. The risk model is based on
several assumptions and estimates, including waterway usage rates, distribution and duration
of specific recreational activities, water ingestion rates for specific activities, and the
infectious dose of specific pathogens. Environmental sampling was conducted in wet and dry
weather. The risk assessment projected a low probability of developing gastrointestinal
illness attributable to recreation (about 1 to 2 per thousand exposures) even for the most
recreational users in areas of the CAWS in close proximity to the District’s WRPs.
4. Limitations of the literature for establishing a CAWS bacterial water quality standard
4.1. Limitations of prior secondary contact studies
The studies of secondary contact discussed above have limitations, including (in one or more
studies) the lack of a comparison of rates of illness to those in a group of unexposed
individuals, the possibility of recall bias, and the fact that rates of illness were not reported.
The dominant activities on the Calumet system of the CAWS are boating and fishing,
3
which
CHEERS Overview
11
July 2008
were not evaluated in the UK river studies. Even the risks for CAWS canoeing can not be
predicted with any precision based on the UK studies of canoeing, because exposure was
likely much greater on a whitewater slalom course than on the low-flow conditions of the
CAWS.
4.2. Limitations of prior primary contact studies
The relevance of studies of primary contact exposure to the establishment of secondary
contact standards is questionable.
4.2.1 The exposures are not comparable given the assumption that smaller quantities of
water ingested (the presumed route of pathogen exposure) during secondary contact
recreation than during primary contact recreation. Risk estimates derived from primary
contact studies would be relevant to modeling risks for secondary contact activities if the
amount of water ingested by swimmers could be compared to that of paddlers or fishers.
Ingestion rates for swimmers have been determined among adults and children swimming
in a pool.
28
If similar estimates were available for secondary contact recreation,
extrapolation of risks from primary to secondary contact could be made, but such
estimates have not been determined.
4.2.2 Additionally, there are no studies comparing rates of illness among swimmers to
those among paddlers, boaters, or fishers in the same body of water. The National
Epidemiological and Environmental Assessment of Recreation (NEEAR) study reported
higher odds of illness among beachgoers who had head-immersion, body immersion, and
any water contact, compared to those who had no water contact.
18
Because water quality
at Great Lakes beaches is so different than at many CAWS locations, and because wading
is so different than kayaking, extrapolating from other surface waters to the CAWS may
not be justified.
5. The epidemiologic study of recreational use of the CAWS
As discussed, the existing literature of risk of illness following primary and secondary contact
water recreation is insufficient for establishing a microbial water quality standard for the CAWS.
Although the GeoSyntech risk assessment suggested a low risk, many of the assumptions used in
the analysis have yet to be validated. In order to develop a scientific basis for establishing a
CHEERS Overview
12
July 2008
standard, on April 19, 2007 the MWRDGC Board of Commissioners voted to contract with the
University of Illinois at Chicago (UIC) to conduct an epidemiologic study of recreational use of
the CAWS. That study is CHEERS, and the remainder of this overview document describes its
components.
CHEERS Overview
13
July 2008
STUDY OBJECTIVES
The overall objective of CHEERS is to investigate illness associated with secondary contact
recreation on the CAWS. Specific aims are:
1) To determine rates of acute gastrointestinal and non-gastrointestinal illness attributable
to CAWS recreation.
2) To characterize the relationship between concentrations of microbes in the CAWS and
rates of illness among recreators.
3) To identify pathogens responsible for acute infections among recreators, and to
explore sources of those pathogens on the CAWS.
The purpose of this study is not to develop regulatory standards. The findings of this research
may provide a scientific basis for the establishment of state or federal water quality standards.
CHEERS Overview
14
July 2008
FIELD STUDY OVERVIEW AND DESIGN CONSIDERATIONS
1. Field Study Summary
A prospective cohort study design is being conducted in which the health of research participants
is evaluated both prior to and following recreation. Three groups of participants are being
enrolled: 1) CAWS recreators (the “CAWS group”), 2) recreators on Lake Michigan and other
general use waters (GUW) (the “GUW group”), and 3) outdoor recreators without water
exposure, such as joggers and cyclists (the “unexposed group”). An overview of study
components is presented in Figure 2. After completing an eligibility screen and an informed
consent process, participants complete two interviewers in the field. The first interview collects
basic demographic information, while the second, administered after recreation to the water-
exposed groups, inquires about water contact. Participants also provide information regarding
their health in general, and about risk factors for acute illness that are unrelated to water
exposure. They are also asked about any open skin wounds and pre-existing infections of the
eyes, ears, and skin. Water is sampled at the location of and on the same days as subject
enrollment; rates of illness will be analyzed as a function of water microbe concentration.
Clinical specimens for microbial analyses are obtained from participants who develop symptoms
of acute gastrointestinal illness (AGI) and non-gastrointestinal illness (NGI). Subjects are
contacted for follow-up telephone interviews at 2, 5, and approximately 21 days after enrollment.
The major study elements are discussed in greater detail in each specific QAPP document.
2. Approach to Choice of Study Methods
The use of well-established methods was strongly preferred over innovation for the
epidemiologic study design. The central components – a prospective cohort design, pre- and
post-recreation evaluations of health, post-recreation evaluations of exposure, the content and
methods of administering surveys, measures of water quality, and the enrollment of a reference
group – are based on the methods employed by the previously discussed studies by Fewtrell,
4
Wade,
17-18
and Colford.
4, 16-18
Water sampling – direct grab samples and with mechanized large
volume sampling – is being conducted using US EPA-approved methods. Novel statistical
methods will not be developed. Rather, the approaches used in the NEEAR and other studies will
be utilized, and analyses will be conducted using SAS, a widely accepted and thoroughly tested
statistical software package.
CHEERS Overview
15
July 2008
Recruitment,
eligibility screen
Pre-recreation survey
(Field Interview A)
Study participant
____activities___
Laboratory
__analyses_
Day of recreation
Recreation
Post-recreation survey
(Field Interview B)
Water sampling for
indicators, pathogens
Follow-up period
Telephone surveys
Days 2, 5, 21
Obtain
clinical specimens
Other
analyses
Culture
Environmental
sampling
Consent
Figure 2. Overview of study components
3. Rationale for Prospective Cohort Design
While randomized controlled trial designs, such as that used in European primary contact
studies,
19-22
have the advantage of the ability to equalize levels of confounders among study
groups, such a design could not be effectively implemented in our setting. Almost all of the
kayakers, canoeists, and boaters who come to the CAWS – particularly for events such as the
Chicago River Flatwater Classic – will have planned in advance and brought their watercraft
CHEERS Overview
16
July 2008
with them. Such individuals would be unwilling to be randomized to a non-water-recreator
group. Rather than addressing confounding by randomization, we will collect data about a
variety of potential confounders and statistically adjust for the confounders in analyses. The
prospective design will minimize recall bias because we will ask participants about water
exposure immediately after recreation, rather than after some become ill.
4. Study groups
Conceptually, there are three possible sources of risk for acute gastrointestinal and non-
gastrointestinal illness among CAWS recreators:
•
“Background” factors that result in AGI and NGI symptoms. For AGI these
include existing population risks of food-borne illness, fecal-orally transmitted
gastrointestinal infections, medication side effects, and lactose intolerance. For
NGI, the relevant factors are population rates of acute respiratory, skin, eye, and
ear symptoms.
•
Acute illness due to recreational contact with water itself. Water can be aspirated,
causing acute respiratory symptoms. Acute respiratory symptom rates have been
noted to be higher among secondary contact recreators on a pristine whitewater
course than among unexposed individuals, suggesting that water contact, rather
than pathogen contact, is responsible for some symptoms (Fewtrell et al. 1992).
Direct water contact promotes breakdown of the protective layer of the skin,
increasing the risk of dermatitis and otitis externa (“swimmers ear”).
•
Infections caused by waterborne pathogens that are acquired through recreational
activities.
AGI is the best-studied health endpoint in studies of water recreation. There are substantial rates
of AGI in the general population. Failure to account for background rates could result in some
cases of AGI in water-exposed recreators to be attributed to water contact or pathogens, rather
than to background factors. Such erroneous attribution would inflate estimated risks of illness
due to microbial pathogen or water contact.
Data from the three groups of recreators will allow us to meet study objective 1,
determining rates of illness attributable to CAWS recreation. We will differentiate the risk of
acute illness following CAWS recreation from the risk attributable to microbial exposure on the
CHEERS Overview
17
July 2008
CAWS by enrolling three groups of study participants: CAWS recreators, GUW recreators, and
unexposed (non-water) recreators. CAWS recreators have all three sources of risk. At
recruitment locations which are not immediately downstream of wastewater treatment facilities,
recreators in the GUW group have risks due to background factors and water contact, but will be
exposed to much lower concentrations of waterborne microbes. The inclusion of the GUW group
will allow the evaluation of a dose-response relationship between water quality and illness rates
that will include a broader range of water quality measures than if only CAWS recreators were
included. Risk for acute illness in the unexposed group, enrolled at the same times and areas as
participants in the two water-exposed groups, will be due to “background” factors only. The
fact that all three groups will consist of people engaging in outdoor recreational activities should
reduce demographic and general health variables from confounding possible associations
observed between illness rates and study group. Additionally, we will be able to examine self-
reported measures of water exposure for various recreational activities, and to use this
information in to help understand the variability of risk among study participants.
5. Estimating background rates for sample size calculations
We estimate that background rates of AGI in the population are approximately 50-75 per 1,000
per month. This estimate is based upon rates of AGI in groups of unexposed individuals in the
primary contact recreation studies
16-18
noted above that employed study designs and methods of
defining health endpoints that are similar to those that will be used in the present study. In the
NEEAR study, the overall rate of AGI among those without water contact was 80/1,000 during
the 10-12 days following the beach visit.
18
However, in that study, the rate of AGI among
unexposed beachgoers at the Indiana Dunes (located near Chicago) was 50/1,000. At the Lake
Erie beach, the rate among the unexposed was also approximately 50/1,000, with the exception
of one day when it exceeded 100/1,000 (personnel communication, T. Wade). In the Mission
Bay, CA study, the rate among the unexposed was again approximately 75/1,000/month.
16
Another estimate of background rates for acute gastrointestinal symptoms comes from a large
hepatitis A post-marketing surveillance study of US adults and children. In that study the rate of
“diarrhea/gastroenteritis” requiring emergency department evaluation was 50/1,000 over a thirty
day period.
29
CHEERS Overview
18
July 2008
6. Study name
Although the name of this study when originally proposed to the MWRDGC was the
“Epidemiologic Study of Recreational Use of the Chicago Area Waterways,” for the purposes of
study publicity and recruitment, the study will be known as “CHEERS,” the Chicago Health
Environmental Exposure and Recreation Study. We wish to avoid biasing potential study
participants regarding the study’s hypotheses, and we will not identify the Chicago River (or
other parts of the CAWS) as the primary focus of the research. Additionally, our promotional
materials and recruitment scripts do not identify those who have no water contact as being a
“control group,” so that all participants perceive that the information that they provide is no less
important than that provided by individuals in the CAWS and GUW groups.
7. Participant recruitment strategies
Three general approaches will be employed to promote enrollment: day-of-recruitment efforts,
advance coordination with organizers of special events, and advance coordination with teams or
clubs. Study participants are also offered financial incentives for their time and effort. The
efforts to recruit study participants are the responsibility of a recruitment manager.
7.1 Recruitment manager
Beginning in 2008, a member of the CHEERS team now functions as a recruitment manager
and has primary responsibility for identification and contact of relevant organizations, and
for the development of plans to work with clubs,/ teams, and organizers of secondary contact
water recreation events in the Chicago area. In January, 2008, a dinner meeting was held
with 25 representatives of yacht clubs, organizers of secondary contact water recreation
events in the Chicago area, and high-school, collegiate, and private rowing and paddling
clubs. The meeting was successful in allowing the research group to form new working
relationships with organizations and to solidify existing relationships in order to promote
recruitment of club/team members in the study. If recruitment goals are not met in 2008, a
similar meeting will be held during the winter of 2009. In order to introduce the study to the
target audience, the recruitment coordinators will also staff a CHEERS booth at non-
recreation events that are attended by water recreation enthusiasts, such as conventions and
sales conferences for water sports equipment.
CHEERS Overview
19
July 2008
7.2 Recruitment strategies
During the 2007 season, participants were recruited using three approaches. The “intercept
interview” was the most frequently used. A recruiting station would be established at a
location at which recreators were expected to be and recreators were approached by staff and
invited to participate in the study. The second approach, event oriented recruiting, was used
on two occasions: The Chicago River Flatwater Classic (a rowing and paddling event) and
the Chicago Shoreline Marathon (a lake kayaking race). This approach involved the
coordination of publicity and recruitment with event organizers. The third approach, club
and team recruiting, was used several times in 2007 to recruit members of three rowing
teams, a kayaking club, and a club that bicycles along Lake Michigan and the North Branch
of the Chicago River.
In the 2008 season we will vary the balance of the recruiting
approaches over the course of the season in an effort to maximize recruitment.
7.2.1 Intercept interviews
In 2007, we relied primarily on intercept interviews at locations on Lake Michigan and
the CAWS.
These efforts were most successful on weekends. The number of
participants recruited was related to the number of recruiting locations that could be
staffed on a given day, and the number of staff available. In 2007, the number of staff
available limited such recruiting to about 4-6 hours per day, generally on either Saturday
or Sunday (but frequently not both on the same weekend) and generally at one location
per day. In 2008, we are have increased duration and staffing of intercept interview
recruitment on dates when large numbers of participants are likely to be recruited, such as
summer weekends and holidays, and have extended the length of the recruiting day from
about five hours to between ten and twelve hours. Separate morning and
afternoon/evening shifts are be staffed by approximately 4-5 people at each of three to
four locations.
The composition of a team (data manager, interviewer/recruiters,
incentives/publicity, and use survey recorder [CAWS locations only]) remains
unchanged. The positions are described in QAPP 2: Survey Methods. These eight teams
(four locations, two shifts per location) are supervised by a field supervisor, one of the
project managers.
CHEERS Overview
20
July 2008
7.2.2 Event-oriented recruiting
It is far more efficient to enroll participants at one large event than fewer participants on
multiple dates. Our recruitment coordinator has identified at least number additional
major events that will allow us to enroll participants at CAWS and general use waters
locations. We will provide the organization hosting the event between $50-$250 to assist
with publicity and distribution of information, such as links to our website and study
information sheets. Several large rowing and paddling events will be held on CAWS and
general use waters in 2008 and canoe/kayak vendors host smaller group outings on a
regular basis. The amount of money provided to the organizers will vary based on the
number of participants in the event, not the number who choose to enroll in the research
study.
7.2.3 Club and team recruiting
Unlike events, in which several hundred people participate a single time, clubs and teams
generally consist of 10-100 members, and have multiple outings per season. A problem
with this type of recruitment is that members are often ineligible to enroll; many of the
clubs and teams row 6-7 days per week and, to meet study eligibility requirements,
participants must not have engaged in water activities 48 hours prior to enrollment. To
address this issue, the recruitment manager works with coaches to set a date for CHEERS
recruiting on which we can sign up team members on their first day out on the water, as
well as on their first time back after spring and summer breaks. We have also simplified
the process of enrolling members of high school rowing teams. Parents will have the
option of providing consent once during the season for enrolling their children in the
study as many times as they are eligible. As will be the case for event organizers, we will
compensate clubs and teams for the time they put into helping coordinate recruitment.
Teams will receive $100 for their participation, regardless of the number of team
members who enroll in CHEERS. If a school has four teams (men’s and women’s,
varsity and novice) the school would receive $400. Additionally, participants will have
the option to donate the $50 incentive to the team or club.
7.3 Participation incentives
Research participants will receive a CHEERS T-shirt and $15 Target gift card on the day of
field evaluation. Following the completion of the third and final telephone follow-up, they
CHEERS Overview
21
July 2008
will be sent a check for $35. CHEERS participants recruited as members of a rowing or
paddling team or club have the option to donate their incentive to the team, or to Friends of
the Chicago River, a UIC research partner. Those who are asked to provide a stool or other
clinical specimen for analysis (based on the presence of specific symptoms on telephone
follow-up) will receive an additional $75. These amounts were established based on the
experience of UIC investigators in prior occupational and environmental epidemiologic
studies. For example, in a study conducted by members of this research team about possible
Helicobacter
transmission in an occupational setting, offering a $25 incentive resulted in 28
out of the 45 eligible workers (62%) providing stool samples and 28 of the 30 who enrolled
in the study (93%).
7.4 Study awareness and publicity
Various efforts have been undertaken to increase awareness of the study among potential
participants.
7.4.1 Logo
A logo has been developed to promote awareness of the study among local water
recreation enthusiasts (Appendix 1). The logo was designed to convey messages of
“outdoors” and “sports” without indicating that a specific body of water or regulatory
issue is the focus of this research, so as not to bias study participants. The CHEERS
study logo is displayed on the banners, T-shirts, and a recruitment flyer (Appendix 2) that
is distributed in advance of group- and event-oriented recruiting, and during other
recruitment.
7.4.2 Informative handouts
7.4.2.1 Recruitment flier
Like the logo, the recruitment flyer (Appendix 2) was designed to interest potential
participants without communicating information that could result in biased enrollment (in
other words, we do not wish to promote enrollment into the study in way that appeals
more to those who perceive water quality to be particularly poor or particularly good).
For example, although it is clear that this research is about water, outdoor recreation, and
health, no message is communicated to suggest that CAWS recreation is either hazardous
or risk-free. Similarly, the flyer does not state that some participants who enrolled to be
CHEERS Overview
22
July 2008
part of a “control group” rather than being in a group of primary interest. Additionally,
although the financial compensation is presented as a range, $50-$125, but no mention is
made of the fact that if participants note specific symptoms, they will receive a $125
rather than $50.
7.4.2.2 General information about CHEERS
The “General Information about CHEERS: For clubs, teams, organizers, and vendors”
sheet Appendix 3) was developed to provide information to people contacted by the
recruitment manager about the possibility of coordinating recruitment efforts. The flier
outlines the basic goal of the study and what participation entails.
7.4.2.3 Water Quality FAQ
Anticipating that our presence will prompt inquiries from the public regarding the quality
of the waterways, we compiled a “Water Quality Frequently Asked Questions” sheet
(Overview Appendix 4), based on information from publicly available government
websites, including the Illinois EPA. The sheet allows study staff to provide standard,
unbiased responses to common questions without offering opinions on water quality or
health risks.
7.4.3 Day of event publicity
On the day of recruitment multi-disciplinary field teams will set up a tent and banners at
the site of recruitment. All CHEERS field team members will wear the CHEERS t-shirt
which, like the banners and flyers, will display the study logo. After their participation in
the field study, each research subject will receive a CHEERS T-shirts. To the degree that
participants subsequently wear the T-shirts, they will be promoting recognition of the
study “brand,” potentially facilitating recruitment of new participants.
7.4.4 Recruitment assistance by Friends of the Chicago River
Friends of the Chicago River (FCR) is a non-profit organization that promotes the health
and use of the Chicago River. FCR is an important partner in this research, helping to
publicize the study, promote recruitment, and provide space at events for enrollment.
FCR estimates that through its newsletter, website, special events, event promotion, club
outreach, and other methods, it will promote awareness of the CHEERS study among
CHEERS Overview
23
July 2008
several thousand area residents. FCR organizes several urban canoe trips per season at
various locations, as well as the “Chicago River Flatwater Classic,” a popular canoeing
and kayaking event. FCR provides information to its members about the CHEERS study,
and will provide specific enrollment information to those who register for the Flatwater
Classic and selected other events.
7.4.5 Website
In July, 2008, a website was launched to inform the public about this research. A goal of
the website is to provide information about the study, and how one might enroll in it.
The web address of the site is www.cheerschicago.org.
8. Study enrollment and locations
8.1 Enrollment locations
8.1.1 CAWS locations
The November 2004 Draft UAA report includes profiles of CAWS use by recreational
activity and by waterway reach. Based on information in that report, the most heavily
used access points on the Cal-Sag Chanel are the boat launches of Alsip and Worth, from
which approximately 7,000 and 4,000 launches occur per season respectively, making
them by far the most active sites of recreation on the Calumet river system downstream
of the Calumet WRP. At Clark Park on the North Branch of the Chicago River and at the
Skokie Rowing Center Boat Launch on the North Shore Channel, the Chicago River
Canoe & Kayak Rental Company estimates that a total of approximately 5,000 launches
occurred in 2004. That number has reportedly increased to over 7,000 launches per year.
Additionally, approximately 200 rowers per week use the CAWS as members of rowing
clubs and school teams. After assessing these and twelve less frequently used access
points on the CAWS in May 2007, we finalized the list of sites for the enrollment of
study participants for the 2007 season. For the 2008 season, we will recruit at two new
locations of the South Branch of the Chicago River (one for rowers, one for fishers), and
at a second location at North Avenue on the west side of the turning basin. On the Cal-
Sag Channel recruiting will also take place at new locations in 2008: Riverdale Marina,
and at an aeration station near the Village of Worth boat launch.
CHEERS Overview
24
July 2008
8.1.2 GUW locations
We will expand the number of “general use waters” locations where recruitment will take
place. In 2007 such recruitment took place at Lake Michigan harbors and beaches, the
Skokie Lagoons, and Crystal Lake.
In 2008 this has been expanded to include
potentially the Fox, Des Plaines, Kankakee, and Du Page Rivers as well as two boating
centers within the Cook County Forest Preserves: Busse Woods and Tampier Lake. The
recruitment manager will work throughout the season to identify additional locations.
8.1.3 Study Area
All 2007 enrollment sites will be included in the 2008 season. The 2008 season will also
incorporate additional CAWS and GUW sites. Enrollment sites visited during the 2007
and 2008 seasons are shown in Figure 3. Site specifications, including geographic
coordinates and the occurrence of water and non-water activities can be found in
Appendix 5 (CAWS locations) and Appendix 6 (GUW locations).
CHEERS Overview
25
July 2008
Figure 3. Recruitment locations 2007, 2008 seasons
CAWS North (NSC, NBCR, MS, SBCR)
BR: Bridge Street, Evanston, IL
SK: Skokie Rowing Center, Skokie, IL
RP: River Park, Chicago, IL
CP: Clark Park, Chicago, IL
NA: North Avenue, Chicago, IL
MS: Main Stem, Chicago, IL
PT: Ping Tom Park, Chicago, IL
CAWS South (Cal-Sag)
WO: Worth Boat Launch, Worth, IL
AL: Alsip Boat Launch, Alsip, IL
RM: Riverdale Marina, Riverdale, IL
General Use Waterways
UDP: Upper Des Plaines River, Wadsworth, IL
DS: Des Plaines River, Libertyville, IL
CL: Cystal Lake, Crystal Lake, IL
SL: Skokie Lagoons, near Winnetka, IL
BS: Busse Woods Boating Center, Elk Grove Village, IL
FR: Fox River, St. Charles, IL
LB: Leone Beach, Chicago, IL
MB: Montrose Beach, Chicago, IL
BH: Belmont Harbor, Chicago, IL
DH: Diversey Harbor, Chicago, IL
SD: Solidarity Dr., Chicago, IL
BN: Burnham Harbor, Chicago, IL
JP: Jackson Park Harbor, Chicago, IL
TL: Tampier Lake Boating Center, near Palos Park, IL
HW: Hammel Woods, Shorewood, IL
CHEERS Overview
26
July 2008
8.2 Scheduling enrollment by location
8.2.1 Theoretical considerations
Field study locations were selected based on the following principles relevant to data
analysis and interpretation: 1) the activities and locations of enrollment of participants in
the CAWS group should reflect actual CAWS usage patterns, by recreational activity and
location; 2) the distribution of recreational activities among GUW group participants
should reflect that observed in the CAWS group; and 3) the demographic characteristics
of unexposed group participants should reflect that of the other two groups. The unit of
“sampling” the population of recreators is done at the level of the recruitment site. At a
given recruiting site, efforts are made to approach every water recreator and every non-
water recreator within the pre-defined “recruitment area” (Appendices 5 and 6) at that
site to avoid any potential selection bias on the part of the recruiters.
In order to ensure that the activities and locations of enrollment into the CAWS group
reflect actual use, we will track enrollment and use by location on the CAWS. Several
locations are relatively busy (Skokie Rowing Center, the east and west sides of the
turning basin at North Avenue, Alsip, and Worth), while others are intermediate (Clark
Park, River/Ronan Park), and others see relatively little use (Riverdale Marina, the Cal-
Sag SEPAs and South Branch locations). We schedule recruitment at these locations in
relation to actual use. In general, recruitment will take place at all busy locations every
week of the summer (in 2008).
The study’s statistical power will be maximized if the participants are evenly distributed
among the CAWS, GUW, and unexposed groups. Given that this is a dynamic cohort
study into which new participants are enrolled every week, we have the opportunity to
increase or decrease the frequency of recruitment of participants into the three groups in
order to produce groups of equivalent size. If recruitment of one group of participants is
lagging far behind the other two, the frequency of scheduling recruitment targeting that
group will be increased.
Maintaining three groups of equivalent size throughout the
study is desirable for two reasons. First, it will produce a sample size that has higher
statistical power. Second, if a community-wide outbreak were to occur (unrelated to
water quality), all three groups would have a similar likelihood of capturing this outbreak
if the groups are of comparable size. For these reasons, after each block of 1,000
CHEERS Overview
27
July 2008
participants is enrolled, the distribution of participants among the three groups is
reviewed. If one or two groups are lagging significantly behind the other(s) (by more
than 15%), the frequency of recruitment efforts targeting those groups are adjusted for the
following month so that the lagging group(s) can catch up in enrollment to the largest
group. This is done based strictly on recruitment rates per site (by group) and not based
on measures of water quality or health events (such data is not available at the time of
schedule revision).
8.2.2 Logistical considerations
Additionally, logistical considerations in the selection of study sites are: 1) waterway
access; 2) the presence of nearby sites of recreational activities that do not involve water,
which would allow the recruitment of participants into the unexposed group on the same
days and at the same approximate location as the recruitment of participants in the
CAWS or lake group; and 3) the physical safety of study personnel.
In the 2007 season, CHEERS enrollment was designed to progress from smaller to larger
enrollment targets during the summer, and from enrolling participants from one access
point per day to two access points per day (by two teams) during periods of anticipated
heavy usage. In 2007, participant recruitment generally took place for 4-6 hours per day.
In 2008, teams will work at four or five recruiting sites per day, with two shifts per site
allowing up to 12 hours of recruitment. Based on the patterns of CAWS usage noted in
the 2007 season, recruitment in 2008 will focus on recruiting rowing teams in clubs in the
early morning or late afternoon, generally on weekdays during the academic year.
Between Memorial Day and Labor Day, recruiting will take place every Friday, Saturday
and Sunday, as well as on holidays at multiple sites per day.
The priorities for setting the recruitment calendar are: 1) organized races or events which
often result in the greatest number of enrolled participants per hour, 2) availability and
eligibility of clubs and teams, 3) more active recruitment sites over less active sites, and
4) increasing frequency of recruitment at sites where enrollment of participants into the
“lagging” group is likely.
CHEERS Overview
28
July 2008
A summary of recruiting efforts during the 2007 season and proposed efforts for the 2008
season are listed in Table 8. The recruiting calendar for 2007 and the current calendar for
2008 can be found in appendices 7 and 8, respectively.
CHEERS Overview
29
July 2008
Table 2. Summary of 2007 recruitment efforts, projected 2008 recruitment efforts
2007
2008
Intercept
interview
locations x days
28
217
Events
2 (Flatwater
Classic, Chicago
Shoreline
Marathon)
9 (Flatwater Classic, Chicago Shoreline Marathon,
DesPlaines River Canoe Marathon, Kane County Canoe
Marathon, Mid-America Canoe Race, Fox River Dragon
Boat Race, Chicago River Dragon Boat Race,
Chicagoland Bass Tournament, canoers and kayakers in
Earth Day clean up)
Teams/Clubs
3 (New Trier,
LPJ, Crystal
Lake)
11 (New Trier, LPJ, Crystal Lake, St. Ignatius,
Northwestern U., U of Chicago, Chicago River Rowing
and Paddling Club, Chicago Rowing Club, Evanston
Ecology Center, Lincoln Park Boat Club, North Park
University)
Typical duration
of field recruiting
per day
4-6 hours
12 hours
Recruiting at 2
locations per day
4
2
Recruiting at 3
locations per day
2
16
Recruiting at 4
or more locations
per day
0
38
8.3 Enrollment targets by exposure group and study year
We plan to enroll a total of 9330 participants, based on considerations outlined in the
Statistical Analysis document (which is not part of this “Overview” document). While we
projected enrollment of 2,010 participants during the 2007 season, we successfully enrolled
886 study participants during the brief 2007 season, of whom 811 were eligible for telephone
follow-up. The recruiting effort has been substantially increased in 2008, with the goal of
recruiting the remaining 8,442 participants. Table 2 displays the overall recruiting targets by
group, by year.
CHEERS Overview
30
July 2008
CAWS
Lake/Lagoon
Unexposed
Annual Total
2007 (achieved)
386
123
377
886
2008 (projected)
2723
2986
2733
8444
Group Totals
3110
3110
3110
9330
Table 3. Study participant recruitment achieved during 2007 and targets for 2008
9. Survey data
9.1 The survey questionnaires being used in this study are derived from those used in the
NEEAR study, conducted by the US EPA and CDC. Like the NEEAR study, we will use
surveys to conduct pre-exposure enrollment, post-recreation exposure assessments, and post
recreation health follow-up by telephone. Modifications to the NEEAR approach are: 1) the
unit of recruitment (and interviewing) will be individuals, rather than family groups, and 2)
exposure questions specific to secondary contact recreational activities have been added. The
study was initially designed to conduct a pre-exposure health assessment questionnaire and a
field clinical exam, and the first 130 participants were enrolled following this protocol.
However, because the physical examination in the field by a clinician was considered a
potential disincentive to participation and little information was gained from it, it was
dropped from the protocol. The survey questionnaires were developed for this study in
conjunction with the University of Illinois at Chicago (UIC) Survey Research Laboratory
(SRL), a national leader in survey development, administration, and analysis. Elements of
the survey portion of CHEERS are described briefly below. Detailed information is
presented in QAPP 2: Survey Methods.
9.2 Questionnaires
A total of four questionnaires will be administered to all study participants:
9.2.1 An eligibility screen
9.2.2 A pre-recreation field questionnaire
9.2.4 A post-recreation field questionnaire
9.2.5 A telephone follow-up questionnaire
CHEERS Overview
31
July 2008
9.3 Questionnaire administration
Questionnaires will be administered in face-to-face interviews, with the exception of the
follow-up questionnaire, which will be administered by telephone. The questionnaires will be
administered using computer assisted interview (CAI) methods, with the exception of the
eligibility screen. The CAIs conducted in the field will be administered used computer-
assisted personal interviewing (CAPI) methods, while the telephone follow-up questionnaire
will be administered using computer assisted telephone interview (CATI) methods. Field
interviews will be conducted using laptop computers and the telephone interview will be
conducted at UIC using desktop computers.
For children under the age of 7, parents will be required to provide proxy responses for the
child; for children ages 8 through 17, parents have the option to serve as the proxy
respondent. In both cases, parents are encouraged to accompany the child during the
interview.
9.4. Health end-points
Using survey methods, we will determine whether each participant does or does not develop
specific health endpoints. Key health endpoints to be identified are acute gastrointestinal
illness and non-gastrointestinal illness:
Acute gastrointestinal illness (AGI)
We will use two of definitions of AGI. One is based on the presence or absence of single
symptoms (such as vomiting or diarrhea). The other is based on a syndrome of two or
more symptoms. Prior studies have defined specific syndromes, and our study
questionnaires and data analyses have been planned to allow comparisons of rates of such
syndromes determined in our study to those in other studies. These definitions include
Cabelli’s “Highly Credible Gastrointestinal Illness”
14
and the definitions used the primary
contact studies.
16-18
Non gastrointestinal illness (NGI)
Definitions for otitis externa, conjunctivitis, acute respiratory tract infections, and skin
infections will be based on responses to questionnaire items. In addition to the above
definitions, “culture positive AGI” will be defined as cases of AGI in which a pathogen is
identified on stool analysis. Similar categories for culture-positive conjunctivitis and
CHEERS Overview
32
July 2008
skin/wound infection will be created though we expect few individuals to have such
infections.
10. Clinical microbiology
10.1 Prior health studies of recreational water contact have relied upon telephone or postal
questionnaires to define health endpoints (such as AGI). In other words, objective measures
of health endpoints have been lacking. One exception is a primary contact study conducted in
the United Kingdom conducted by Jones, et al.
30
In that randomized controlled trial of
exposure to seawater, both water-exposed and unexposed participants were asked to bring
stool samples three days before, three days after, and three weeks after the trial date. A total
of 781 specimens were obtained from 276 participants, the vast majority of whom had no
gastrointestinal symptoms. Two samples tested positive for
Salmonella spp,. (
one sample was
obtained prior to the trial date), one for
Campylobacter spp.
(again, obtained prior to the trial
date), none for
Cryptosporidium
, seven for
Giardia
, and five for enterovirus. Because of the
small numbers of positive stool specimens, differences in the rates of infection by study
group (exposed vs. unexposed) were not presented. In part because of the lack of positive
findings in this study, investigations of the pathogens responsible for endemic (as opposed to
outbreak-associated) recreational water illness were not conducted.
Based on the experience of Jones, we intend to only collect stool samples 1) after recreational
activities, 2) from individuals with symptoms, and 3) by dispatching study personnel to pick
up samples from the participants’ homes. After participants who report specific symptoms at
telephone follow-up are asked to provde a stool specimen, they will be told that they will
receive an extra $75 for their efforts. This is expected to provide additional motivation for the
collection of samples.
10.2 Selection of pathogens
Pathogens of interest have been identified by reviewing recent publications by the
Waterborne Disease and Outbreak Surveillance System of the US Centers for Disease
Control and Prevention.
24,25
Additionally, data on pathogens in the CAWS have been
evaluated.
27
Members
of
the
UIC
research
team,
two
infectious
disease
physician/epidemiologists and the director of a university hospital microbiology laboratory,
have assisted in defining the pathogens of interest, as presented in Table 3.
CHEERS Overview
33
July 2008
Bacteria
Virus
Parasites
Salmonella
Shigella
Edwardsiella
Yersinia
Aeromonas
Plesiomonas
Campylobacter
Table 4: Pathogens to be detected in stool samples
E. coli
0157:H7
Norovirus
Rotavirus
Enterovirus
Enteric adenovirus
Entamoeba histolytica
Giardia lamblia
Cryptosporidium spp.
Cyclorospora
10.3 Timing of follow-up phone interviews
Based on information from an authoritative textbook of infectious diseases,
31
we devised a
chart of the latency between exposure and AGI onset (Figure 4) and well as the optimal
timing for collecting stool samples, given the period of shedding organisms in stool (Figure
5). This information was used to select days 2, 5 and 21 following recreation to conduct
telephone follow-up and collect stool samples.
11. Human Research Subject Protections
This research study has been approved by the UIC Office for Protection of Research Subjects,
Institutional Review Board (IRB). The UIC IRB protocol number is 2007-0436. Human
research protection issues and the IRB process are described in detail in QAPP #2: Survey
methods.
Further details about clinical sample collection analyses are described in QAPP #3: Clinical
Microbiology and Evaluations.
CHEERS Overview
34
July 2008
Figure 4: Usual incubation period ranges for selected etiologic agents
Figure 5: Shedding period and optimal collection period for select etiologic agents
CHEERS Overview
35
July 2008
12. Overview of Water Sampling
12.1 Purpose and types of water sampling
Water sampling and analysis will support study objectives 2 (characterizing the relationship
between concentrations of water microbes and rates of illness) and study objective 3 (exploring
sources of pathogens on the waterways). What follows is an overview of water sampling and
analysis. Detailed information is provided in QAPP 1: Water sampling and Analysis.
Two types of water sampling will take place: direct sampling and large volume sampling. The
large volumes samples will be collected by the use of pumps which will collect water for
continuous flow centrifugation (CFC) and for viral filtration . Direct sampling will be used to
collect water for analyses of densities of indicator micrboes
E. coli,
enterococci, and coliphages.
CFC will be employed to collect water samples for analyses of
Giardia
and
Cryptosporidium
.
Filtration samples will be analyzed for norovirus. Samples will be archived for potential future
analyses.
12.2 Locations for Water Sampling
Water sampling locations will depend upon enrollment locations on any given date. For each
enrollment location we will sample water every two hours at the approximate site of water entry
(“access point sampling”). CAWS water will also be sampled upstream and downstream of the
nearest upstream WRP, at the beginning and end of the enrollment day (“WRP sampling”).
Additionally, for locations on the North Branch of the Chicago River, water samples will also be
taken from the North Branch upstream of the North Branch dam. This location is important
because at this location water that does not have an upstream WRP enters the CAWS from the
North Branch of the Chicago River.
Categories of sampling locations were developed to support the analyses required for meeting
specific study objectives. Table 5 presents the primary uses of water quality measures collected
by location category. Health outcomes will be modeled using access point indicator densities,
and also (separately) using downstream sampling site indicator organism densities. While access
point measures may prove to be better predictors of health outcomes, sampling from a variety of
access points will likely not prove practical in establishing water quality standards for the
CAWS. Microbe densities measured in sampling collected at the fixed “downstream” sites could
be a convenient measure used to define a sampling plan to comply with possible regulatory
CHEERS Overview
36
July 2008
requirements in the future. This distinction parallels the ongoing debate in recreational water
illness epidemiology over whether the “ecologic exposure” or “personal exposure model” is
preferable. Recent work indicates that in the primary contact setting any difference between the
two approaches is small,
32
though it is not clear whether this will prove to be true for CAWS
recreation.
Primary purpose
Access point
Upstream of
WRP
Downstream of
WRP
Upstream of NB
dam
Predicting health events
√
Regulatory
√
Illness-source linkage
√
√
√
Table 5. Primary purpose of water sampling, by location.
The collection of water samples both upstream and downstream of the WRPs, as well as above
the North Branch Dam (for North Branch access points) will allow the exploration of sources of
pathogens responsible for illness among CAWS recreators. Table 6 presents locations for CAWS
water sampling at sites other than participant recruitment locations.
WRP
Location
GPS coordinates
Nearest MWRD
monitoring stations
North Side
Upstream
Bridge Street
N 42°03.398’
W87°42.025’
35, 101
Downstream
Lincoln Ave.
N 41°59.570’
W87°42.605’
102
North Branch NB Dam
N 41°58.426’
W87°42.286’
37
Calumet
Upstream
Beaubien Woods
N 41°38.983’
W87°35.493’
55, 56
Downstream
Riverdale Marina
N 41°39.368’
W87°38.830’
76
Table 6. Locations for water sampling on the CAWS other than recruitment sites.
CHEERS Overview
37
July 2008
On each day of participant enrollment and field evaluation, water will be sampled at the locations
listed in Table 7.
Enrollment site
At access point
Upstream of
WRP
Downstream of
WRP
Upstream of
NB Dam
Skokie Rowing Ctr.
√
Bridge Street
Lincoln Avenue
River Park
√
Bridge Street
Lincoln Avenue
√
Clark Park
√
Bridge Street
Lincoln Avenue
√
North Ave. launches
√
Bridge Street
Lincoln Avenue
√
Worth Boat Launch
√
Beaubien
Woods
Riverdale Marina
Alsip Boat Launch
√
Beaubien
Woods
Riverdale Marina
Calumet Boat
Launch
√
Beaubien
Woods
Riverdale Marina
Table 7. Water sampling locations, by site of participant enrollment.
13. Field Team Organization
The management structure of the overall epidemiologic study is described in a separate
document, the “Quality Management Plan.” Each field team consists of approximately twelve
UIC personnel, who perform logistical, water sampling, participant recruitment, and evaluation
functions. The staffing level varies with daily recruiting targets. On days on which participants
will be enrolled at two locations, two teams are simultaneously in the field. On those days, the
water sampling and survey supervisors, water sampling staff, and equipment courier serve both
locations. Each site has a dedicated data manager plus survey personnel and recruiters..For the
2008 season, twelve-hour recruiting efforts will employ 2 six-hour shifts of recruiters and
interviewers.
Position (number in this capacity)
Water sampling supervisor (1 per day)
Direct method water sampling staff (2-4 per shift)
Filtration method water sampling staff (2 per shift)
Water chemistry and quality monitoring (1 per shift)
Courier/driver (1 per shift)
Survey supervisor (1 per day)
Recruiter/interviewer (2-4 per site)
CHEERS Overview
38
July 2008
Field data manager (1 per site per shift)
14. Project management
The management structure of CHEERS reflects the need to coordinate and ensure the quality of
the diverse and interdependent elements of this project. Figure 6 outlines the positions and roles
of members of the project management team. All work will be done at UIC, with the exception
of the microbial measures of water quality, certain elements of study publicity performed by
Friends of the Chicago River and some stool analyses to be performed by the Illinois Department
of Public Health (IDPH). The study director, project coordinator, project managers, quality
manager, and field coordinator comprise the leadership team of the project and have direct
responsibility for its execution. Additionally, several UIC SPH faculty members have played
critical roles in protocol development and will continue to provide ongoing consultation. All
personnel are employed by the UIC School of Public Health unless otherwise indicated. Other
important members of the research team and the roles that they play are included in the project-
specific QAPP documents.
14.1 Study director
The study director, Samuel Dorevitch, MD, MPH, will have overall responsibility for the
development and implementation of the CHEERS study, as well as providing deliverables to the
MWRDGC. Specific responsibilities of the study director include the hiring and supervising of
project managers, establishing subcontracts with entities external to UIC, complying with human
subjects research protection requirements, and communicating with the MWRDGC.
14.2 Quality Manager
Peter Scheff, PhD, is the CHEERS quality manager. His responsibilities will include the
development of the organization-wide QMP, as well as the four individual Quality Assurance
Project Plans (QAPPs) for each component of the overall study. He has worked with the study
director and assistant study directors to establish data quality objectives for individual projects.
He will review the quality control data of the laboratories conducting water and medical sample
analyses, conduct quality audits, and communicate with quality managers of the Survey Research
Laboratory (SRL), UIC microbiology laboratory, IDPH microbiology, and microbial water
quality laboratories.
CHEERS Overview
39
July 2008
14.3 CHEERS Project Manager
Sara Wuellner, MS, is the CHEERS project manager. The CHEERS project manager will have
field, administrative, data management and quality monitoring functions. The CHEERS project
manager will serve as a field supervisor and coordinate the three primary field data collection
activities (water, survey, and clinical). The program manager will be responsible for ensuring
that sufficient staff are available to conduct the field study, and will manages an online
scheduling and staffing system. She will also track and consolidate incoming data from the
laboratories and ensure the timely addition of the data to the central CHEERS database. The
project manager will have responsibilities for organizing and maintain study related documents
(laboratory reports, data, IRB documents) and for report writing.
14.4 Survey Data Manager
The survey data manager will ensure the training of field team members and develop survey
questionnaires based upon those used by the US EPA/CDC NEEAR study. Preethi Pratap, PhD,
serving in this capacity, will also coordinate the review and programming of the questionnaires
to be performed by SRL. She will also be responsible for training the field and telephone survey
staff. Dr. Pratap will ensure the smooth transfer of data from field surveys to SRL, and from
SRL to the telephone follow-up team. At field study events, she will serve as field data
coordinator, and will ensure the accurate compilation of consent documents, the assignment of
case ID’s, unique identifiers for study participants, and the smooth flow of participants through
the study process. Throughout data collection periods, the Survey Project Manager will track
study enrollment, study completion by participants, and attrition from the study. The Survey
Project Manager will coordinate staffing of the survey teams with the field coordinator.
14.5 Clinical Project Manager
Jacqueline Wuellner, RN, MPH, as the Clinical Project Manager will be responsible for the
staffing and training of clinicians who will conduct field and home clinical evaluations, and the
staff who will collect stool samples from homes of study participants. The Clinical Project
Manager will be responsible for communications with the managers of the microbiology
laboratories that will analyze medical samples. Together with the study Quality Manager, she
will be responsible for establishing the identification and tracking procedures for medical
samples.
CHEERS Overview
40
July 2008
14.6 Water Project Manager
Margit Javor, MS, is the Water Project Manager, and will be responsible for the development of
the water sampling protocol, standard operating procedures (SOPs), and the training of field staff
to implement the protocol and SOPs. The water data manager will work closely with the field
manager and quality manager to review data from the analytic laboratories to identify and
promptly address deviations from the data quality objectives for water quality measurements.
The water data manager will be responsible for staff that performs quality monitoring and water
chemistry measures, as well as the training of all water sampling staff. The Water Project
Manager will communicate with the laboratories that analyze water samples to ensure the prompt
delivery of chain of custody forms and laboratory results.
14.7 Field manager
The field manager will be responsible for the logistics of fieldwork. Todd Schoonover, MS,
CIH, will ensure the smooth functioning of the water sampling portion of the study, and will be
responsible for procuring and maintaining major study pieces of equipment, such as vehicles,
boats, pumps, centrifuges, and refrigerators. He will ensure the transport of water samples to the
commercial analytic laboratories. The field manager works closely with the Water Project
Manager to ensure the implementation of the water sampling protocol by field staff. This will
involve preparing all necessary timetables, labels, materials, and equipment for each day of field
sampling. Mr. Schoonover will provide the water sampling schedule to the analytic laboratories
and provide timely notification regarding any modifications to that schedule. The decision to
cancel a day of field work due to a high probability (80% chance) of thunderstorms will be made
by the field manager.
14.8 Supply manager
Nicholas “Buck” Hanson, MPH, will be the project supply manager. He will be responsible for
training and supervising staff who prepare the equipment and supplies for the field study. He
will send mailings (including stool sample collection kits, when indicated) to study participants.
He will monitor study supply inventories, order additional supplies as needed, and ensure
delivery of supplies to the field. He will also have certain data management responsibilities.
CHEERS Overview
41
July 2008
14.9 Biostatistician
Li Liu, PhD, will be responsible for merging study data from diverse sources into a single dataset
for analysis Dr. Liu will perform interim analyses following each recreation season, as well as a
final analysis after all health data has been collected. She will review data analysis plans
developed by members of the research team who will conduct parts of the data analysis, and
provide consultation.
14.10 Fiscal management
Anita Shaperd, of the Division of Epidemiology and Biostatistics at the UIC SPH, will
coordinate the fiscal aspects of the study, including purchasing, accounting, and subcontracting.
The fiscal manager will work closely with the personnel/human resources manager in the
Division to hire and pay study personnel.
14.11 Internal Consultants
Daniel Hryhorczuk, MD, MPH; Mark Dworkin, MD, MPH; Ronald Hershow, MD, MPH; and
Leslie Stayner, PhD, have all assisted with the development of the study protocol. They will
continue to advise on the implementation, analysis, and interpretation of results of the study.
14.12 WERF Science Advisory Board
A group of independent, nationally-recognized experts on water microbiology, the epidemiology
of recreational water-borne illness, infectious diseases, waste water treatment, and related fields,
was convened by the Water Environment Research Foundation (WERF) to conduct peer review
of the study before it was launched in the field. The same group now functions as a science
advisory board, reviewing study progress, quality data and proposed changes to the study
protocol. Lola Olabode of WERF coordinates the review/science advisory group.
14.13 Friends of the Chicago River
Friends of the Chicago River (FCR) works to preserve, protect and foster the vitality of the
Chicago River. FCR is trusted and respected by users of the Chicago River and will work to
promote publicity about the CHEERS study among river users. Margaret Frisbie, Executive
Director of FCR, will be responsible for working with UIC to disseminate the publicity message
among river users.
CHEERS Overview
42
July 2008
14.14 MWRDGC Liaison
Thomas Granato, PhD, will be the MWRDGC’s contact for communications with UIC. He will
coordinate the MWRDGC’s review of this protocol and QAPPs, the external scientific review, a
local stakeholder review, and WERF/Science Advisory Board-UIC meetings. Dr. Granato will
ensure the transfer of data collected by the MWRDGC, such as water quality measures
monitored along the CAWs, the CSOs, water flow through the controlling works and pumping
stations, and effluent discharge from the WRPs.
CHEERS Overview
43
July 2008
Study director
S. Dorevitch, MD, MPH
Field study support
Quality Manager
P. Scheff, PhD
Survey project
manager
P. Pratap, PhD
Clinical project
manager
J. Wuellner, RN
Water project
manager
M. Javor, MS
Fiscal Management
A. Shaperd
Data Analysis
L. Liu, PhD
UIC SPH
consultants
MWRDGC liaison
T. Granato, PhD
CHEERS Project Manager
S. Wuellner, MS
Field manager
T. Schoonover,
MS, CIH
Recruitment
manager
A. DeLaquil, BS
WERF Science
Advisory Board
L. Olabode
Supply manager
N. Hanson, MPH
Personnel Manager
K. Fodor
Figure 6: CHEERS project management
15. Communications Plan
15.1 General
The study director, quality manager, project managers, field manager, and their assistants
meet each week to discuss the data quality objectives and any issues or concerns regarding
field work and logistics. The study maintains an internal website for sharing documents,
housing training materials, and posting schedules. All study managers have access to the
website. In addition to this meeting of the core leadership team, “water group” meetings and
“interview group” meetings will be held on a weekly basis, with the project coordinator and
assistant study directors planning upcoming events and reviewing quality data (such as
unusual occurrence reports) with project staff. Communications between the project
managers and the project staff is supported by a “Shiftboard” website. That site is used for
scheduling and general communications purposes. A central element of the CHEERS
training manual includes lines of communications and all personnel are aware of whom to
contact with questions.
15.2 Field work
Pre-paid cellular phone cards are provided to field supervisors and onsite survey data
managers to facilitate field communications and to allow supervisors to coordinate activities
and address problems as quickly as possible. Members of the interview/recruitment team
bring questions or concerns to the enrollment onsite data manager. In the event that the data
managers have questions or concerns, they contact the day’s field interview supervisor.
Members of the water sampling team bring concerns to the field water supervisor. Questions
from the field supervisors re brought to the project coordinator, who consults with the study
director when necessary. All field staff are provided with a list of contact names and phone
numbers that includes daily field supervisor and study director contact information, as well as
contact Information for laboratories and municipalities with which we work. Before each
shift, a briefing is conducted by the field supervisor to address any new or ongoing issues.
CHEERS Overview
44
July 2008
References
1.
CDM. Use Attainability Analsysis (Draft). 2004.
2.
Illinois EPA. Pre-filed testimony of Rob Sulski. 2007.
3.
CDM. Chicago Area Waterway Use Attainability Analysis. 2007.
4.
Fewtrell L, Godfree AF, Jones F, Kay D, Salmon RL, Wyer MD. Health effects of white-
water canoeing. Lancet 1992; 339:1587-9.
5.
Fewtrell L, Kay D, Salmon RL, Wyer MD, Newman G, Bowering G. The health effects
of low-contact water activities in fresh and estuarine waters. Journal of the Institute of
Water and Environmental Management 1994; 81:97-101.
6.
Lee JV, Dawson SR, Ward S, Surman SB, Neal KR. Bacteriophages are a better indicator
of illness rates than bacteria amongst users of a white water course fed by a lowland
river. Water Sci Technol 1997; 35:165-70.
7.
Gray JJ, Green J, Cunliffe C, Gallimore C, Lee JV, Neal K, et al. Mixed genogroup
SRSV infections among a party of canoeists exposed to contaminated recreational water.
J Med Virol 1997; 52:425-9.
8.
Appleton CC, Bailey IW. Canoeists and waterborne diseases in South Africa. S Afr Med
J 1990; 78:323-6.
9.
Taylor MB, Becker PJ, Van Rensburg EJ, Harris BN, Bailey IW, Grabow WO. A
serosurvey of water-borne pathogens amongst canoeists in South Africa. Epidemiol
Infect 1995; 115:299-307.
10.
Pruss A. Review of epidemiological studies on health effects from exposure to
recreational water. Int J Epidemiol 1998; 27:1-9.
11.
Wade TJ, Pai N, Eisenberg JN, Colford JM, Jr. Do U.S. Environmental Protection
Agency water quality guidelines for recreational waters prevent gastrointestinal illness?
A systematic review and meta-analysis. Environ Health Perspect 2003; 111:1102-9.
12.
Stevenson AH. Studies of bathing water quality and health. Am J Public Health 1953;
43:529-38.
13.
Cabelli VJ, Dufour AP, Levin MA, McCabe LJ, Haberman PW. Relationship of
microbial indicators to health effects at marine bathing beaches. Am J Public Health
1979; 69:690-6.
CHEERS Overview
45
July 2008
14.
Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. Swimming-associated gastroenteritis and
water quality. Am J Epidemiol 1982; 115:606-16.
15.
Dufour AP. Bacterial indicators of recreational water quality. Can J Public Health 1984;
75:49-56.
16.
Colford JM, Jr., Wade TJ, Schiff KC, Wright CC, Griffith JF, Sandhu SK, et al. Water
quality indicators and the risk of illness at beaches with nonpoint sources of fecal
contamination. Epidemiology 2007; 18:27-35.
17.
Wade TJ, Calderon RL, Brenner KP, Sams E, Beach M, Haugland R, et al. High
sensitivity of children to swimming-associated gastrointestinal illness: results using a
rapid assay of recreational water quality. Epidemiology 2008; 19:375-83.
18.
Wade TJ, Calderon RL, Sams E, Beach M, Brenner KP, Williams AH, et al. Rapidly
measured indicators of recreational water quality are predictive of swimming-associated
gastrointestinal illness. Environ Health Perspect 2006; 114:24-8.
19.
Fleisher JM, Jones F, Kay D, Stanwell-Smith R, Wyer M, Morano R. Water and non-
water-related risk factors for gastroenteritis among bathers exposed to sewage-
contaminated marine waters. Int J Epidemiol 1993; 22:698-708.
20.
Fleisher JM, Kay D, Salmon RL, Jones F, Wyer MD, Godfree AF. Marine waters
contaminated with domestic sewage: nonenteric illnesses associated with bather exposure
in the United Kingdom. Am J Public Health 1996; 86:1228-34.
21.
Kay D, Fleisher JM, Salmon RL, Jones F, Wyer MD, Godfree AF, et al. Predicting
likelihood of gastroenteritis from sea bathing: results from randomised exposure. Lancet
1994; 344:905-9.
22.
Wiedenmann A, Kruger P, Dietz K, Lopez-Pila JM, Szewzyk R, Botzenhart K. A
randomized controlled trial assessing infectious disease risks from bathing in fresh
recreational waters in relation to the concentration of Escherichia coli, intestinal
enterococci, Clostridium perfringens, and somatic coliphages. Environ Health Perspect
2006; 114:228-36.
23.
Jessop EG, Horsley SD, Wood L. Recreational use of inland water and health: are
Windermere and Coniston water a health hazard? Public Health 1985; 99:338-42.
CHEERS Overview
46
July 2008
24.
Dziuban EJ, Liang JL, Craun GF, Hill V, Yu PA, Painter J, et al. Surveillance for
waterborne disease and outbreaks associated with recreational water--United States,
2003-2004. MMWR Surveill Summ 2006; 55:1-30.
25.
Yoder JS, Blackburn BG, Craun GF, Hill V, Levy DA, Chen N, et al. Surveillance for
waterborne-disease outbreaks associated with recreational water--United States, 2001-
2002. MMWR Surveill Summ 2004; 53:1-22.
26.
MWRDGC. Expert review report regarding United States Enviornmental Protection
Agency's water quality criteria for bacteria - applicaiton to secondary contact criteria.
Chicago, IL, 2006.
27.
GeosyntechConsultants. Dry and wet weather risk assessment of human health impacts of
disinfection versus no disinfection of the Chicago Area Waterways System (CWS). 2008.
28.
Dufour AP, Evans O, Behymer TD, Cantu R. Water ingestion during swimming activities
in a pool: a pilot study. J Water Health 2006; 4:425-30.
29.
Black S, Shinefield H, Hansen J, Lewis E, Su L, Coplan P. A post-licensure evaluation of
the safety of inactivated hepatitis A vaccine (VAQTA, Merck) in children and adults.
Vaccine 2004; 22:766-72.
30.
Jones F, Kay D, Stanwell-Smith R, Wyer M. Results of the first pilot scale controlled
cohort epidemiological observation into the possible health effects of bathing in seawater
and Landland Bay, Swansea. J Inst Water Env Management 1991:91-8.
31.
Mandell G, Bennet J, Dolin R. Principles and Syndromes of Enteric Infections. In:
Principles and Practices of Infectious Diseases 6ed. Philadelphia, PA: Elsevier Inc.; 2005.
32.
Wymer LJ, Dufour AP, Calderon RL, Wade TJ, Beach M. Comment on "Derivation of
numerical values for the World Health Organization guidelines for recreational waters".
Water Res 2005; 39:2774-7.
CHEERS Overview
47
July 2008
CHEERS Overview Document
Appendix 1: Study Logo
CHEERS Overview Document
Appendix 2: Recruitment Flier
IRB approval
box
$TAR?$A
F F ffi
S VA
L
sxprnes
JUN
?
'1
?ilijfi
JUN
2
0
Z00g
UitllV#i$;Ti
$F
tLr-rr{{Jlti
fft
CH|CAG0
lfil$Tt'ru
ri$$,rAL
i{EVt
Ew
g0AR0
?#nrt{*^ft
{ft{{&##,
-$&#ffi:{#'
PLEASE
NOTE:
THE
CHEERS
STUDY
IS
IN
PROGRESS
TODAY
AT
THE LAKE
As
part
of our
efforts
to improve
public
health,
the
lJniversity
of
Illinois
at Chicago
(UIC)
School
of
pubiic
Health
is conducting
a
three-year
study
called
"CHEERS,"
'lhe
Chicago,
Health.
Environmental
Exposure
and
Recreation
Stucly.
In this
study,
researchers
will evaluate
the
health
of
people
who exercise
o.,i.Jc,orr.
some
who have
water
contact,
and some
who
do
not.
This
research
will take
place
on
different
clays,
at dif-ferept
locations
on or
near
Lake
Michigan,
the Chicago
and
Calumet
River
systems'
and
other
rivers
apd
lagoons
in the
Chicagg
area.
If
you
participate
in
two short
survey
interviews
today,
you
will
receive
a
$15
gift card
and
a
T-shirt
today.
After
completing
three
10
minute
telephone
follow-
up
intervier",
on.i
the
next
three
weekso
a
check
for
$35
check
wilt
be
mailed
to
you
after
your last
home
telephone
interview.
If
you
are
selected
for
a
follow-up
home
visit
by
research
nurses'
you
will
receive
a
check
for
an
additional
$75.
purpose
of
the Survey.
Toclay's
survey
is
part
of a
research
project that
will
help
to better
understand
the
ielationship
between
outdoor
recreation,
water
quality, and,
people's
health'
When
the
research
project
is completed,
the
results
will
help
develop
better
guidelines
for recreational
water
quality.
your
Cooperation
is
Extremely
Valuable
and
Will
be
Greatly
Appreciated.
of
course,
your
participation
in
today's
survey
is
voluntary
-
whether
you
are
interviewed
today
is entirely
up
to
you' All
ihe
int'brmation
that
you
provide
will
be
kept
confidential.
what
to
Expect
Today.
All
interviewers
are
wearing
CHEERS
t-shirts
and
name
badges.
If
you
are
eligible.
statf
will
interview
your for
about
3
minutes.
The
second
part
of
the
interview
will be
conducted
afti,
yo.r finish
your outdooi
activity
today-
This
will
take
about
8-
10
rninutes'
When
you
go home.
We
will
contact
you
three
times
over
the
next
three
weeks.
to ask
you
about
your
health.
on
completion
of
the
last
telephone
interview,
we
will
send
you
a
$35
check.
If
you
are
selected
fbr
a
home
visit.
we may
request
a
stool
sample,
or
research
staff
may
visit
you at
home'
If
you were
to
get sick.
those
results
couid
irelp
you
and
your doctor
by
identifying
germs that
may
have
made
you
ill'
you
would
receive
an
additional
check
for
$75
for
the
home
visit'
Local
Support
for
GHEERS:
Several
cities,
including
chicago,
Evanston,
Skokie,
worth.
and
Alsip
are
helpir-rg
ulC
with this
project.
For
any
questions
call
the
CHEERS
project coordinator
at
(312)
99G2094'
wE
THANK
yoil
rN
ADVANCE
FoR
YouR
TIME
AND
cooPERATIoN!!
#tr'ffi
CHEERS
flyer,
Lake,
Versioti
1,112312008
CHEERS Overview Document
Appendix 3: Information for clubs, team
organizers and vendors
1
?
CHEE Ca Ha a E Ea
and it is being conducted by
Dr. Sam Dorevitch of The University of Illinois at Chicago (UIC) School of Public Health.
?
This study is being funded by the Metropolitan Water Reclamation District of Greater Chicago.
?
CHEERS is a research study about the health of people in the Chicago area who participate in outdoor
activities. We are interested in canoers, kayakers, rowers and fishermen on waters in the Chicago area, as
well as, people engaging in outdoor activities
a ’ a
(such as bicycling and jogging) near
local lakes, lagoons and rivers.
?
Our goal is to assess the health of people who participate in different outdoor activities on and around local
lakes, lagoons, rivers and channels.
?
We want to know if health is linked with water quality, especially among people like you who participate in
outdoor recreational activities in and around the Chicago waterways.
?
The results of this study could be used for developing better environmental water quality standards to
improve public health.
?
The study does not involve swimmers because many research studies have evaluated the link between water
quality and swimming. This study addresses other forms of water recreation.
?
No information provided by research participants during the research will be disclosed to non-research staff.
We respect the privacy of research participants. All computer files with will be password-protected. When
the results of the research are published or discussed in conferences, no information will be included that
would reveal the identity of study participants.
?
The research study will last about 3 years. Individuals who will enroll will answer a few questions in person
when they enroll in the
. W’ a
them by phone 2, 5 and 21 days from the date of enrollment to
check on their health. When they have completed the last telephone interview, 21 days later they will be
eligible to partake in the study again.
?
We will explain the study to potential participants in detail. Any questions they may have will be answered.
Then, we will ask them a few questions to see if they are eligible to be in this study.
?
Anyone who participates will be given a free CHEERS T-shirt and a $15 Target gift card after finishing the
surveys. At the end of 21 days of participation we will send participants a $35 check for their time and effort.
?
Some people will be selected for an optional home visit. We will provide those selected with an extra $75.
?
This is not an official recruiting document. Anyone who wants to be in the study will be given a consent
form, and we will answer any questions they may have about this research.
?
We look forward to the opportunity to work with your organization on this environmental health study! For
any questions, please contact Amelia DeLaquil at adelaquil@gmail.com.
General information about CHEERS: For
clubs, teams, organizations, and vendors
CHEERS Overview Document
Appendix 4: Water Quality Frequently Asked
Questions sheet
Water
Qualitv
Information
for the
Public
I
{#'$Y## {'&{f*,##W #,W#M'{#
IRB approval
box
sranrsA
F
p
R
S
VA
L
rxprnrs
JUN 2 i
;n;;c
JU"i\i
Z
0
Z00g
-
-
I
[tTi+i,
l,$'*it'
#?lE
fi
'
rgi'f;A
* u
The Chicaso
River and Calumet
River svstem
Tlrese river systems
are
managed by the Metropolitan
Water Reclamation
District of Greater
Chicago.
Swimming,
jet
skiing
and
water
skiing
not allowed in these
waterways,
but
activities
such
as boatirrg,
fishiug, and
rowiug are allowed. For
more inforrnatiott about the
waterways.
visit:[1p1rryWy.$_!-r._LSi]gaA!:qa\$lC1L4y1qg
For
more infbrrnation
about the Metropolitan
Water
Reclamatiou District,
visit;
It1p...-,1ltUr.
._Ln
s,rd gc:.tlst. i I.
usi
Lakes
and Beaches
For
more intbrnratiorr
about beach
health arrd beaclr
closirrgs
irr
Chicago.
please
visit the
rvebsite
of the Chicago
Park District Beach
Report
http:i,/c!ricasep41:[di.lllig'1.C"e]ff/iftdel.cfnl
rseactiorti
inclefC.ltU/-fqWaglj-Q.-niir-v-ig*-1q-p-Q$jlgn-Q
For
more
information about beach
health,
please
visit to
website
of
the Illinois Department
of
Public
Health Beach
Information
Irttl,rr.uruujdurlo!.
For
rnore irrforr.natiou
about
water quality on
Lake Michigan,
visit
the
website
of the
A I I i a n c e
fo r t
h
e G
reat [,ake
s :
!i!-h;lly'
w
W.glg-?i-tl
iik"e-,:.,Ql.gi.
[atine
fish
causht
in Illinois
rivers and
lakes
General
information,
2007 :
!-rup:ll-r,lw-rr
jslr-dstilir-1s.p-sljaskrqrl$d&$A!vrisvZ"elQ0?.Q2.-0j+:-df
Great
lakes tlsh:
ltllpt'iu:yl
l
The Chicago
River system:
!rtm:/lw--r.y:r.idpli.,ltalqi.-d,U,llstt-\hqallhlfdtitdyr,c-lt!9r1g-111.!1'9r,,rr
CHEERS Overview Document
Appendix 5
Recruitment sites: CAWS
CHEERS Overview: CAWS recruiting locations
1
2008 Recruiting Site Specifications-CAWS
Water Activity
Non-water Activity
Site
GPS
Coordinates
Canoeing
Kayaking
Rowing
Powerboating
Fishing
Run/walking
Bicycling
Golfing
Playing ball
Recruitment Area and Tent
Location
CAWS: Calumet System
Village of Alsip
Boat Launch
N: 41°39.947’
W: 87°45.342’
9
9
Village of
Worth Boat
Launch and
adjacent
aeration station
N: 41°40.763’
W: 87°48.157’
N: 41°40.704’
W: 87°47.765’
9 9 9 9 9
Riverdale
Marina
N: 41°39.393’
W: 87°38.733’
9
9
CAWS: North Branch Chicago
River
North Ave, East
N: 41°54.607’
W: 87°93.283’
9
9
CHEERS Overview: CAWS recruiting locations
2
North Ave,
West
N: 41°54.564’
W: 87°39.539’
9
Clark Park
N: 41°56.589’
W: 87°41.701’
9
9
9 9
9
West River
Park/Ronan
Park
N: 41°58.426’
W: 87°42.286’
9
9
9
Skokie Rowing
Center
N: 42°01.668’
W: 87°42.572’
9
9 9
9 9
CAWS: Main Stem
Columbus &
Wacker
N: 41°53.295’
W. 87°37.217’
9
9
9
CAWS: South Branch Chicago
River
Ping Tom Park
N: 41°51.820’
W. 87°38.039’
9
9 9
9 9
CHEERS Overview: CAWS recruiting locations
3
CHEERS Overview: CAWS recruiting locations
4
CHEERS Overview Document
Appendix 6
Recruitment sites: GUW
CHEERS Overview: GUW recruiting sites
1
Water Activity
Non-water Activity
Site
GPS
Coordinates
Canoeing
Kayaking
Rowing
Powerboating
Fishing
Run/walking
Bicycling
Golfing
Playing ball
Recruitment Area and Tent
Location
GUW: Lake Michigan
Belmont Harbor
N: 41°56.475’
W: 87°38.230’
9
9
9
9
Burnham
Harbor
N: 41°51.628’
W: 87°36.778’
9
9
9
9
Diversey
Harbor
N: 41°55.511’
W: 87°38.072’
9
9
9
Jackson Park
Harbor
N: 41°46.439’
W: 87°34.227’
9 9 9 9 9
Leone Beach
N: 42°0.466’
W: 87°39.431’
9
9
9
CHEERS Overview: GUW recruiting sites
2
Montrose
Harbor/Wilson
Beach
N: 41°57.450’
W: 87°38.302’
9
9 9 9 9
9
Solidarity Drive
N: 41°51.995’
W: 87°36.593’
9
9
9
9
GUW: Area Waterways
Busse Lake
Boating Center
N: 42°01.467’
W: 88°00.900’
9
9
9 9 9 9
Crystal Lake
N: 42°14.107’
W: 88°22.170’
9
Fox River, St.
Charles
N: 41°54.975’
W: 88°18.855’
9
9
9
Des Plaines
River,
Libertyville –
Mt. Prospect
N: 42°17.321’
W: 87°56.256’
9
9
CHEERS Overview: GUW recruiting sites
3
Lincoln Park
Boat Club
N: 41°55.452’
W: 87°37.927’
9 9
9 9 9
Hammel
Woods,
Shorewood
N: 41°31.353’
W: 88°11.647’
9
9
Skokie Lagoons
N:42°07.368’
W: 87°46.367’
9 9
9 9
9
Tampier Lake
Boating Center
N: 41°39.037’
W: 87°53.868’
9
9
9 9 9 9
CHEERS Overview: GUW recruiting sites
4
CHEERS Overview Document
Appendix 7
2007 Recruiting Schedule
DATE
SITE
8/4/2007
CAWS: Alsip Boat Launch
8/11/2007
GUW: Skokie Lagoons
8/12/2007
CAWS: Skokie Rowing Center
8/17/2007
CAWS: Worth Boat Launch
8/18/2007
GUW: Skokie Lagoons
8/19/2007
CAWS: Skokie Rowing Center
8/24/2007
GUW: Montrose Beach
8/25/2007
GUW: Diversey Harbor
8/26/2007
CAWS: Clark Park
8/31/2007
CAWS: River Park
9/1/2007
GUW: Montrose Beach
9/8/2007
GUW: Leone Beach
9/8/2007
CAWS: Skokie Rowing Center
9/9/2007
CAWS: Skokie Rowing Center
9/9/2007
CAWS: Clark Park
9/15/2007
GUW: Montrose Beach
9/16/2007
CAWS: Clark Park - Ping Tom (Flatwater Classic)
9/22/2007
CAWS: Worth Boat Launch
9/22/2007
CAWS: Alsip Boat Launch
9/23/2007
GUW: Jackson Park Harbor
9/24/2007
CAWS: Skokie Rowing Center
9/29/2007
GUW: Montrose Beach
9/30/2007
GUW: Montrose Beach
10/1/2007
CAWS: Skokie Rowing Center
10/6/2007
GUW: Montrose Beach
10/7/2007
GUW: Jackson Park Harbor
10/9/2007
CAWS: Skokie Rowing Center
10/13/2007
GUW: Montrose Beach
10/14/2007
GUW: Crystal Lake
10/16/2007
GUW: Crystal Lake
10/23/2007
CAWS: North Ave @ Kingsbury
11/5/2007
CAWS: North Ave @ Kingsbury
CHEERS Overview Document
Appendix 8
2008 Recruiting Schedule
CHEERS Overview-Appendix 8
1
Date
Location
Notes
3/10/2008 CAWS: North Ave @ Kingsbury
3/18/2008 CAWS: North Ave @ Magnolia
3/22/2008 CAWS: North Ave @ Magnolia
team canceled
3/24/2008 CAWS: Skokie Rowing Center
3/26/2008 CAWS: North Ave @ Magnolia
3/31/2008 CAWS: Skokie Rowing Center
4/1/2008 CAWS: Skokie Rowing Center
4/5/2008 GUW: Diversey Harbor
4/7/2008 CAWS: North Ave @ Kingsbury
4/13/2008 GUW: Diversey Harbor
4/14/2008 CAWS: North Ave @ Magnolia
4/19/2008 GUW: DuPage River - Hammel Woods
4/20/2008 GUW: Skokie Lagoons
4/21/2008 CAWS: Skokie Rowing Center
4/22/2008 CAWS: Skokie Rowing Center
4/23/2008 GUW: Crystal Lake
4/26/2008 GUW: Diversey Harbor
4/27/2008 GUW: Upper Des Plaines River
5/3/2008 GUW: Skokie Lagoons
5/4/2008 GUW: Montrose Beach
5/10/2008 GUW: Jackso Park Harbor
5/11/2008 GUW: Burnham Harbor
rain cancellation
5/12/2008 CAWS: North Ave @ Magnolia
5/13/2008 CAWS: North Ave @ Magnolia
canceled
5/17/2008 GUW: Skokie Lagoons
5/18/2008 GUW: Des Plaines River - Libertyville
5/22/2008 CAWS: North Ave @ Magnolia
5/22/2008 GUW: Jackso Park Harbor
5/24/2008 CAWS: Skokie Rowing Center
5/24/2008 CAWS: River Park
5/24/2008 CAWS: Clark Park
5/24/2008 CAWS: North Ave @ Magnolia
5/25/2008 CAWS: Alsip Boat Launch
5/25/2008 CAWS: Riverdale Marina
5/25/2008 CAWS: Worth Boat Launch
5/26/2008 GUW: Montrose Beach
5/26/2008 GUW: Diversey Harbor
5/26/2008 GUW: Jackso Park Harbor
5/30/2008 CAWS: Alsip Boat Launch
rain cancellation
5/30/2008 CAWS: Riverdale Marina
rain cancellation
5/30/2008 CAWS: Worth Boat Launch
rain cancellation
5/31/2008 GUW: Busse Lake Boating Center
5/31/2008 GUW: Diversey Harbor
CHEERS Overview-Appendix 8
2
5/31/2008 GUW: Leone Beach
5/31/2008 GUW: Montrose Beach
6/1/2008 GUW: Crystal Lake
6/1/2008 GUW: Skokie Lagoons
6/6/2008 CAWS: Clark Park
rain cancellation
6/6/2008 CAWS: Skokie Rowing Center
rain cancellation
6/7/2008 GUW: Fox River - St. Charles
6/7/2008 GUW: Tampier Lake Boating Center
6/8/2008 GUW: Busse Lake Boating Center
6/8/2008 GUW: Fox River - St. Charles
6/9/2008 CAWS: Skokie Rowing Center
6/10/2008 CAWS: Skokie Rowing Center
6/13/2008 CAWS: Alsip Boat Launch
6/13/2008 CAWS: Riverdale Marina
6/13/2008 CAWS: Worth Boat Launch
6/14/2008 GUW: Belmont Harbor
6/14/2008 GUW: Diversey Harbor
6/14/2008 GUW: Montrose Beach
6/14/2008 GUW: Skokie Lagoons
6/15/2008 CAWS: Clark Park
6/15/2008 CAWS: North Ave @ Magnolia
6/15/2008 CAWS: River Park
6/15/2008 CAWS: Skokie Rowing Center
6/17/2008 CAWS: North Ave @ Kingsbury
6/20/2008 CAWS: Alsip Boat Launch
6/20/2008 CAWS: Riverdale Marina
6/20/2008 CAWS: Worth Boat Launch
6/21/2008 CAWS: Clark Park
6/21/2008 CAWS: Evanston Ecology Center
6/21/2008 CAWS: North Ave @ Magnolia
6/21/2008 CAWS: River Park
6/21/2008 CAWS: Skokie Rowing Center
6/22/2008 CAWS: Alsip Boat Launch
6/22/2008 CAWS: Riverdale Marina
6/22/2008 CAWS: Worth Boat Launch
6/22/2008 GUW: Tampier Lake Boating Center
6/27/2008 CAWS: Clark Park
6/27/2008 CAWS: River Fishing Festival
6/27/2008 CAWS: Skokie Rowing Center
6/28/2008 CAWS: Alsip Boat Launch
6/28/2008 CAWS: Riverdale Marina
6/28/2008 CAWS: Worth Boat Launch
6/28/2008 GUW: Busse Lake Boating Center
6/29/2008 CAWS: Clark Park
6/29/2008 CAWS: North Ave @ Magnolia
6/29/2008 CAWS: Skokie Rowing Center
CHEERS Overview-Appendix 8
3
6/29/2008 GUW: Skokie Lagoons
7/4/2008 GUW: Burnham Harbor
7/4/2008 GUW: Leone Beach
7/4/2008 GUW: Montrose Beach
7/4/2008 GUW: Solidarity Drive
7/5/2008 CAWS: Clark Park
7/5/2008 CAWS: North Ave @ Magnolia
7/5/2008 CAWS: River Park
7/5/2008 CAWS: Skokie Rowing Center
7/5/2008 GUW: Lincoln Park Boat Club
7/6/2008 CAWS: Alsip Boat Launch
7/6/2008 CAWS: Riverdale Marina
7/6/2008 CAWS: Worth Boat Launch
7/6/2008 GUW: Busse Lake Boating Center
7/7/2008 CAWS: River Fishing Festival
7/11/2008 CAWS: Clark Park
7/11/2008 CAWS: North Ave @ Magnolia
7/11/2008 CAWS: River Fishing Festival
7/11/2008 CAWS: Skokie Rowing Center
7/12/2008 GUW: Lake Arlington
rain cancellation
7/12/2008 GUW: Diversey Harbor
rain cancellation
7/12/2008 GUW: Lincoln Park Boat Club
7/12/2008 GUW: Montrose Beach
rain cancellation
7/13/2008 CAWS: Clark Park
7/13/2008 CAWS: North Ave @ Magnolia
7/13/2008 CAWS: Skokie Rowing Center
7/13/2008 GUW: Skokie Lagoons
7/18/2008 GUW: Belmont Harbor
7/18/2008 GUW: Busse Lake Boating Center
7/18/2008 GUW: Diversey Harbor
7/18/2008 GUW: Montrose Beach
7/19/2008 CAWS: Alsip Boat Launch
rain cancellation
7/19/2008 CAWS: Worth Boat Launch
rain cancellation
7/19/2008 GUW: Tampier Lake Boating Center
rain cancellation
7/20/2008 CAWS: Clark Park
7/20/2008 CAWS: North Ave @ Magnolia
7/20/2008 CAWS: River Park
7/20/2008 CAWS: Skokie Rowing Center
7/20/2008 GUW: Skokie Lagoons
7/25/2008 CAWS: Clark Park
7/25/2008 GUW: Diversey Harbor
7/25/2008 GUW: Montrose Beach
7/25/2008 GUW: Solidarity Drive
7/26/2008 CAWS: Clark Park
7/26/2008 CAWS: North Ave @ Magnolia
7/26/2008 CAWS: Ping Tom Park
CHEERS Overview-Appendix 8
4
7/27/2008 CAWS: Alsip Boat Launch
7/27/2008 CAWS: Worth Boat Launch
7/27/2008 GUW: Tampier Lake Boating Center
Overview Appendix 8: 2008 CHEERS recruiting schedule
CHEERS Overview-Appendix 8
5
CHEERS: The Chicago Health, Environmental
Exposure and Recreation Study
QUALITY MANAGEMENT PLAN
Samuel Dorevitch, MD, MPH
Division of Environmental and Occupational Health Sciences
Division of Epidemiology and Biostatistics
University of Illinois at Chicago School of Public Health
2121 W. Taylor, M/C 922
Chicago, IL 60612
__________________________________
______________
Samuel Dorevitch, Study Director
Date
__________________________________
_______________
Peter A. Scheff, Project Quality Manager
Date
Table of Contents
Section
Page
I. INTRODUCTION
1
II. MANAGEMENT AND ORGANIZATION
2
III. QUALITY SYSTEM COMPONENTS
11
IV. PERSONNEL QUALIFICATION AND TRAINING
16
V. PROCUREMENT OF ITEMS AND SERVICES
17
VI. DOCUMENTS AND RECORDS
17
VII. COMPUTER HARDWARE AND SOFTWARE
18
VIII. PLANNING
18
IX. IMPLEMENTATION OF WORK PROCESSES
19
X. ASSESSMENT AND RESPONSE
19
XI. QUALITY IMPROVEMENT
20
List of Acronyms
CAWs
Chicago Area Waterways
CDC
Centers for Disease Control and Prevention
CSOs
Combined Sewer Overflows
DQO
Data quality objective
FCR
Friends of the Chicago River
IEPA
Illinois Environmental Protection Agency
IRB
Institutional Review Board
MWRDGC
Metropolitan Water Reclamation District of Greater Chicago
NEEAR
National Environmental and Epidemiological Assessment of
Recreational Water
QA
Quality assurance
QAPP
Quality Assurance Project Plan
QC
Quality Control
QM
Quality manager
QMP
Quality Management Plan
SPH
School of Public Health
SRL
Survey Research Laboratory
UAA
Use attainability analysis
UIC
University of Illinois at Chicago
USEPA
United States Environmental Protection Agency
WRP
Water Reclamation Plant
INTRODUCTION
This document was designed to conform with US Environmental Protection
Agency (EPA) quality requirements, which are in turn based on ANSI/ASQC E4-1994,
Specifications and Guidelines for Environmental Data Collection and Environmental
Technology Programs
. The QMP is designed to conform with EPA Requirements for
Quality Management Plans, EPA QA/R-2, which is based upon Part A of the ANSI
standard. The QMP provides a system-wide framework for conducting the three projects
described in their respective Quality Assurance Projects Plan (QAPP) documents. The
QAPPs, in turn are designed to conform with EPA Requirements for Quality Assurance
Project Plans, EPA QA/R-5, which is based upon Part B of the ANSI standard. Each
QAPP, in turn, is grounded in the principle of obtaining data of sufficient quality and
quantity to achieve the objectives of that particular project. Data quality objectives were
developed for each project using the EPA’s Guidance on Systematic Planning Using the
Data Quality Objectives Process, EPA QA/G4.
II.
MANAGEMENT and ORGANIZATION
1.
Organization’s policy on quality assurance
This Quality Management Plan (QMP) describes the systematic approach to
addressing quality developed by the UIC team for the conduct of the CAWs
epidemiologic study. This QMP documents the management practices, including quality
assurance (QA) and quality control (QC) activities, used to ensure that the results of
technical work are of the type and quality needed for their intended use. The University
of Illinois at Chicago School of Public Health, in general, and this research group in
particular, embrace the principles and practices of quality management, recognizing that
the data collected for this study are only of value to the degree that they meet quality
objectives. The systematic approach to quality is outlined below.
The overall objective of the quality system is to ensure that each of the three
component “projects” of the epidemiologic study produce data that will achieve their
specific objectives. The quality of data obtained by the three individual projects will be
promoted by establishing and implementing organization-wide practices for quality
control, personnel training, procurement of supplies and equipment, contracting to
extramural entities elements of the study, documentation, data management, work
process implementation, assessment, and quality improvement. Furthermore, this will
promote a culture of attention to quality and adherence to protocol throughout the
organization.
It is with the recognition that this research could potentially impact
environmental regulation, that we use US EPA quality guidelines to achieve quality
objectives.
Resources have been committed to support quality management at both
organization and project levels. On the organization level, the salary of the Quality
Manager and other staff who perform quality monitoring functions will be supported by
this project. Additionally, the directors of each of the three projects and the director of
the epidemiologic study will commit a substantial portion of their time addressing quality
on organization- and project-levels. Space, resources and personnel will be available for
all quality practices needed for data management, record keeping, procurement, and
personnel. Within individual projects, the budget will support all necessary training,
documentation, performance evaluations (such as use of field blanks, replicate samples,
positive controls, monitoring telephone survey staff), and instruments necessary to
monitor and enhance data quality.
2.
Organization of the research team
The organizational chart and description of responsibilities are presented in the CHEERS
Overview document.
3. Authorities of the Quality Manager and other QA staff
The Quality Manager (QM) will have authority to evaluate quality aspects of all data
generating activities. Project Managers will review all quality data with the QM, whether
the data were generated by UIC personnel or by subcontractors. The quality manager
will be independent of those who generate, compile and evaluate environmental data.
The Project Managers, as well as the QC manager of UIC’s subcontractors, know that
they will be asked to provide quality monitoring data to the UIC QM. Their work with
UIC is contingent on their compliance with requests from the QM additional quality data.
Likewise, they will be required to respond to quality improvement plans proposed by the
UIC QM. QM will have authority to conduct quality audits, review training and
documentation practices, and recommend modifications to the study protocols.
4. Technical activities supported by the QA system
4.1. Specific programs that require quality management controls
4.1.1. Analyses of water samples for
E. coli
and enterococci
4.1.2. Analyses of water samples for pathogens and coliphages
4.1.3. Participant enrollment and attrition
4.1.4. Survey data collection
4.1.5. Clinical evaluations of participants
4.1.6. Microbiologic evaluations of clinical specimens
4.1.7. Data management practices
4.2. Oversight of delegated, contracted or other extramural programs needed to assure
quality data
Extramural laboratories will produce data for this study. Those laboratories
include:
4.2.1. A commercial laboratory that will analyze water samples for
E. coli
and
enterococci (described in QAPP #1: Water sampling and analysis).
4.2.2. A commercial laboratory that will analyze water filtrate for pathogens and
coliphages (described in QAPP #1: Water sampling and analysis).
4.2.3. The Illinois Department of Public Health will analyze stool samples for
norovirus. (described in QAPP #3: Clinical examinations and microbial
evaluations)
4.3. Where and how internal coordination of QA and QC activities among the group’s
organization units needs to occur
Internal QA and QC activities among the organization’s units will be coordinated by
the QM through regular quality assurance meetings held in room 210 of the UIC SPH
West (the CHEERS study center). During the initial weeks of the field study, such
meetings will occur following each day of data collection. The frequency of quality
assurance meetings will subsequently decrease to once per week if ongoing quality
monitoring indicates that data quality objectives are being met. Such meetings will
be held during the data collection season only.
At weekly meetings, the QA staff, the study director, the Project Managers, and
the Field Manager will review the number of participants enrolled, by site; the
number of telephone follow-up calls completed, the number of home visits, the
number of positive cultures obtained from clinical specimens. Any problems with
equipment calibration or performance will be discussed. Additionally, unusual
occurrence reports will reviewed, and results of performance evaluations that did not
meet data quality objectives will be discussed. The performance of the system of
using notebook computers in the field, and the transfer of data from the field, to the
UIC Survey Research Laboratory (SRL), to the telephone call center, and back to
SRL will be reviewed.
At monthly reviews, quantitative summaries of water quality data will be presented,
as will results of positive clinical specimen cultures, and numbers of participants
enrolled by location, by study group. Results of QC data from water and clinical
microbiology laboratories will be presented.
Annual reviews are discussed on the following page under “Quality System
Components.”
5. Assuring implementation of quality system in all environmental programs
All management and staff of the epidemiologic study will undergo an orientation session
that addresses the importance of the quality system to this research. In that session,
either the study director, the project coordinator or the Quality Manager will explain now
critical it is that all activities in the study be conducted by protocol. Staff will be
encouraged to either contact a manager for help, or to refer to the relevant protocol or
operating procedure, rather than “guessing” or improvising about how to handle
uncertainty. All Project Managers and assistant project managers will receive a copy of
this quality management system. Personnel will be told that quality audits will occur
regularly, and that their practices and documentation will be reviewed. By providing
timely feedback to study staff about ongoing quality monitoring, a culture of quality will
be promoted.
6. Processes for solving disputes
The QM, rather than the study director, will be final authority for any disputes regarding
quality system requirements, procedures, assessments or corrective actions.
III.
QUALITY SYSTEM COMPONENTS
1. Principal components of the system and roles and implementation responsibilities for:
1.1. Documentation
Each project supported by this QMP will have its own QAPP which establishes
project-specific documentation requirements.
System-wide quality documentation
will include reports of weekly project managers’ meetings with the QM in attendance,
as well as annual summaries of quality monitoring. Copies of the QMP and QAPP
will be made available to all study personnel, and will be posted on a secure internal
website using UIC’s BlackBoard system.
1.2. Annual reviews and planning
Following the completion of data collection for the 2007 water recreation season, data
quality will be comprehensively reviewed. Participant recruitment, water sampling
and analysis, clinical microbiology results, and survey data, as well as the QC data
compiled by internal (UIC) and external laboratories will be reviewed. Results of
performance evaluation sample analyses (replicates, field blanks, positive controls),
holding times, and other project-specific quality benchmarks will be summarized.
Trends and deviations from data quality objectives will be reviewed. Quality
improvements for the 2008 water recreation season will be based on the annual
quality reviews. The QM and his staff will be responsible for the quality reviews,
which will be presented to the study director, the Project Managers, the quality
managers of laboratories within and external to UIC, to the study’s internal
consultants, and to the MWRDGC liaison. In addition to study managers and staff,
internal consultants, quality personnel of internal and external laboratories, internal
consultants, and the MWRDGC liaison will be invited to participate in the annual
reviews.
1.3. Management assessments
For each major data stream (water, survey and clinical), an “Unusual Occurrence
Report” will be completed each day of field data collection. Completing the form will
be the responsibility of the individual serving as a field data manager (for survey
data), the Field Manager (for water collection data), or the Clinical Project Manager
(for clinical microbiology data). The unusual occurrence report will be completed
after discussions with each member of the study team at the end of the day.
Qualitative information regarding possible deviations from protocol, unexpected
events, or problems in documentation, data collection, handling, transport will be
recorded. Additionally, Project Manages and the study director will meet with the
QM and the study director to identify and address system-wide and project-specific
quality problems. They will also participate in annual quality reviews and in quality
improvement planning.
1.4. Training
Job descriptions for study positions will define eligibility for hire. Subsequent to
hire, additional training will be required. Job descriptions and subsequent training
requirements will be developed by the study director, Project Managers, and will be
reviewed by the QM.
1.5. Systematic planning of projects
Projects within the larger study have been developed using the EPA “Guidance on
systematic planning using the data quality objective process” and the “EPA
Requirements for Quality Assurance Project Plans.” Additionally, input has been
sought from the MWRDGC, UIC consultants, and environmental researchers external
to this project. Additionally, during the design phase of the epidemiologic study,
overviews of the research were presented to Mr. Ephraim King of US EPA, Director
of the Office of Science and Technology in the Office Water, and his staff; to the
Science Advisory Board coordinated by the Water Environment Research
Foundation; at an Illinois EPA’s Use Attainability Analysis stakeholder meeting; at a
local advisory group on July 9, 2007; at the initial Science Advisory Board meeting
(“initial peer review”) organized by WERF on July 17-18, 2007; and at a follow-up
Science Advisory Board meeting on February, 27, 2008.
1.6. Project-specific quality documentation
Each QAPP supported by this Quality Management Plan includes documentation
requirements. Accurate and secure documentation is necessary to ensure the validity,
accuracy, precision, and integrity of the data.
Each Project Manager will be
responsible for ensuring compliance with documentation requirements of their
projects. Documentation requirements common to all projects will include chain of
custody documentation, storage of the results of laboratory analyses, and ensuring the
confidentiality of study participants. The QM will be responsible for regularly
reviewing compliance with documentation and other requirements of each project. .
1.7. Project and data assessments
All projects, as well as the study overall will be the subject of weekly, monthly and
annual assessments. The comparison of actual data quality to data quality objectives
will be assessed at these evaluations.
2. Tools for implementing each component of the quality system
2.1. This system-wide Quality Management Plan will be the basis for establishing and
reviewing quality management practices throughout each project within the larger
epidemiologic study.
2.2. Audits
2.2.1. Quality system audits will be performed by the QM and the project
coordinator. These audits will include qualitative evaluations of the field
and laboratory quality control systems. During the system audit, the quality
control activities actually performed or scheduled will be compared with
those specified in the three
2.2.2. QAPPs. The results of the system audits will include the reviews of the
following:
•
Completeness of the field records,
•
Completeness of the Chain-of-Custodian forms,
•
Safe storage the documents and backup of the computer files,
•
Adherence to training requirements
•
Instrument status and calibration records,
•
The status of other relevant laboratory equipment,
•
Identification of invalid data based on the specified accuracy, precision,
and comparability,
•
Any corrective actions and the results.
2.2.2. Performance Audits
A performance audit is a quantitative evaluation of the data collection and analysis
aspects of the project. For the projects involving microbial analyses of water samples and
microbial analyses of clinical specimens, data from performance evaluation samples
(such as replicates, blanks, and positive control or spike samples) will be analyzed. For
survey data, “mock subjects” will be enrolled in the study and will provide pre-
determined answers to specific questions, and the way such responses are recorded by
survey staff will be reviewed. The results will be used to evaluate the performance of the
data generation system.
2.3. Training plans
The training plans for staff will be reviewed by the QM. Documentation of the
implementation of training within the overall study will be reviewed by the QM. Any
deficits in training will be identified and brought to the attention of the Quality Manager
and the appropriate Project Manager.
2.4. QA Project Plans
One QAPP has been developed for each of the following major study elements:
•
Water sampling and analysis
•
Survey methods
•
Clinical examinations and microbiologic evaluations
These QAPPs will describe project management, data generation and acquisition,
assessment and oversight, and data validation and usability. The key function of each
QAPP is to ensure that data quality and quantity generated within each project will be
sufficient to meet study objectives. Each Project Manager will be responsible for
ensuring implementation of the QAPP specific to their project. The QA manager will
review each QAPP, and conduct the necessary reviews to ensure that data quality
objectives for each QAPP are being met.
2.5. Data verification and validation
Each QAPP includes plans to ensure data verification and validation. The Project
Managers and quality management staff will review all data generated or acquired to
determine if validation rules have been violated, and if so, to identify factors that may
have resulted in invalid data. Corrective actions will then be identified by the Quality
Manager in conjunction with the Project Manager and project coordinator.
3.
Components of the organization that develop Quality Plans
Project Managers, the study director, and the QM develop quality plans, in collaboration
with quality managers of internal and extramural laboratories that will provide services to
the study.
IV.
PERSONNEL QUALIFICATIONS AND TRAINING
1. Policy regarding training for management and staff
All personnel performing work for the Epidemiologic Study of Recreational Use of the
Chicago Area Waterways study will be qualified for all assigned tasks and will be given
appropriate training to ensure proper performance of all duties.
2. Processes for ensuring appropriate qualifications and training
All personnel must be qualified for the roles they will perform in this study. Each role in
the project will have a job description that will include the necessary knowledge, skill,
certification, accreditation, licenses or other formal qualification. These job descriptions
will be developed for project staff by each Project Manager for their personnel. Job
descriptions for personnel for whom more than one Project Manager will have authority,
will be developed by the relevant Project Managers. Job descriptions for managers in
this project will be developed by the study director. Job descriptions will be developed
using a template that will include the necessary knowledge, skills, and certification. In
addition to the skills required for hire in a position, a list of training requirements for each
role in the project will specify the necessary training following hire.
3. Retraining needs
Project specific training requirements for all CHEERS staff is outlined in the individual
QAPPS. Retraining will be required for staff that, on performance evaluations are
deficient. The performance evaluations will vary from project to project. For the survey
project (QAPP 2) this will involve mock interviews and interview monitoring. For the
water sampling and analysis project (QAPP 1) this will involve evaluations of split
samples and observations of sampling technique. For the clinical evaluations, it will
involve classifying photos of conjunctivitis by severity grade. For all projects, problems
noted on unusual occurrence reports (such as breaches in protocol, chain of custody
problems, etc) either retraining or disciplinary action or both will be initiated.
V.
PROCUREMENT OF ITEMS AND SERVICES
In order to ensure high quality data, the projects with the epidemiologic study will
procure items and services after determining whether performance standards or
certifications exist for a particular purchase that are relevant to data quality. We have:
- selected EPA certified laboratories to conduct water analysis
- selected clinical laboratories that are CLIA-certified
- selected equipment such as GPS devices, bar-code scanners, instruments to
measure basic water quality parameters after reviewing EPA and other Federal
guidelines.
- evaluated tablet and laptop computers to be used in the field surveys will be tested
during the pilot study. We will assess battery life, screen visibility in bright
outdoor light, field performance, including portability and ease of use
- field tested the bar-code printing and reading technology
VI.
DOCUMENTS AND RECORDS
Study-wide we have a uniform that requires appropriate storage of documents. Each
QAPP has listed the personnel responsible for the development, storage and distribution
of project specific documents and records. For example, consent form that will have Case
IDs and names of study participants will require special storage and restricted access. In
general paper-based records will have more than 1 copy that will be stored in binders in
two different places. We will maintain copies of documents that are free of personal
identifiers and raw data in the CHEERS study center (room 210, UIC SPHW). This
would include protocols, references, literature, and quality monitoring reports. Paper-
based data will be kept in a locked study storage room (room 417, UIC SPHW) and a
second copy will be maintained in the office of the manager of the project that generated
the data. We will use the UIC Blackboard system which insures multiple CHEERS staff
members can work with, or discuss, the same version of a document at the same time.
All computer files with identifiers will be password protected. All computer files,
databases, reports will be backed up on a secure UIC server.
VII. COMPUTER HARDWARE AND SOFTWARE
The University of Illinois, Chicago (UIC) has selected high quality hardware from
reputable manufactures that we will purchase centrally through the UIC system. Software
used for data analysis, the SAS system, has been extensively tested and is widely used
in environmental and epidemiologic research conducted by and for the US EPA.
VIII. PLANNING
The initial planning for this project began in February, 2007 when MWDGC personnel
and UIC faculty first communicated about this study. The initial weeks of planning
involved defining study objectives and selecting an appropriate study design to meet
those objectives. Over the subsequent weeks, in consultation with MWRDG personnel,
UIC faculty and staff, and external resources, the specific projects of the study were
developed. Planning of individual projects used the EPA’s systematic planning process
called the Data Quality Objectives (DQO) Process mentioned in the
EPA Guidance for
the Data Quality Objectives Process (QA/G-4)
(EPA 2000).
The study objectives, study sites, study timetable are presented in the “Overview”
document, as are considerations that went into the design of the study.
IX. IMPLEMENTATION OF WORK PRCESSES
The QM and each Project Manager will be responsible for ensuring implementation of
the QAPP as written. After new personnel are trained, the Project Managers will
supervise their field activities and monitoring adherence to protocol. The QM will
review QC data that will identify possible breaches in adherence to protocol, or needs to
modify the protocol. Each Project Manager, with QM have identified processes within
each project that will require review. Any modification to the protocol will require the
approval of the study director and quality manager, as well the notification of the
MWRDGC liaison.
X. ASSESSMENT AND RESPONSE
Project specific assessment plans and response actions are outlined in the individual
QAPPS. In addition, Section III discusses the overall quality system components
including audits and response actions for each QAPP. In addition, any unusual
occurrence report will be submitted by the responsible Project Manager to the QM within
24 hours. These reports will be reviewed and an appropriate response action will be
decided by the QM and/or the study director.
At weekly meetings, the QA staff, the study director, the Project Managers, and the
field coordinator will review any problems with equipment calibration or performance,
unusual occurrence reports, and results of performance evaluations that did not meet data
quality objectives will be discussed.
At monthly reviews, quantitative summaries of water quality data will be presented, as
will results of positive clinical specimen cultures, and numbers of participants enrolled by
location, by recreational group. Results of QC data from water and clinical microbiology
laboratories will be presented. All available study personnel and will be present at
monthly reviews.
Annual reviews are discussed in Section III.
XI.
QUALITY IMPROVEMENT
The QM and each Project Manager and program manager will be responsible for
identifying, planning, implementing, and evaluating the effectiveness of quality
improvement activities as described in each QAPP. At all quality reviews, potential
threats to data quality will be identified and addressed. The range of responses will be
determined and efforts will be made to improve quality through system-wide
improvements.
Thus, a quality issue identified in the water project may prompt
improvements in that project, and also in the survey or clinical evaluation project.
CHEERS QAPP-1
- ii -
July 2008
Table of Contents
A. PROJECT MANAGEMENT
1. Distribution List
1
2. Project/Task Organization
1
3. Problem Definition/Background
4
4. Project Task/Description
5
5. Quality Objectives and Criteria
13
6. Special Training/Certification
22
7. Documents and Records
23
B. DATA GENERATION AND ACQUISITION
24
1. Sampling Process Design (Experimental Design)
24
2. Sampling Methods
25
3. Sample Handling and Custody
33
4. Analytical Methods
35
5. Quality Control
38
6. Instrument/Equipment Testing, Inspection, and Maintenance
40
7. Instrument/Equipment Calibration and Frequency
40
8. Inspection/Acceptance of Supplies and Consumables
41
9. Non-direct Measurements
43
10. Data Management
44
C. ASSESSMENT AND OVERSIGHT
44
1. Assessments and Response Actions
44
D. DATA VALIDATION AND USABILITY
45
1. Data Review, Verification, and Validation
45
2. Verification and Validation Methods
45
References
47
CHEERS QAPP-1
- iii -
July 2008
List of Tables
Table
Description
Page
Table 1
Contact information for water sampling project
2
Table 2
Table 3
Table 4
List of microbes and their quantifying methods
Summary of sample collection on days of participant
enrollment for two years of data collection
Spike levels, by location
6
8
14
Table 5
Summary table for QA/QC Requirements for methods 1600
and 1603 (Enterococci & E.coli)
15
Table 6
Summary table for QA/QC Requirements for method 1602:
Male-specific (F+) and Somatic Coliphage in water
16
Table 7
Summary table for QA/QC Requirements for method 1623:
Cryptosporidium and Giardia
20
Table 8
Locations of water sampling by participant enrollment site;
CAWS sites
25
Table 9
Size of direct sampling bottles
26
Table 10
Suggested range of sample volumes (mL) for fecal coliform
tests using the membrane Filter Method
36
Table 11
Dilution volumes, by sampling location, weather condition,
and indicator
37
Table 12
Microbiology lab equipment monitoring
41
Table 13
Quality of reagent water used for microbiology testing
42
List of Figures
Figure
Description
Page
Figure 1
Water sampling organization
1
CHEERS QAPP-1
- iv -
July 2008
List of Appendices
Appendix
Description
1
Laboratory certification, EnviroTest/Perry
2
Laboratory certification, SMI
3
EPA reference method 1603;
E. coli
in water by membrane
filtration
4
EPA reference method 1600; Enterococci in Water by
Membrane Filtration
5
EPA reference method 1602; Male–specific (F+) and
Somatic Coliphage in Water by Single Agar Layer (SAL)
Procedure
6
Method for serotyping F(+)RNA coliphages (Hsu, 1995)
7
EPA’s Reference Method 1623 (
Cryptosporidium, Giardia
)
8
Method for detection of Norovirus
9
Properties of continuous fluid centrifugation (Zuckerman
2006)
10
EPA approval of CFC for Method 1623
11
Matrix spiking materials
12
Water Quality Sampling Field Data Sheets (FDS)
13
Field Log Book
14
Continuous flow centrifugation (CFC) system
15
SMI Virus sampling kit
16
SMI SOP for CFC
17
Chain-of-Custody records (EnviroTest/Perry)
18
Chain-of-Custody records (SMI)
19
Water sampling location codes
CHEERS QAPP-1
- 1 -
July 2008
A. PROJECT MANGAMENT
1. Distribution list
Current and revised versions of the Water Sampling and Analysis QAPP are to be distributed to
the following individuals:
i. Samuel Dorevitch, UIC
ii. Peter Scheff, UIC
iii. Margit Javor, UIC
iv. Sara Wuellner, UIC
v. Todd Schoonover, UIC
vi. Thomas Granato, MWRDGC
2. Project/Task Organization
Key personnel participating in the water sampling and analysis for the epidemiology study and
their specific roles/responsibilities are listed below. The organization chart indicating the lines of
communication is included in Figure 1.
Figure 1: Water sampling organization
Study Director
S. Dorevitch
Quality Manager
P. Scheff
Water Project Manger
M. Javor
Field Manager
T. Schoonover
Enviro-Test/Perry Laboratories
M. Lenos
Water project
manager assistants
Scientific Methods, Inc.
F. Hsu
M. Hayes
Assistant to the
Water sampling staff
field manager
CHEERS QAPP-1
- 2 -
July 2008
Name
Project Title or Role
Telephone Number
Samuel Dorevitch
Study Director
(312) 355-3629
Peter Scheff
Quality Manager
(312) 996-0800
Margit Javor
Water Project Manager
(312) 413-1390
Todd Schoonover
Field Manager
(312) 413-0305
Mirka Lenos
Enviro-Test/Perry Lab contact
(630) 734-9530
Fu-Chih Hsu
Scientific Methods, Inc.
(574) 277-4078
Matt Hayes
Scientific Methods, Inc.
(574) 277-4078
Table 1: Contact information for water sampling project
Responsibilities
1. Study Director: Samuel Dorevitch, MD, MPH
The Study Director will have overall responsibility for the development and implementation of
the CHEERS study, as well as providing deliverables to the MWRDGC. Specific responsibilities
of the Study Director include the hiring of project staff, establishing subcontracts with entities
external to UIC, complying with human subject research protection requirements, and
communicating with the MWRDGC and with the public as needed.
2. Water Project Manager: Margit Javor, MS
The Water Project Manager will be responsible for developing the water sampling protocol and
selecting, based on performance data, laboratories that will analyze water samples. The Water
Project Manager will work with the Field Manager to ensure implementation of protocols and
will also be responsible for developing training materials, observing field sampling as needed,
and monitoring the field sampling procedures. If deviations from the data quality objectives for
water analysis are noted, the Water Project Manager will collaborate with the Quality Manager
and Field Manager to identify field practices or protocol elements responsible for such deviations
and to develop solutions.
CHEERS QAPP-1
- 3 -
July 2008
3. Quality Manager: Peter Scheff, PhD
The responsibilities of the Study Quality Manager will include assisting with the development of
the Quality Assurance Project Plan (QAPP), establishing data quality objectives, reviewing the
quality control data of laboratories conducting water analyses, conducting quality audits, and
communicating with laboratory Quality Managers.
4. Field Manager: Todd Schoonover, MS, CIH, CSP
The responsibilities of the Field Manager will include field training of water samplers, oversight
of the surface-water collection, and ensuring that sampling occurs according to written standard
operating procedures by trained personnel. The Field Manager will plan and prepare paperwork
for each field sampling event and ensure that samples arrive at the lab within the holding-time
specified in the relevant analytic methods. The Field Manager will provide updated information
to the commercial laboratories regarding the water sampling schedule, including the anticipated
number of samples for each analytic method and the expected date and time of sample receipt.
5. Commercial Water Quality Laboratory for indicator bacteria: Enviro-Test/Perry Laboratory
(contact: Mirka Lenos).
This commercial water quality laboratory will perform analyses of the water samples for
E. coli
and enterococci. The laboratory will be responsible for adhering to US EPA protocols and
maintaining Illinois Department of Public Health certification (Appendix 1) as an analytic
laboratory for microbial water quality in drinking water. The laboratory will provide results of
microbial water quality measures to the Water Project Manager and invoices to the Fiscal
Manager.
6. Commercial Water Quality Laboratory for pathogens and coliphages: Scientific Methods
Incorporated (SMI)
6.1 Laboratory and technical issues (contact: Fu-Chih Hsu, PhD)
Dr. Hsu will be responsible for maintaining Indiana State Department of Health laboratory
certification (Appendix 2). He will also be responsible for teaching the Water Project Manager
CHEERS QAPP-1
- 4 -
July 2008
and the Field Manager to operate the continuous fluid centrifugation (CFC) and virus sampling
systems, and also for water pathogen and coliphage analyses conducted by SMI. He will provide
results of microbial water quality measures and of internal QC analyses to the Water Project
Manager.
6.2 Coordination of sample receiving and reporting of results (contact: Matt Hayes)
Mr. Hayes will prepare for laboratory analyses based on the schedule received by the Field
Manager. Mr. Hayes will send laboratory results to the Water Project Manager and invoices to
the Fiscal Manager.
7. Water sampling staff
7.1 Water Sampling Specialists
Water Sampling Specialists will be responsible for implementing matrix spike sampling on an
ongoing basis. This function may also be performed by the Field Manager or by personnel with
responsibility for filtration sampling. Water Sampling Specialists will also maintain and use
instruments for measuring water chemistry parameters, such as dissolved oxygen, pH,
conductivity, turbidity, and temperature.
7.2 Water Sampling Technicians
Water Sampling Technicians will be responsible for collecting samples in accordance with SOPs
and documenting any deviations from the SOPs.
3. Problem Definition/Background
The primary purpose of the research study’s water sampling activities is to provide a measure of
concentration of microbes in the water to which study participants may be exposed. By
collecting water samples at the approximate times and locations of water recreation, we aim to
identify and characterize water quality measures that predict the risk of illness among people
engaging in secondary contact water recreational activities. Samples will be analyzed both for
conventional indicators of water quality, as well as for pathogens that may cause recreational
waterborne illness.
CHEERS QAPP-1
- 5 -
July 2008
Concentrations of
Escherichia coli
and enterococci are commonly used as indicators of water
quality and samples will be collected and analyzed for these bacteria as part of the study.
However, there are limits to the information gained from measurements of
E. coli
and
enterococci. First, recreational waterborne illness is not thought to be caused by enterococci and
is rarely caused by specific pathogenic strains of
E. coli
. Rather, disease is caused by pathogens
such as enteric viruses
Giardia spp.
and
Cryptosporidium spp.,
and bacteria such as
Salomnella
spp., Shigella spp.,
and
Aeromonas spp.
Second, the CAWS risk assessment (1) found that
bacterial indicators and pathogens were, at best, weakly associated with one another. In one of
the few epidemiologic studies of paddlers, measures of coliphages were shown to be better
predictors of acute illness than were bacterial indicators.(2) Additionally, coliphage typing
allows the tracking of sources of fecal contamination and the differentiation of human from non-
human sources of water microbes.(3, 4) To characterize exposure, we will collect water samples
throughout each day of participant enrollment at various locations using EPA-approved methods
to analyze for indicator bacteria, coliphages, and waterborne pathogens. Samples taken using
filtration methods will be archived (frozen) for possible future analyses that can provide a
molecular link between pathogens obtained from clinical and water samples.
4. Project/Task Description
4.1 Overview of water sampling
As discussed in the “Overview” document, the objective of the water sampling is to provide
accurate and precise measures of microbe concentrations in the CAWS and general-use
waterways (GUW) to address study objectives 2 and 3. In order to characterize the relationship
between recreator illness and microbe concentrations in the CAWS (study objective 2), it is
necessary to estimate exposure to waterborne microbes among recreators. This will be
accomplished by analyzing water samples collected along the waterways at multiple time points
throughout the day of participant enrollment. To meet study objective 3 (identifying pathogens
responsible for illness and exploring their sources on the CAWS), we will measure three
pathogens;
Giardia, Cryptosporidium,
and norovirus. Potential future analyses of archived water
samples collected upstream and downstream of WRPs may permit testing water samples for
specific pathogens (at a species or genotype level) responsible for acute infections among
CHEERS QAPP-1
- 6 -
July 2008
recreators. Moreover, if a water quality standard were to be applied to the CAWS, compliance
with such a standard may require sampling water at specific fixed locations. The results of spatial
and temporal variability of water quality on the CAWS, to be established by intensive sampling
on a limited number of occasions and by ongoing “standard” sampling during participant
enrollment, will provide information that could be translated into regulatory requirements.
Two categories of microbial analyses will be performed: 1) those for indicator microbes and 2)
those for pathogens. Samples will be collected by the direct method for indicator bacteria,
E. coli
and enterococci, as well as for male-specific and somatic coliphages. Coliphages will be
serotyped (Groups I-IV) to identify the relative contribution of human and non-human sources of
coliform bacteria at various locations along the waterways. Pathogen analyses will be performed
on samples collected using continuous flow centrifugation (CFC) and with a viral sampling kit.
The pathogens to be analyzed are:
Giardia
spp.
, Cryptosporidium
spp., and norovirus. In 2007,
samples were also analyzed for
Pseudomonas
spp.,
Salmonella
spp.,
and Shigella
spp. This was
discontinued in 2008 based on questions about the precision, accuracy and validity of the 2007
analyses of these bacteria. Following discussions with the CHEERS Science Advisory Board in
February 2008, large volume sampling for these pathogens was discontinued. The pairing of
indicator and pathogen sampling will build on the risk assessment’s analysis of the relationship
between these two categories of microbes on the CAWS.
CHEERS QAPP-1
- 7 -
July 2008
Microbe
Method
Appendix
Indicators
E. coli
EPA 1603
Appendix 3
enterococci
EPA 1600
Appendix 4
Male specific colipahge
EPA 1602
Appendix 5
Male specific RNA coliphage serotyping
Hsu, 1995
Appendix 6
Pathogens
Giardia
EPA 1623
Appendix 7
Cryptosporidium
EPA 1623
Appendix 7
Norovirus
RT-PCR
Appendix 8
Pseudomonas
SM 9213E
discontinued after 2007
Salmonella
EPA 1682
discontinued after 2007
Shigella
SM 9260E
discontinued after 2007
Table 2. List of microbes and their quantifying methods
Water sampling will occur at two categories of locations; Access points and WRP-oriented
locations. Access points are the locations at which water recreators (research subjects) begin
their water recreation. For boaters, paddlers and rowers, this is the “put in” or launch location.
For streamside fishers, the access point is where they are fishing. Access point sampling will
take place on the CAWS and general use waters. WRP-oriented sampling will take place
upstream and downstream of the WRPs. This will occur at CAWS locations only.
Table 3 provides an overview of the sampling schedule for indicators and pathogens at access
points and at WRP-related sites on the CAWS, and at general use access points. The differences
between 2007 and 2008 sampling strategies are described below.
In addition to microbial evaluations, environmental observations and water chemistry measures
will be recorded, as described below (Sections B 2.3 and B 2.4).
CHEERS QAPP-1
- 8 -
July 2008
2007
2008
Location
Indicator
sampling
Pathogen
sampling
Indicator
sampling
Pathogen
sampling
CAWS
Access point
5 every 2 hrs
1-2 per 6-hr day
1 every 2 hrs
1-2 per 12-hr
WRP: Upstream
2 per 6-hr day
1-2 per 6-hr day
2 per 12-hr
day
1-2 per 12-hr
WRP:
Downstream
2 per 6-hr day
1-2 per 6-hr day
2 per 12-hr
day
1-2 per 12-hr
General Use
River access point
Not done
Not done
3 every 2 hrs 1-2 per 12-hr
Lake/lagoon
access
5 every 2 hrs
1-2 per 6-hr day
2 every 2 hrs 1-2 per 12-hr
Table 3. Summary of sample collection on days of participant enrollment for two years of
data collection
4.2 Frequency and location of water sampling
An overview of sampling intensity (number of samples per round, frequency of sampling) is
presented in Table 3, which contrasts the 2008 methods and those employed in 2007.
4.2.1 CAWS sampling
4.2.1.1 Access points: Number of samples collected by direct method for indicator
microbes.
During the 2007 enrollment season, samples were collected at 5 points near each access point:
left, right, and center stream at the access point plus center stream 0.5 mile upstream and center
stream 0.5 mile downstream of the access point. The results of “Preliminary Water Sampling
Study 2: Spatial and temporal variability of CAWS indicator organisms” (described in detail in
the July, 2007 QAPP: Water Sampling and Analysis), suggested that sampling at one cross-
sectional location is sufficient to characterize concentrations across the waterway. Analyses of
CHEERS QAPP-1
- 9 -
July 2008
the entire 2007 dataset demonstrated that concentrations of all indicator microbes were
statistically indistinguishable whether collected at left, center or right sampling sites across
cross-sections of CAWS locations. After discussion with the CHEERS Science Advisory Board
in February, 2008, sampling was revised to collecting two samples per single cross-sectional
location (left, center or right) at each time point of sampling. Analysis of the 2007 data also
suggests that, for most locations, concentrations do not differ significantly within a mile of each
other along the length of the waterway with one exception; The Skokie Rowing Center, which is
immediately upstream of the North Side WRP.
With the exception of the Skokie Rowing
Center site, the upstream and downstream sampling points were omitted from the 2008 sampling
strategy. This allowed us to refine our sampling strategy for the 2008 season and collect samples
from a single cross-section at the access point, using a telescopic pole with a sample collection
cup secured to the end of the pole. Since the 2007 data suggest that concentrations may vary by
time of day, we will continue collecting samples at the access point every two hours.
4.2.1.2 CAWS Sampling: Water reclamation plant-oriented sampling
Samples will be collected once during each 6-hour shift of fieldwork at locations upstream and
downstream of the nearest upstream WRP. Because results from the preliminary water study
examining the spatial and temporal variability of the indicator organisms in the CAWS showed
no statistically significant differences in river cross-sectional concentrations of the indicator
microbes, access point samples will be collected from a single cross-sectional location.
4.2.1.3 CAWS Sampling: Large volume sampling for pathogens
Large volume sampling for pathogens will be performed by two methods: continuous flow
centrifugation (CFC) and viral filtration sampling. Large volume water samples will be collected
at the access point, as well as upstream and downstream of the nearest upstream WRP. These
samples will be collected from the cross-sectional location used for recreator access at access
points. At WRP-oriented sampling locations, these samples will be collected at either the left-
stream or right-stream shore, depending on logistical considerations. Pathogen samples will be
collected at each of these three points at least once each day of CAWS participant enrollment.
Three options for the collection of water samples for filtration during the 2008 season were
identified: A) Bringing the CFC into the field to sample the water directly from the shore of the
river, B) Pumping water into a 20L cubitainer to be brought back to the UIC water quality lab
CHEERS QAPP-1
- 10 -
July 2008
and processed by the CFC, and C) transporting the cubitainer to the analytic laboratory,
approximately 100 miles away, for CFC processing and analysis. Option A was not viable
because there were not enough CFC available for use at multiple field locations. After
discussing these options with the Scientific Advisory Board, it was decided that during the 2008
season, water would be collected in a 20L cubitainer and brought back to the UIC Water lab for
processing in the CFC.
4.2.2 Sampling at general-use waters
Water will be sampled every two hours at access points using a telescopic pole. There will be no
WRP-oriented samples as there are no water reclamation plants operating on these waterways.
At lake and lagoon locations, two samples will be collected every two hours by telescopic pole
from shore. Large volume water samples for pathogens will be collected at access points at least
once each day of general use participant enrollment. When possible, a second set of pathogen
samples will be collected 6-12 hours later.
The exact locations of water sampling, with GPS coordinates, are found in the Overview
document.
4.3 Water analysis
4.3.1 Grab samples
4.3.1.1 Analysis for E. coli
Escherichia coli
will be analyzed at the commercial laboratory using US EPA Method 1603, a
single-step membrane filter procedure which uses the modified membrane-Thermotolerant E.
coli (mTEC) agar. The red or magenta colonies that grow on the surface of the membrane filter,
after a two-stage incubation period, provide a direct count of E. coli in water. The EPA
reference method for E. coli analyses can be found in Appendix 3 of this QAPP document.
4.3.1.2 Analysis for enterococci
Enterococci indicator bacteria will be analyzed at the commercial laboratory using US EPA
Method 1600, a single-step membrane filter procedure which uses the membrane-Enterococcus
Indoxyl-b-D-Glucoside (mEI) Agar. All colonies greater than or equal to 0.5mm in diameter
with a blue halo are counted in the surface water samples. The EPA reference method for
enterococci analyses can be found in Appendix 4 of this QAPP document.
CHEERS QAPP-1
- 11 -
July 2008
4.3.1.3 Analysis for coliphage
Male-specific (F+) and somatic coliphages will be analyzed at the commercial laboratory using
US EPA Method 1602, a single agar layer procedure in which magnesium chloride, log-phase
host bacteria, and molten tryptic soy agar are added to the sample. Circular lysis zones (plaques)
are counted after an overnight incubation period, allowing for enumeration of coliphage in water.
The EPA reference method for coliphage analyses can be found in Appendix 5 of this QAPP
document. Serotyping of F(+) RNA coliphages will be performed according to the method of
Hsu 1995, which can be found in Appendix 6 of this QAPP document.
4.3.2 Large volume sampling for pathogens
Large volume sampling methods will be used to collect samples for analysis of pathogens.
Continuous flow centrifugation (CFC) will be used to collect samples for detection of
Giardia
spp
. and
Cryptosporidium spp.
The performance of this method has been previously reported to
yield good recoveries (Zuckerman 2006), as detailed in Appendix 9. CFC sampling has received
US EPA approval for use (Appendix 10). In 2007 this method was also used fo
r Shigella spp.,
Salmonella spp, and Pseudomonas
. The CFC system allows for automated sampling of large
volumes by drawing water through an inlet tube using a peristaltic pump at a specified sample
flow rate. As the sampled water passes through the continuous flow centrifuge, the pathogens
are concentrated in the CFC bowl. We compared two methods of sampling large volumes of
water, and the CFC system was chosen as the preferred method of pathogen sampling because of
its ease of use in the field and because it is an EPA approved method for Cryptosporidium (see
appendix 10). A second filtering system will be used to collect water samples for analysis of
norovirus and potentially other waterborne viruses.
4.3.2.1 Analysis for
Cryptosporidium
and
Giardia
Samples will be analyzed for
Cryptosporidium
and
Giardia
using US EPA Method 1623,
utilizing filtration, immunomagnetic separation, and immunofluorescence assay microscopy.
The samples are filtered and the filtered materials are centrifuged, resulting in pellets containing
cysts and oocysts which are magnetized to allow for separation from the extraneous materials.
The cysts and oocysts are stained on slide wells with antibodies able to be detected using
fluorescence and differential interference contrast microscopy. Oocysts and cysts are then
identified qualitatively based on size, shape, color, and morphology. The total number of objects
CHEERS QAPP-1
- 12 -
July 2008
on the slide provides a count of the pathogens present in the water samples. The EPA reference
method for Giardia and Cryptosoporidum analyses can be found in Appendix 7 of this QAPP.
4.3.2.2 Analysis for
Salmonella
In 2007 samples were analyzed for
Salmonella
spp. using US EPA Method 1682:
Salmonella
in
Sewage Sludge (Biosolids) by Modified Semisolid Rappaport-Vassiliadis (MSRV) Medium.
4.3.2.3 Analysis for
Shigella
In 2007 samples were analyzed for
Shigella
spp. using standard method 9260E, (APHA, 2005)
4.3.2.4 Analysis for
Pseudomonas aeruginosa
In 2007 samples were analyzed for
Pseudomonas
using standard method 9213E, Membrane filter
Technique for
Pseudomonas aeruginosa
(APHA, 2005)
4.3.3 Selection of commercial laboratories
Based on the results of a head-to-head comparison of two commercial laboratories that included
evaluation of the performances (described in detail in the July 2007 QAPP: Water and Sampling
Analysis document), up-to-date laboratory SOPs, capacity, turnaround time, data product,
accessibility (weekends, holidays), distance and cost, Enviro-Test/Perry Laboratories was
selected as the commercial lab to analyze samples for enterococci and
E. coli
using US EPA
Methods 1600 and 1603, respectively. Scientific Methods, Inc. will conduct the analyses for
coliphages (Method 1602) with serotyping of F+ RNA coliphages;
Cryptosporidium
and
Giardia
(Method 1623), and norovirus. In 2007, CFC samples were also analyzed for
Shigella
(Method
1682),
Salmonella
(SM 9260E) and
Pseudomonas aeruginosa
(SM 9213E). Additionally, SMI
will make available the CFC and virus sampling kits required for this study, and will archive
water samples for potential future analyses.
4.4 Quantitative polymerase chain reaction pilot study
4.4.1 Overview
A qPCR pilot study has been designed to address the following objectives: 1) to establish the
precision and accuracy of qPCR for CAWS samples collected at areas of high and low
enterococci concentration, 2) to characterize the relationship between qPCR and USEPA method
1600 for enterococci and 3) to evaluate the impact of freezing CAWS samples on pPCR results.
Because measures of enterococci by qPCR are thought to correlate with viral pathogens in
CHEERS QAPP-1
- 13 -
July 2008
surface waters, we will also measure other indicators and pathogens at the same times and places
where samples are collected for qPCR measurement of enterococci .
qPCR enterococci testing does not address the needs of the Illinois EPA and, for that reason, will
not be part of the basic set of tests for indicator microbes (namely
E. coli
, enterococci, and
coliphages) performed on all water samples. However, it would increase the relevance of the
epidemiology study if it were possible to compare results obtained in this study to those which
use qPCR testing. A small scale study is in development that would allow us to meet the
objectives listed above. A commercial laboratory (Mycometrics) that has experience in
analyzing surface water sampling for enterococci by aPCR and has worked closely with the US
EPA in developing draft methods for such analyses, will analyze our samples. qPCR testing will
not become part of the basic package of indicator analyses performed as part of the ongoing
epidemiologic study.
5. Quality Objectives and Criteria
In order to monitor laboratory performance, study personnel will send to the lab performance
evaluation samples such as method blanks, matrix spikes and sample splits. The laboratories are
required to adhere to the quality control procedures provided in the US EPA Methods 1600,
1602, 1603, and 1623 documents. These procedures include initial and ongoing precision and
recovery tests, media sterility checks, and record maintenance.
5.1 Indicator bacteria water samples
5.1.1 Method Blanks
For each method of analysis, at least one field blank will be included with every 20 samples sent
to the laboratory. Blanks consisting of sterile buffer solution will be prepared by the water
sampling technicians at the end of the first round of sampling every day of sampling.
5.1.2 Replicate Samples
For each method of analysis, at least one sample for every 20 samples collected will be split in
the field among three bottles, two of which will be sent to the lab to evaluate precision, the third
will be prepared with the matrix spike material (see section 5.1.3).
CHEERS QAPP-1
- 14 -
July 2008
5.1.3 Matrix Spikes
For each method of analysis, the third bottle of the split sample (see section 5.1.2) will be
prepared with the matrix spike material by the water sampling technician. This will occur for at
least 5% of the samples collected each day. See Appendix 11A-C for spike materials details.
Location group
E. coli
(cfu/100mL)
Enterococci
(550cfu/100
mL)
Coliphages
(Somatic: 10,00pfu/mL;
F+: 1,000 pfu/mL)
Giardia &
Cryptosporidum
(160 (oo)cysts)
GUW: Lake Michigan
550 x 1
1
1 mL
1/20L
GUW: other
550 x 1
6
1 mL
1/20L
CAWS-N
10,000 x 1
10
1 mL
1/20L
CAWS-S
550 x 1
4
1 mL
1/20L
Table 4: Spiking levels by location.
5.1.4 Method Performance
Laboratories are required to meet (or exceed) all method performance specifications of EPA
Methods 1600, 1602 and 1603. Performance requirements of Methods 1600 and 1603 are
described in detail in the method documents and are summarized in Table 5. Performance
requirements of Methods 1602 is described in detail in the method document is summarized in
Table 6.
CHEERS QAPP-1
- 15 -
July 2008
Method 1603 (E.coli)
Both Methods
QC Measures
Method 1600 (Enterococci)
Procedure
Criteria (BioBall)
Note
Frequency
Procedure
Criteria (BioBall)
IPR
(Init.
Precision &
Recovery)
4x100mL PBS
+spikeATCC#11775
1x50mL MB
Mean %Recovery:
Detect-144%
Precision RSD 61%
Chart with OPR
Before sample
collection start (once)
4x100mL PBS
+spikeATCC#19433
1x50mL MB
Mean %Recovery:
85-106%
Precision RSD 14%
OPR
(Ongoing
Precision &
Recovery
1x100mL PBS
+spikeATCC#11775
1x50mL MB
%Recovery:
Detect-
144%
Chart with IPR
through all time
1/20 sample (5%)
1x100mL PBS
+spikeATCC#19433
1x50mL MB
%Recovery:
78-113%
MS
(Matrix
Spike)
1xXXmL +spike ATCC#11775
1xXXmL unspiked
1x50mL MB
%Recovery:
17-117%
Control chart for
matrices
(Rec. based on
disinfect. ww)
1/20 sample (5%)
1xXXmL +spike
ATCC#19433
1xXXmL un-spiked
1x50mL MB
%Recovery:
63-110%
MB
(Method
Blank)
1x50mL PBS or dilution
W>>mTEC
Absence of growth
Every day when sample
analyzed
1x50mL mEI agar
Absence of growth
Positive Control
1xXXmL diluted E. coli
(ATCC#11775)
Growth
Count
Every day when sample
analyzed (+ new batch
of media)
1xXXmL diluted
Enterococci (ATCC#19433)
Growth
Negative Control
1xXXmL diluted E. faecalis
(ATCC#19433)
Absence of growth
Every day when sample
analyzed (+ new batch
of media)
1xXXmL dil E. coli
(ATCC#11775)
Absence of growth
Filter sterility
check
1 filter placed on TSA
plate>incubate
Absence of growth
Every day when sample
analyses
1 filter placed on TSA
plate>incubate
Absence of growth
Filtration Blank
1x50mL PBS filtered >TSA
plate>incubate
Absence of growth
Every day before
beginning sample
analyses
1x50mL PBS filtered >TSA
plate>incubate
Absence of growth
Media Sterility
Check
1 from each batch of medium
Absence of growth
Every day when sample
analyses
1 from each batch of
medium
Absence of growth
Media
Verification
All 4 media tested with positive
and negative controls
Absence of growth
When new batch or
reagents used
All 3 media tested with
positive and negative
controls
Absence of growth
Colony
Verification
1 typical colony/ 10 positive
samples
1 atypical colony/10 positive
samples
E. coli
Verify
1/10 positive samples
(or 10/month) for both
typical and atypical
colonies
1 typical colony/ 10 positive
samples
1 atypical colony/10 positive
samples
E. faecalis
Table 5. Summary table for QA/QC Requirements for methods 1600 and 1603 (Enterococci & E.coli)
CHEERS QAPP-1
- 16 -
July 2008
Criteria
Both Methods
QC Measures
Procedure (both separately)
Male-specific
Somatic
Note
Frequency
IPR (Init. Precision
& Recovery)
4x100mL Reag W +spike 80PFU
(bulk spike!)
1x100mL MB
Mean % Recovery:
9-
130%
Precision RSD 46%
Mean % Recovery: 86-
177%
Precision RSD 23%
Chart with OPR
Before sample
collection start (once)
OPR (Ongoing
Precision &
Recovery
1x100mL Reag W +spike 80PFU
1x50mL MB
%Recovery: 4-135%
%Recovery: 79-183%
Control chart after 5
value;Ave R% &s (Stdev);
% R interval plot R-2s;
R+2s; update on a reg.
basis
1 with each analyzed
batch (batch<=20
samples)
MS (Matrix Spike)/
MSD (duplicate)
2x100mL +spike 80PFU
1x100mL unspiked
%Recovery: Detect-120%
Precision (RPD) 57%
%Recovery: 48-291%
Precision (RPD) 28%
Control chart for matrices
and update on a reg. basis
First received source
and 1/20 sample (5%)
MB (Method
Blank)
1x100mL Reagent W > same anal
as samples
No coliphage PFU
No coliphage PFU
1 with each batch of
sample
Positive Control
>OPR serves it
See OPR
Table 6. Summary table for QA/QC Requirements for method 1602: Male-specific (F+) and Somatic Coliphage in water
CHEERS QAPP-1
- 17 -
July 2008
5.2 Filtration samples for pathogens
5.2.1 Method blanks
Sterile buffer solutions prepared in the UIC SPH laboratories will be used to process virus and
CFC blanks. To ensure that at least one field blank will be included with every 20 samples sent
to the laboratory, blanks will be prepared by the water sampling technicians immediately upon
collection of the final sample in a group to be sent to the lab. Thus, each shipment of samples
will include one field blank.
5.2.2 Matrix spikes
Study personnel will spike one sample aliquot for
Giardia
and
Cryptosporidium
for
approximately every 10 samples sent to the laboratory for analysis. In 2007, BioBalls spikes
were used. In 2008 spike materials from the Wisconsin State Hygiene Laboratory will be used.
For coliphage analyses spike material provided by the Scientific Methods, Inc. was found in
2007 to produce more consistent results than those obtained as BioBalls from BTF, Inc. In 2008
only Scientific Methods, Inc. material will be used. The target concentration of the male-specific
coliphage spike material is 10,000 pfu/mL and for somatic coliphages, 1,000 pfu/mL. A volume
of 1mL from both male-specific and somatic coliphages spike material is pipetted into the
specified samples. Once each week, 60 liters of water will be collected for matrix spiking,
completed in the UIC laboratory. The matrix to be spiked will rotate weekly from among the
following four groups: CAWS-North, CAWS-South, Lake Michigan, and other general use
waters. Samples from CAWS-North, CAWS-South, Lake Michigan, and other general use
waters are spiked at a frequency of approximately one spike per 10 samples for each of 1600,
1602, and 1603 methods.
CHEERS QAPP-1
- 18 -
July 2008
Spiking 1600 / 1603 samples (Indicator bacteria)
1600 = enterococci
1603 =
E. coli
•
Identify spike samples from FDS
•
Match sample method with correct spike material from above
•
Open sample
•
Adjust volume to 170ml line if necessary
•
Open correct BTF bioball vial
•
Insert ball into sample
•
Close lid tightly
•
Agitate sample
•
Circle sample in the FDS, write # of vials added and your initials
Spiking 1602 samples (Indicator viruses, coliphages)
•
Identify spike sample from FDS
•
Take “Coliphages” label container from refrigerator and put it in the chemical hood
•
Open container, identify 2 test tubes inside (MS2 male specific and somatic coliphages)
•
Open sample
•
Adjust volume to 450ml if possible (compare to the empty bottle in hood with volume lines)
•
Attach a 1mL pipette to pipetter
•
Open the MS2 male specific test tube, mix material with the pipette and pipette exactly 1mL
material into the sample (touch the pipette tip to the upper neck of sample bottle to remove
the remaining material in the pipette)
•
Cap the spike material
•
Repeat the procedure with the somatic coliphage using clean pipette
•
Close sample bottle lid tightly
•
Agitate sample
•
Circle sample in the FDS, write down sample volume, number of coliphages from the test
tubes and your initials
•
Put spike material back to refrigerator
Spiking CFC samples
(Parasites:
Giardia
and
Cryptosporidium
)
•
Take “Parasites” label container from refrigerator and remove one vial from it
•
Agitate vial with circular movement for ~30seconds and remove parafilm
•
Identify spike sample (20L container) from FDS
•
Empty contents of vial into sample and rinse vial at least 3 times with sterile buffer (pour
buffer to tube, cap and shake, repeat)
•
Close sample and agitate by rolling it from one side to the other
•
Circle sample in the FDS, write down spike tube number and your initials
CHEERS QAPP-1
- 19 -
July 2008
5.2.3 Method Performance
Laboratories are required to meet (or exceed) all method performance specifications of EPA
Methods 1623. These are described in detail in the method document and summarized in Table
7.
CHEERS QAPP-1
- 20 -
July 2008
Criteria
Both Parasites
QC Measures
Procedure
Cryptosporidium
Giardia
Note
Frequency
IPR (Init. Precision
& Recovery)
4x20L ReagW +spike ~100-500
Cryptooocysts and ~100-500
Gia cysts
1x20L MB
Mean % Recovery:
24-100%
Precision: RSD 55%
Mean % Recovery: 24-
100%
Precision: RSD 49%
Examine & doc slides for each sample;
1
st
3 Crypto & 1
st
3 Gia ID, characterize
(size, shape, DAPI, DIC cat.)
50%Undamaged, morf. intact oocysts
&cysts (200x;400x magnification)
Examination form fill
Before sample collection
start (once if meet criteria)
4 spikes + 1 MB
OPR (Ongoing
Precision &
Recovery
1x20L ReagW +spike ~100-500
Crypto oocysts and ~100-500
Gia cysts
1x20L MB
%Recovery:
11-100%
%Recovery:
14-100%
Examine & doc slide; 1
st
3 Crypto & 1
st
3
Gia ID, characterize (size, shape, FITC,
DAPI, DIC cat.) 50%Undamaged, morf.
intact oocysts &cysts (200x;400x
magnification)
Report form must be filled
1 each week or 1 for every
20 sample if more than 20
samples/week;
1 spike + 1MB
MS (Matrix Spike)/
MSD (duplicate)
1 or 2x20L (split) sample
+spike ~100-500 Crypto
oocysts and ~100-500 Gia cysts
1x20L Un-spike sample matrix
%Recovery:
13-111%
Precision (RPD) 61%
%Recovery:
15-118%
Precision (RPD) 30%
Control chart for matrices when 5 sample
available and update on a reg. basis
P-2s to P+2s (mean recov: P; std: s)
First received source and
1/20 sample (5%) for
source
1-2 spikes + 1 un-spike
MB (Method Blank;
negative control)
1x20L Reagent W > same anal
as samples
No interfering organisms
with Crypto oocysts
No interfering organisms
with Giardia cysts
If criteria meets, acceptable MB
1 with IPR; 1 with OPR
weekly with samples
Positive Control
>OPR serves it
See OPR
Table 7. Summary table for QA/QC Requirements for method 1623: Cryptosporidium and Giardia
CHEERS QAPP-1
- 21 -
July 2008
5.3 Basic water quality parameters
Conductivity, turbidity, dissolved oxygen, pH and temperature will be measured by field
sampling personnel at the time of microbial sampling using portable instruments.
Parameters will be measured at least once per day at each sampling location.
5.3.1 Turbidity
Turbidity will be measured with an HP Scientific MicroTPW portable turbidimeter. This
instrument uses a white light (tungsten) source and conforms to USEPA Method 180.1.
Resolution is 1% of full-scale (10, 100 or 1100 NTU) with an accuracy of ±2%.
5.3.2 Conductivity
Conductivity
will
be
measured
using
an
Oakton
Acorn
CON6
Conductivity/TDS/Temperature portable field meter, which measures conductivity from
0.0μS/cm to 200mS/cm with resolutions of 0.1μS, 1μS and 0.01mS and relative accuracy
of ±1% full scale.
5.3.3 DO, pH, temperature
Dissolved oxygen, pH and temperature will be measured with an Accumet AP84 portable
pH/dissolved oxygen meter. The DO range is 0.00 to 20.00mg/L with a resolution of
1.5% of saturation and an accuracy of 1.5% of full scale. The pH range is -2.00 to
16.00pH with a resolution of ±0.01pH and an accuracy of ±0.01pH. The temperature
probe will be calibrated against a NIST standard. These instruments will be used and
calibrated per the manufacturer’s instructions.
5.4 QA/QC Procedures
Implementation of the QA/QC procedures for surface water evaluation will be
established through the following steps:
5.4.1 Ensure that each field team member is familiar with the provisions of the
QAPP. The Water Project Manager will ensure that each field team member is familiar
with the field sampling SOPs and QAPP prior to implementation of field activities.
5.4.2 The Data Quality Manager, with the assistance of the Water Project Manager,
will regularly perform a QA review of field activities and field data sheets to ensure that
all procedures are followed.
5.4.3 The Data Quality Manager will verify that all contracted laboratories have a
written description of their QA activities and a QA plan describing the QA management
CHEERS QAPP-1
- 22 -
July 2008
of day-to-day routine operations. UIC personnel will conduct telephone interviews and
visit the laboratories before any project samples can be evaluated.
5.4.4 The laboratories contracted to evaluate samples are required to adhere to
defined quality assurance procedures to ensure that generated analytical data are
scientifically valid and are of known and acceptable precision and specificity.
5.4.5 Quality control charts will be maintained for every parameter measured for the
study including blanks, calibration factors, and measures of precision for each parameter.
The Data Quality Manager will initially perform weekly reviews and specify corrective
action as needed. Once acceptable performance is established, this review will be
conducted monthly or more often if needed.
6. Special Training/Certification
6.1 Surface water sampler qualification
A high school diploma and one year of college level laboratory course work is required.
Samplers should be physically fit and not afraid of water (know how to swim).
Medications causing dizziness are unadvisable.
6.2 Description of Training
Surface water samplers must participate in a 2-hr in-class training and a 2-hr field
training conducted by the Assistant Project Director, Water Data.
6.2.1 Laboratory Training
During the in-class training section, water samplers are instructed about the surface water
sample collection technique and handling of sample containers for microbiological
analysis following the protocol. Health effects of possible pathogens and techniques for
safely handling contaminated water are emphasized. Water samplers learn the use and
calibration of portable meters to measure basic water quality parameters (principle of
operations is included). The parameters to be measured are: water and air temperature;
dissolved oxygen, pH; turbidity and conductivity.
6.2.2 Field Training
Field training will be held at one of the sampling sites. Samplers will learn to sample for
microbes, prepare sample bottles for transportation, measure water quality parameters
and record all data on Field Data Sheets. Safety on waterways will be emphasized.
CHEERS QAPP-1
- 23 -
July 2008
6.2.3 Performance Evaluation
The performance of trainees will be evaluated visually after repeating sampling and
sample handling five times. Water quality parameters are measured and documented five
times by each sampler and calibration of meters before and after the measurements.
Relative standard deviation should be within 5%. Sampler’s performance will be
evaluated. If he or she used proper sampling technique and demonstrated satisfactory
variability of basic water quality parameters, he or she will be approved for field
sampling. The performance of trainees will be documented in a Training Log-book.
6.2.4 Frequency of Training
Initial training will occur at the time of hire. Refresher training will take place if quality
monitoring identifies potential problems (positive field blanks, high variability in split
samples).
7. Documents and Records
7.1 Water quality sampling Field Data Sheets (FDS) must be completed on-site at the
time sampling occurs. Appendix 12 presents a sample FDS. The FDS has been
developed to facilitate both completion in the field and the subsequent merging of
laboratory results with data regarding the time, place, and conditions of water sampling.
Identification of site sample number, date of collection, sample volume analysis required
and sample type (blank, split, spike) will be pre-printed on the FDS. The time of sample
collection, sample volume, sampler’s name, water quality parameters, and environmental
conditions will be recorded by the field technician at the time of sample collection.
Special notes about unusual observations require documentation. Completed FDS must
be submitted to the Assistant Project Director promptly (within one day). FDS will be
maintained for 5 years after completion of the project.
7.2 A separate Field Log Book (Appendix 13) is maintained for each site and filled in
by the Water Project Manager using information in the FDS. The Field Log Book is kept
in a safe place and not allowed to be taken to the field where it could be
damaged/destroyed (rain, wind, dropped in river). It is maintained for 5 years after
completion of study.
CHEERS QAPP-1
- 24 -
July 2008
7.3 The custody of water samples is documented when the sampler and the sample
courier sign the Chain of Custody (COC) record at the time of transfer. A copy of the
COC will be faxed to the Water Project Manager and the Project Coordinator within 48
hours of sample receipt.
7.4 The contracted laboratories send results of lab analyses electronically that are then
maintained by the Water Project Manager. Results will be sent as both an Excel file and
as a PDF document bearing the laboratory letterhead and signature of the commercial lab
manager. Upon receiving results, hard copies are printed out, computer back-up disks
made, and copies distributed to the Project Coordinator. Laboratory results will be
maintained for at least 5 years after completion of the project.
B. DATA GENERATION AND ACQUISITION
1. Sampling Process Design (Experimental Design)
1.1 CAWS sample locations
The location(s) for enrolling participants into this epidemiologic research study on a
given date will determine the locations of water sampling at the CAWS and will reflect
the frequency of actual CAWS usage, by recreational activity and location.
For each enrollment location we will sample water at the approximate location of water
entry every 2 hours. Additionally, water will be sampled upstream and downstream of the
nearest upstream WRP twice a day (before the beginning of event and at the end). For
research participant enrollment locations on the North Branch of the Chicago River,
water samples will also be taken from the North Branch upstream of the North Branch
Dam. Exact locations of water sampling are presented in Appendix 5 and Appendix 6 of
the Overview document. Table 8 presents a summary of water sampling locations by
CAWS enrollment sites.
CHEERS QAPP-1
- 25 -
July 2008
Enrollment site
Water sampling locations
At access point
Upstream of
WRP
Downstream of
WRP
Upstream of NB
Dam
Skokie Rowing Ctr.
√
Bridge Street
Lincoln Avenue
River Park
√
Bridge Street
Lincoln Avenue
√
Clark Park
√
Bridge Street
Lincoln Avenue
√
North Ave sites
√
Bridge Street
Lincoln Avenue
√
Main stem
√
Bubbly Creek, Ping Tom
√
Bridge Street
Lincoln Avenue
√
Calumet Boat Launch
√
Beaubien Woods
Riverdale Marina
Worth Boat Launch
√
Beaubien Woods
Riverdale Marina
Alsip Boat Launch
√
Beaubien Woods
Riverdale Marina
Table 8: Locations of water sampling, by participant enrollment site, CAWS sites
1.2 General Use sampling locations
For enrollment sites of the GUW group (Lake Michigan, the Skokie Lagoons, Crystal
Lake, and the Des Plaines, Fox, and Kankakee Rivers), water sampling locations will be
determined by the site and type of study participants’ recreational activities. Samples
will be taken at the launch site for boaters and paddlers except for beach-launch paddlers.
Water sampling for fishing subjects will happen at the point of recruitment (where they
are fishing).
The interval between sample collection at a given site on a given date will be every two
hours, the same as the interval between CAWS sampling. Samples will be collected
using a telescopic pole at launch sites (for boaters and paddlers) and near the study
participants (in the setting of fishers from shore).
2. Sampling Methods
2.1 Collection of Surface Water Samples for Indicator Organisms
2.1.1 A grab sample (discrete aliquot representative of a specific location/time) will
be collected using a telescopic pole fitted with a bottle holder, collecting the surface layer
only (up to 10 cm of depth) directly into sterile high-density polyethylene sampling
bottles.
CHEERS QAPP-1
- 26 -
July 2008
Size of sample bottle
Sampling Site
E. coli
Enterococci
Coliphage
CAWS
200 mL
200 mL
500 mL
General Use
200 mL
200 mL
500 mL
Table 9: Size of direct sampling bottles
The volumes (Table 9) were chosen based on the suggestion from literature that [6] “the
minimum volume collected should be three to four times the amount required for the
analysis”.
2.1.3 Preservation of surface water samples will not be required for the indicator
organism analyses as none of the sources contain residual chlorine. (None of the
Wastewater Treatments Plants chlorinate effluents and Lake Michigan has no detectable
level of chlorine residual.) Sampling bottles will be used without preservative (e.g.
sodium thiosulfate or other).
2.1.4 Samples will be collected from the channel/river cross-section (left-stream or
right-stream, facing upstream) location nearest the access point. In addition, sampling
approximately ¼ mile upstream and ¼ mile downstream of Skokie Rowing Center will
be collected in conjunction with access point sampling at that location. Sampling sites
will be mapped for each event and provided to the sampler with the Field Data Sheets.
2.1.5 Method blanks, field splits, and matrix spikes will be prepared at every access
point where samples are being collected. Details are provided in section B.5. Five
percent of samples sent to the lab for analysis will be field blanks. The number of field
blanks collected will be determined by the total number of samples collected. On days
when more than one field blank will be collected, blanks will be collected at more than
one sampling site.
2.1.6 At least 5 percent of samples or 1 per method per day, whichever is greater,
sent to the lab for analysis will be matrix spike samples. The number of matrix spike
samples will be determined by the total number of samples collected. All samples will be
spiked at a single location each day; locations will vary by day.
CHEERS QAPP-1
- 27 -
July 2008
2.2 Water sampling by field filtration
Scientific Methods, Inc. (SMI) of Granger, IN has made available to the research team a
continuous flow centrifugation (CFC) system for use in the field or laboratory (Appendix
14). This system has Tier 2 EPA approval for use in Cryptosporidium analyses
(Appendix 10). Additionally, SMI has developed a “Virus Sampling Kit” consisting of a
2L per minute pump and a ViroCap 5-inch filter (Appendix 15). SMI will train UIC
personnel to use the CFC and virus sampling systems according to each system’s
Standard Operating Procedures (SOP) (see Appendix 16 for CFC SOP).
CFC samples are collected with non-filtering diaphragm water sampling pumps at each
location. Samples are collected into 20 liter sterile cubitainers using new tubing for each
sample. Samples are either processed by centrifugation upon delivery to the laboratory or
refrigerated until processed. Following processing, CFC bowls are refrigerated and
shipped cold.
All analyses will be performed by SMI as follows: Aliquots from the CFC bowl will be
used for analyses of
Giardia
and
Cryptosporidium
oocysts.
Direct method, CFC, and simple filtration samples will be analyzed for E. coli and
enterococci by EPA reference methods 1603 and 1600, respectively. CFC and simple
filtration samples will be analyzed for microbes using methods listed in Table 2.
2.2.1 SMI will perform all QA and QC procedures required for each method and
follow internal QA practices required for certification by the Indiana Department of
Public Health for analyses of drinking water sources.
2.2.2 Laboratory results will be transmitted electronically to the director of the Water
Sampling and Analysis project by e-mail.
2.2.3 CFC and virus sampling in the epidemiologic study
2.2.3.1 Water samples for the analysis of pathogens will be collected every day
that direct method water sampling will take place (in other words, on days that CAWS
group and Lake group participants are enrolled). For WRP-oriented sampling, water will
be collected first upstream of the WRP, and approximately 30 minutes later from the
downstream location. One access point sample, one sample upstream of the WRP, and
one sample downstream of the WRP will be collected for pathogen analysis, in parallel
with the direct method sampling. Throughout the 2007 season, samples were filtered
CHEERS QAPP-1
- 28 -
July 2008
directly from the waterway by the CFC. Because of a nation-wide limited supply of CFC
systems, for the 2008 season samples are collected in 20L cubitainers and then
transported to the UIC lab to be filtered through the CFC. This method of collection was
chosen in consultation with the scientific review committee. The CFC sampling will take
place streamside/lakeside, as close as possible to water as permitted by safety
considerations. Study staff will monitor the filter systems to ensure that the pumps are
functioning properly and that the sampling tubing extends at least 10 feet from the
water’s edge.
2.2.4 Quality monitoring
2.2.4.1 Blank samples
Sterile, buffered water samples from the UIC SPH laboratories will be used to prepare
virus and CFC blanks for analysis.
2.2.4.2 Virus replicates
Every other week, 1 set of replicate virus samples will be created by filling a 200L drum
with water, at either a CAWS or general use location. Two 100L samples for viral
analyses will be collected, one right after the other.
2.2.4.3 CFC replicates
Every other week, two aliquots from the same CFC bowl will be sent for bacterial and
protozoal analyses.
2.2.4.4 Aliquoting of CFC samples will be as described in the SOP. One aliquot
will be kept at SMI -80°C.
2.2.4.5 Sample handling and transport will be as outlined in Section B.3, below.
2.3 Water Quality Parameters for Field Data Sheets
At each sampling location basic water quality parameters will be measured and recorded
on the FDS (Appendix 12). Turbidity will be measured with an HP Scientific MicroTPW
portable turbidimeter. DO (dissolved oxygen), pH and temperature will be measured with
an Accumet AP84 portable pH/dissolved oxygen meter. Conductivity will be measured
using an Oakton Acorn CON6 conductivity meter. All meters will be operated and
maintained according to the manufacturer’s instructions. Meters will be calibrated in the
UIC lab with certified standard materials before readings are taken for pH and DO
(Accumet AP84 pH/DO/mV meter) and turbidity (Micro TPW) and conductivity (Oakton
CHEERS QAPP-1
- 29 -
July 2008
Acorn CON6) at the site. During a full day of sampling recalibration is necessary.
Calibrations are documented in the log books for equipment maintenance and calibration.
2.4 Meteorology Data for Field Data Sheet
Cloud cover, current precipitation and precipitation in the preceding 72 hours will be
recorded on the FDS (Appendix 12).
2.5 Equipment/Materials
The checklist of equipment and materials necessary for water sampling and safety is
listed below:
•
20 Liter sterile LDPE cubitainer
•
New 0.5” ID sample transfer tubing
•
Shurflo non-filtering viton diaphragm sampling pumps
•
Safety gloves, powder-free
• Sterile sample bottles (plastic, high-density polyethylene (HDPE), 250-mL)
• Sterile sample bottles (plastic, high-density polyethylene (HDPE), 1-L)
• 500-mL sterile water (pyrogen-free) for field blank at each site and each day
• Ziploc bags (7 in x 8 in for holding sample bottles)
• Paper towel
• Coolers (at least two for two different laboratories)
• Ice Packs
• Duct Tape
• Waterproof pen (several)
• Sample-bottle labels (waterproof, preprinted with data available)
• Accumet AP84 portable pH/dissolved oxygen meter
• HP Scientific MicroTPW portable turbidimeter
•
Oakton Acorn CON6 conductivity meter
• Field Logbook
• Chain-of-Custody Records (from laboratories, Appendices 7-9)
• (Water Quality Sampling) Field Data Sheets
• Clipboard with plastic cover (protect from water splash)
• Site location information, including maps and photographs
• Tape measure
CHEERS QAPP-1
- 30 -
July 2008
• Insect repellent/sunscreen (optional)
• Small first aid kit (optional)
• Whistle (to summon help in emergency,optional)
• Refreshments/drinking water (optional)
• Rope
• Camera/film (optional)
• Bottle of tap water (for rinsing exposed skin)
• Trash bag (to collect used safety gloves and other trash)
• CHEERS study contact list
• Cellular telephone
2.6 Collection of Surface Water Samples using Telescopic Pole
2.6.1 Select a bottle with a pre-printed label. Labels are coded based on the type and
location of sample being collected. Make sure that you are choosing the correct type and
location for sampling.
2.6.2 Verify that the bottle looks clean and remains sealed before starting to sample.
If sterility is questionable, deface the label and do not use the bottle.
2.6.3 Write the time and sampler's initials on the label with a waterproof pen
2.6.4 Put safety gloves on and remove the bottle cap carefully, ensuring that you do
not touch the inside of the cap or bottle.
2.6.5 Position the bottle in the holder at the end of the telescopic pole. Ensure that the
bottle is secure in holder and holder is tightly affixed to pole.
2.6.6 While standing at the access point, extend the pole making sure that all sections
are fully extended and tight fitting.
2.6.7 Immerse bottle (holding it about 45 degree) facing upstream so that water
surface and the upper 10 centimeter (~4 inches) layer are included in the sample (avoid
surface debris if possible).
2.6.8 Raise bottle out of water, retract pole.
2.6.9 Remove bottle from holder.
2.6.10 Pour off excess water until the water level is at the fill line or leave about 1-in
air space (for proper mixing purpose before analysis).
CHEERS QAPP-1
- 31 -
July 2008
2.6.11 Recap the bottle (tight enough) and dry off the bottle with paper towel.
2.6.12 Place sample in zip-lock bag and put it in insulated cooler.
2.6.13 Fill in the Field Data Sheet with times and initials (same as on bottle).
2.6.14 Fill in the Chain-of-Custody (COC) record (Appendix 17,18).
2.6.15 Turn over the coolers to the assigned custodian for transportation to the
analyzing laboratory. He/she must sign the Chain-of-Custody record.
2.6.16 Wait until the cooler is sealed with tamper proof tape.
2.6.17 Return Field Data Sheets to Water Project Manager as soon as possible.
2.7 Collection of Field Split samples
2.7.1 Field splits are used to estimate sampling and laboratory analysis precision.
Field split samples are to be collected the same way as the regular samples except that a
higher volume sterile sample container (1-liter) will be used for sampling. The 1-liter
container is used to collect water and split between three regular sterile sample
containers. Regular sample bottles must be labeled and ready for filling by the time of
sampling. All bottles must be kept closed, to be opened for sample filling only and using
aseptic technique. Before splitting, the sample must be shaken up. One staff member will
open the sample bottle and the other one will fill them (from the 1-liter container).
2.7.2 Collect a minimum of two sets of field replicate samples every day of field
sampling. A minimum of 5% of all samples will include a split. Preferably, this should
take place at the access point closest downstream to a WRP.
2.7.3 When sampling will take place at a given access point more the once, the splits
will be prepared at the end of the sampling day.
2.7.5 Follow the steps of “Collection of surface water samples” (from 2.5.1.1 to
2.5.1.11) for the collection of field split samples. They are to be processed the same way
as regular samples sent to the laboratory.
2.8 Preparation of Field Blanks
2.8.1 Sterile buffer will be available for the preparation of field blanks.
CHEERS QAPP-1
- 32 -
July 2008
2.8.2 At least one field blank for every 20 samples of each method will be prepared
on each day of sampling, the total number to be determined by the number of samples
collected. If more than one blank is needed, then blanks will be prepared at more than
one location.
2.8.3 Field blanks will be prepared at the end of the sampling day.
2.8.4 Procedure for preparing field blanks:
2.8.4.1 Locate the sterile bottle of water and the “field blank” coded sample bottles.
2.8.4.2 Initial and note the time on the sample bottle label.
2.8.4.3 Uncap the sample bottles and fill them up with the sterile water until fill-line.
2.8.4.4 Continue the procedure from the 13th step of the “Collection of surface water
samples” (these are handled like regular samples)
2.9 Procedures for 20 liter cubitainer sample collection
2.9.1 Wear new gloves
2.9.2 Connect new sample transfer tubing to sample pump
2.9.3 Position inlet tubing ~6” below surface of source water but not in bottom or
debris
2.9.4 Open pre-labeled 20 Liter cubitainer
2.9.5 Insert tubing into cubitainer
2.9.6 Fill cubitainer near full and cap
2.9.7 Record sample time, volume, and sampler initials on label and data sheet
2.9.1 Transfer to UIC laboratory
2.10 Virus sample collection
2.10.1 Wear new gloves
2.10.2 Remove inlet and outlet caps from pre–labeled filter
2.10.3 Connect new sample transfer tubing to filter inlet
2.10.4 Position inlet tubing ~6” below surface of source water but not in bottom or
debris
2.10.5 Connect tubing from filter outlet to sample pump
2.10.6 Turn pump on and filter 100 liters from water source
2.10.7 Replace filter inlet and outlet covers after sample collection
CHEERS QAPP-1
- 33 -
July 2008
2.10.8 Record sample time, volume, and sampler initials on label and data sheet
2.10.9 Store in cooler and transfer to UIC laboratory
2.11 Safety Precautions for Samplers
2.11.1 Rivers are under the direct influence of treated wastewater discharge. Fecal
contamination or pathogens may be present.
2.11.2 Use disposable safety gloves when collecting samples (have a bag with you
for collecting contaminated ones)
2.11.3 Wash your hands thoroughly after sampling; use hand sanitizer when hand
washing facilities are unavailable.
2.11.4 Do not touch your eyes, ears, nose, or mouth until you’ve washed your hands
2.11.5 Operating in and around bodies of water carries the inherent risk of drowning.
Collecting samples in cold weather, especially around cold water bodies, carries the risk
of hypothermia and collecting samples in extremely hot and humid weather carries the
risk of dehydration and heat stroke. Sampling team members should wear adequate
clothing for protection in cold weather and should carry an adequate supply of water or
other liquids for protection against dehydration in hot weather.
3. Sample Handling and Custody
3.1 Sample Handling
All requirements for methods 1600 and 1603 for sample containers, preservation
techniques, sample volumes and holding times will be met. Specifically, all bacteria
samples will be held at 1-4°C during transit to the laboratory, and the time between
sample collection and the initiation of analysis will not exceed 6 hours. The laboratory
will provide certified-clean sample containers. Separate sample containers for QC
samples will also be provided by the analytical laboratory.
3.2 Sample Identification
Samples are to be identified on the sample container with a separate identification label.
All labeling will be done in indelible/waterproof ink. Any errors will be crossed out with
a single line, dated, and initialed.
3.3 Sample Custody
CHEERS QAPP-1
- 34 -
July 2008
After collection and identification, samples will be maintained under chain-of-custody
procedures specified by the analytical laboratory. Proper sample custody procedures will
be used to ensure that samples have been obtained from the locations stated and that they
have reached the laboratory without alteration. A sample is considered to be in a person's
custody if the sample is:
• in a person's actual possession;
• in view after being in a person's possession;
• locked so that no one can tamper with it after having been in physical custody; or
• in a secured area, restricted to authorized personnel
All samples will be accompanied by a Chain-of-Custody Record. When transferring
samples, the individuals relinquishing and receiving the sample will sign and date the
record. Once the samples have been received by the laboratory, a designated laboratory
person will check all incoming samples for integrity and note any observations on the
original Chain-of-Custody Record. Each sample will be logged into the laboratory system
by assigning it a unique laboratory sample number. This number and the field sample
identification number will be recorded on the laboratory report. The laboratory will
maintain a file of all the documents (e.g., Chain-of-Custody forms) pertinent to sample
custody and sample analysis protocol. For Chain-of-Custody forms, the laboratory
maintains a file copy and the completed original will be returned to the Water Project
Manager as part of the final analytical report. This record will be used to document
sample custody transfer from the sampler to other personnel or the laboratory.
3.4 Sample Packaging, Shipment, and Tracking
All samples will be delivered directly to the analytical laboratories by study staff, lab
staff, or commercial courier service.
3.5 Labeling/Identifying Water Samples
3.5.1 Samples will be identified on the sample container with pre-printed waterproof
identification labels. Sampling times and initials will be added with waterproof ink. After
securely affixing them, labels will be covered with transparent tape.
3.5.2 Sample identification code on the labels will code for location, sample type,
required analysis, sample number, required procedure, date and time.
3.5.3 Sampling location codes are listed in Appendix 19.
CHEERS QAPP-1
- 35 -
July 2008
3.5.4 Sample Type Code
• Blank (sterile water): BK
• Sample (regular sample): SP
• Field replicate (duplicate): FR
• Matrix spike sample: MS
• Matrix spike Sterile Water (blank spike): BS
3.5.6 Sample number code
This number will be given to each sample starting from 1111 based on the Field Log
Book’s serial number
3.5.7 Required procedure with the sample:
• No filtration performed in field: N
• Simple filtration performed in field: F
• Centrifugation performed: C
3.5.8 Date and time of sample collection
• Date will be preprinted on label: month-day-year
• Write time using military time (e.g. 3:08 PM would be 1508)
3.5.9 Example of coding:
Sampling at Alsip, for indicator bacteria, 15th sample in the Field Log Book, directly
sampled (no procedure), and collected on June 12th, 2:45PM;
Code: AL-SP-IB-15N-06-12-07-1445
3.5.10 Necessary information and appearance of label
3.5.10.1 Sample Identification Code
3.5.10.2 Name or initials of samplers (if initials shown on the label, a separate
sheet should be maintained showing the sampler’s full name and initials (to be able to
identify him/her)
3.5.10.3 Date and time of sampling
3.5.10.4 Requested Analyses (fully printed, no abbreviation)
3.5.10.5 Analysis method numbers (e.g. 1600, 1602, 1603)
4. Analytical Methods
CHEERS QAPP-1
- 36 -
July 2008
Water samples will be transported to a commercial EPA-certified analytical laboratory
for measurement of indicator organisms. Based on the results of the first preliminary
water sampling study (detailed in the 2007 QAPP: Water Sampling and Analysis
document), Enviro-Test/Perry Laboratories was chosen as the commercial lab to analyze
samples for E. coli and enterococci, using US EPA Methods 1603 and 1600, respectively.
SMI will analyze samples for coliphages using US EPA Method 1602 as they are the
only lab in the Chicago area able to perform the analysis. Laboratory certification and
initial sample receipt protocols are found in Appendices 18-19.
The analytic laboratories will dilute samples using specific sample volumes using
membrane filtration method for indicator organisms. We developed a series of 5 dilution
volumes to be performed for all samples, based on sampling site (CAWS vs. lake/lagoon)
for the following reasons:
1) For E. coli, the recommendations of “Microbiology Methods” text [3] are shown
below in Table 10. Although this provides information about fecal coliforms,
approximately 80-90% of the coliforms in the CAWS are E. coli. The “Range Covered”
is consistent with Dry Weather Risk Assessment values for the dry season, with higher
densities anticipated in the wet season.
2) For Enterococci analyses, we have chosen a lower dilution range based on the
expectations that densities will be at least ten-fold lower than the E. coli density, as noted
in the Dry Weather Risk Assessment.
Sample
Source
100
30
10
3
1
0.3
0.1
0.03
0.01
Lakes,
Reservoirs
x
x
x
Bathing
Beaches
x
x
x
x
x
River Water
x
x
x
x
x
CFU/100-mL
Range
covered*
20-
60
67-
200
200-
600
667-
2,000
2,000-
6,000
6,670-
20,000
20,000
-
60,000
66,670-
200,000
200,000-
600,000
* “CFU range covered” is calculated using membranes with 20-60 colonies (acceptable
range)
CFU/100-mL = # of colonies counted x 100 / Volume of sample filtered, in mL
CHEERS QAPP-1
- 37 -
July 2008
Table 10. Suggested range of sample volumes (mL) for fecal coliform tests using the
membrane Filter Method
Based on the knowledge of the pollution level of indicator organisms in CAWS, we have
decided to request the analyses of 5 dilution levels from each river samples.
The dilutions to be performed on samples in this study, based on location of sampling are
presented in Table 11 below. If filter clogging or interference is found to occur with the
100mL dilution volumes, that dilution volume will require reduction.
Filtration/Dilution
Location
Condition
E. coli (EPA 1603)
Enterococci
(EPA 1600)
Lake Michigan (off
shore and harbors)
Dry and wet
weather
100; 30; 10; 3; 1 mL 100; 30; 10; 3; 1 mL
Dry weather
Skokie Lagoons
100; 30; 10; 3; 1 mL 100; 30; 10; 3; 1 mL
Wet weather
30; 10; 3; 1; 0.3 mL 30; 10; 3; 1; 0.3 mL
Dry weather
North Shore
30; 10; 3; 1; 0.3 mL 100; 30; 10; 3; 1 mL
Channel – Bridge St
Wet weather
10; 3; 1; 0.3; 0.1 mL 30; 10; 3; 1; 0.3 mL
Dry weather
CAWS – North Side
10; 3; 1; 0.3; 0.1 mL 30; 10; 3; 1; 0.3 mL
Wet weather
3; 1; 0.3; 0.1; 0.03
mL
10; 3; 1; 0.3; 0.1 mL
Dry weather
CAWS – South Side
100; 30; 10; 3; 1 mL 100; 30; 10; 3; 1 mL
Wet weather
30; 10; 3; 1; 0.3 mL 30; 10; 3; 1; 0.3 mL
Table 11: Dilution volumes, by sampling location, weather condition, and indicator
CHEERS QAPP-1
- 38 -
July 2008
5. Quality Control
5.1 Interference and Potential Problems for Sampling
5.1.1 Two main sources of possible interference and problems during surface water
sampling can involve cross-contamination and improper sampling.
5.1.2 Improper sampling arises from unclean and non-sterile sample containers,
improper sampling technique or improper shipment procedures. Improper sampling can
be reduced by using standardized procedures for collecting, handling and shipping
samples and following the procedures of the SOP step-by-step. The commercial
laboratories will provide certified sterile sample bottles. If bottle sterility is questionable,
the sampler must deface the label or write on the bottle “non-sterile” when no label is
attached and put it aside. When temperature of sample measured at the lab is above 20°C,
sample analysis will be refused by the laboratory. Improper sampling technique may be
detectable from too high standard deviation between replicates. Statistical evaluation will
be used to examine the data for outliers.
5.1.3. Cross-contamination from sampling equipment is greatly reduced by using the
direct sampling method (collection of samples directly into the sterile container). If the
sampler accidentally touched the inside of the bottle, he/she must not use it.
5.2 Quality Assurance/Quality Control Samples
5.2.1 “Field Blanks” will be processed at every sampling day along with the regular
samples. For that purpose, sterile buffered water is taken into the field in a sealed
container. Two sterile sampling containers will be filled with it at the sampling site. They
will be marked according to the code of “Field Blank” and sent to the laboratory to be
analyzed with the regular samples. Analysis should result in "0" bacteria counts for
blanks (errors, contamination). See procedure under 2.7. Field blanks will be processed
close to the end of sampling events for the day.
5.2.2 “Field Replicates” and “Matrix Spikes” will be prepared for each method
during every sampling day, which will produce a minimum of one replicate and one
matrix spike for every 20 samples. Water will be collected in a 1 liter bottle and then
CHEERS QAPP-1
- 39 -
July 2008
distributed among four 250mL bottles. Two of the four bottles will be spiked, the other
two will serve as field replicates. Field replicates and matrix spikes will be analyzed with
the regular samples. Replicates should have comparable bacteria counts (precision of
sampling and analysis). See procedure under 2.6 for field replicate sampling.
5.2.3 Temperature will be checked and documented upon arrival at the laboratory.
Pistol-grip infrared thermometers are used in both laboratories that allow for touch-free
measurement of cooler and bottle temperature. Their accuracies are 1% what is ±2°F or
±1°C. Once releasing the button, readings display for 7 seconds which allows time to
record data.
5.3 QA/QC Procedures: Implementation of the QA/QC procedures for surface water
evaluation will be established through the following steps:
5.3.1 Ensure that each field team member is familiar with the provisions of the
QAPP. The Water Project Manager will ensure that each field team member is familiar
with the field sampling SOPs and QAPP prior to implementation of field activities.
5.3.2 The Data Quality Manager, with the assistance of the Water Project Manager,
will regularly perform a QA review of field activities, field data sheets and forms to
ensure that all procedures are followed.
5.3.3 The Data Quality Manager will verify that all laboratories contracted have a
written description of their QA activities and a QA plan describing the QA management
of day-to-day routine operations.
5.3.4 The laboratories contracted to evaluate samples are required to adhere to
defined quality assurance procedures to ensure that generated analytical data is
scientifically valid and are of known and acceptable precision and specificity.
5.3.5 Quality control charts will be maintained for every parameter measured for the
study including blanks, calibration factors, and measures of precision for each parameter.
The Data Quality Manager will initially perform weekly reviews and specify corrective
action as needed. Once acceptable performance is established, this review will be
conducted monthly or more often if needed.
CHEERS QAPP-1
- 40 -
July 2008
6. Instrument/Equipment Testing, Inspection, and Maintenance
Equipment maintenance and repair will be performed as required for each instrument.
Preventive maintenance for all equipment includes inspection before use, cleaning as
necessary during use, and thorough cleaning and inspection after use. Rechargeable
batteries are checked before use and recharged after use. For equipment using disposable
batteries, replacement batteries will always be stocked. Maintenance and repairs will also
occur when corrective action needs are identified. If the instrument cannot be repaired or
recalibrated, the instrument will be replaced.
6.1 Corrective Actions
Corrective actions involving field instruments, including pH, turbidity, DO and
temperature will be implemented by the UIC field personnel and documented in field
logs. Corrective actions for the analytical laboratory may include the following:
6.1.1 Reanalyzing the samples if holding time criteria permits;
6.1.2 Re-sampling and re-analyzing;
6.1.3 Evaluating and amending sampling procedures, and/or evaluating and
amending analytical procedures; and
6.1.4 Accepting the data and acknowledging the level of uncertainty.
7. Instrument/Equipment Calibration and Frequency
Each instrument will be calibrated following the specific manufacturer's
recommendations. Laboratory instruments should be calibrated prior to each use or on a
scheduled, periodic basis as specified in the analytical methods. Analyzing laboratories
both provided documentation of their equipment/instrument calibration data.
Typical microbiology lab instruments/equipment:
CHEERS QAPP-1
- 41 -
July 2008
Item
Action
Frequency
Accuracy
Bench surface
Monitor
for
contamination
Weekly
-
Thermometers
Check accuracy
Semiannually
0.1° C
Balances
Service and recalibrate
Annually
Balances, weights
Check accuracy
Monthly
pH meters
Standardize
Each use
0.1 pH value
Autoclave
Check performance
Monthly
Refrigerator
Check temperature
Daily
Freezer
Check temperature
Daily
Incubator
Check temperature
Twice daily
Air in workplace
Monitor bacterial density Monthly
Dilution water bottle
Check pH and volume
Each use
Table 12: Microbiology lab equipment monitoring
8. Inspection/Acceptance of Supplies and Consumables
Commercial water quality laboratories (EnviroTest/Perry and SMI) have established
rigorous QA/QC systems for all aspects of microbiologic analyses. Copies of their log-
books for rinse/dilution water sterility and quality (pH, conductivity, chlorine residual)
checks, media storage and preparation, media performance check, membrane filter
acceptance QA and copies of other documentation are available for us (and we have
copies of pages from those log books)
8.1 Dilution/Rinse Water Sterility Check
Prepared rinse water needs to be checked for sterility on a per lot basis prior to use. The
sterility check is accomplished by adding 50ml water to 50ml of a non-selective broth
and incubating the mixture. The absence of growth indicates sterility. Water failing the
sterility check is re-sterilized (and re-checked) or disposed. Table 13 below presents
information about the sterility checks, their monitoring frequency, and their acceptable
limits.
CHEERS QAPP-1
- 42 -
July 2008
Test
Monitoring Frequency
Maximum Acceptable
Limit
Heterotrophic Plate Count
Monthly or for new source
<1000cFU/mL,
Student’s t≤2.78
Conductivity
Continuous or each use
>0.5megohm resistance
or <2μmhos/cm @
25
o
C
pH
Each use
5.5-7.5
Total Organic Carbon (TOC)
Monthly
< 1.0 mg/L
Heavy metals, single (Cd, Cr,
Cu, Ni, Pb, Zn)
Annually (more frequently if
problematic)
< 0.05 mg/L
Heavy metals, total
Annually (more frequently if
problematic)
< 0.1 mg/L
Ammonia/organic nitrogen
Monthly
< 0.1 mg/L
Total Chlorine Residual
Monthly or with each use
< 0.01 mg/l
Table 13: Quality of reagent water used for microbiology testing [8]
8.2 Media Sterility Check
New lots of media received from the vendor as well as new batches of media prepared in
the lab need to be evaluated prior to use. Positive and negative controls are analyzed. The
positive control uses a culture that is intended to be the detected analyte of the procedure.
The negative control uses a culture that is not intended to be the detected analyte of the
procedure. Unacceptable lots/batches cannot be used for the analysis of samples.
8.3 Positive/Negative Controls
The method blank is a negative control that goes through all applicable analytical steps
and is used to document non-contamination of the analytical process. This control uses
sterile buffered dilution water to verify the sterility of the media, equipment and
techniques used to process environmental samples in a particular batch. The method
blank is considered a batch control parameter. Samples associated with a method blank
indicating high bias must be re-prepared and analyzed (if possible).
CHEERS QAPP-1
- 43 -
July 2008
The lab control sample is a positive control that goes through all applicable analytical
steps. This control uses a known culture to verify the ability of the entire analytical
procedure (i.e. media, equipment and techniques) to properly identify the presence of the
target organism in a particular batch of environmental samples. The lab control sample is
considered a batch control parameter. Samples associated with a failing lab control
sample must be re-prepared and analyzed (if possible). Where reanalysis is unavailable,
the data reported to client is associated with a narrative explaining the QC failure.
8.4 Media Storage
All opened containers of dehydrated media are stored at room temperature in a desiccator
unless otherwise recommended by the manufacturer. All prepared media is stored at 4
degrees C in an appropriate container until expiration date.
8.5 Filtration Unit Sterilization
Filtration units are sterilized using an autoclave. A thermometer is used to verify the
appropriate temperature of the autoclave environment. Additionally, sterilization tape is
used to indicate proper operation of the autoclave. Method Blanks (MBLKs) are
processed on each filtration unit prior to the first client-submitted sample as well as after
every 10 samples and the last sample. The results from these blanks are used to assess
and indicate clean filtration units prior to use on samples as well as throughout an in-
going run.
9. Non-Direct Measurements
Meteorology data will be obtained for the sampling sites from the closest stations.
10. Data Management
Field data sheets need to be inspected by the sampler for completion/correct information
and given to the Water Project Manager at the end of sampling day or on the following
day in case of late afternoon sampling. The Water Project Manager will review it for
errors and store them in a locked room. Water quality parameters will be entered in an
excel spreadsheet.
Laboratory analyses data for indicator organism will be received by the Water Project
Manager.
CHEERS QAPP-1
- 44 -
July 2008
C. ASSESMENT AND OVERSIGHT
1. Assessments and Response Actions
Reviews of data quality will be performed regularly, as outlined in the system-wide
quality management plan (QMP). This project includes several streams of quality
monitoring data, and we will use a graded approach to review them.
1.1 At weekly quality reviews, we will note and develop ways to prevent deviations in
the protocol that have occurred in the preceding week. This could include:
• rejection of samples by analytic laboratories
• intervals between sample collection and analysis that exceed 6 hours
• field blanks with quantifiable amounts of E. coli or enterococci
• Failure to collect samples at the correct locations or other breaches in protocol
1.2 At monthly quality reviews, summary statistics of field blanks, split samples, and
internal laboratory QC data will be reviewed. Summaries of mean densities of indicator
organisms by sampling location (CAWS vs. general use waters) will be reviewed and
outliers will be identified and reviewed.
1.3 At annual quality reviews, all quality data will be reviewed, as will summaries of
indicator and pathogen measures. Emphasis will be placed on system-wide quality issues,
with the goal of developing quality improvement strategies for the next recreation season.
D. DATA VALIDATION AND USABILITY
1. Data Review, Verification and Validation
Reduction of analytical results will be done using calculations recorded on analytical data
sheets. The laboratory QA manager will verify that the appropriate analytical method is
followed and the data are calculated properly. The laboratory QA Manager or his/her
designee will validate the data by comparing the raw data to the reported results. In
addition, the results of calibration and internal QA/QC checks will be compared with the
project acceptance criteria to assess the usefulness of the data. The laboratory analytical
reports will contain the following information:
1.1 Raw data, including results of calibration and internal QC checks;
1.2 Analytical data results;
CHEERS QAPP-1
- 45 -
July 2008
1.3 Units of measurement;
1.4 Client and sample identification;
1.5 Sample analysis dates;
1.6 Summary of any problems encountered;
1.7 QC data (matrix spikes, blanks,
ORPs); and
1.8 QA reviewer’s signature
2. Verification and Validation Methods
Upon receipt of hardcopy sample results for a monitoring sample, the Water Project
Manager will verify that the following information is included:
2.1 Sample result summary sheet, which should include the following:
2.1.1 Sample identification information
2.1.2 Sample result
2.1.3 Laboratory quality control checklist (or other verification from the laboratory
that all QC specifications were met)
2.1.4 Method Bench Sheet completed by the laboratory with primary sample
processing and analysis data associated with the sample
2.1.5 Laboratory comments. Comments may include any applicable data qualifiers.
The following is a list of potential data qualifiers:
2.1.5.1 Sample arrived at the laboratory in unacceptable condition (i.e., leaking)
2.1.5.2 Sample holding time exceeded
2.1.5.3 Sample holding temperature not within acceptable range
2.1.5.4 Unacceptable blank sample result
2.1.5.5 Unacceptable positive or negative control result
2.1.5.6 Media sterility checks were not acceptable
2.1.5.7 Method incubation times or temperatures were not within acceptable range
2.1.5.8 Membrane filtration: Too much sediment on the filter
2.1.5.9 Membrane filtration: Confluent growth of non-target organism
2.1.5.10 Membrane filtration: Colonies too numerous to count (TNTC)
2.1.5.11. Membrane filtration: Pre- or post- filtration series sterility check not
acceptable
CHEERS QAPP-1
- 46 -
July 2008
If laboratory forms are missing, incomplete, or incorrect, the Water Project Manager will
contact the laboratory project manager immediately to discuss and request resubmission
of the missing forms and/or spreadsheets.
CHEERS QAPP-1
- 47 -
July 2008
References
1.
Geosyntech Consultants. 2008. Dry and wet weather risk assessment of human
health impacts of disinfection versus no disinfection of the Chicago Area Waterways
System (CWS).
2.
Lee, J. V., S. R. Dawson, S. Ward, S. B. Surman, and K. R. Neal. 1997.
Bacteriophages are a better indicator of illness rates than bacteria amongst users of a
white water course fed by a lowland river.
Water Sci Technol
35(11-12):165-170.
3.
Love, D. C., and M. D. Sobsey. 2007. Simple and rapid F+ coliphage culture,
latex agglutination, and typing assay to detect and source track fecal contamination.
Appl
Environ Microbiol
73(13):4110-8.
4.
Noble, R. T., S. M. Allen, A. D. Blackwood, W. Chu, S. C. Jiang, G. L. Lovelace,
M. D. Sobsey, J. R. Stewart, and D. A. Wait. 2003. Use of viral pathogens and indicators
to differentiate between human and non-human fecal contamination in a microbial source
tracking comparison study.
J Water Health
1(4):195-207.
4. U.S. Environmental Protection Agency, ERT, 1994. Surface Water Sampling, SOP#:
2013 (11/17/94)
5 U.S. Environmental Protection Agency, ERT, 1994. General Field Sampling
Guidelines, SOP#: 2001 (08/11/94)
6 U.S. Environmental Protection Agency, R&D, 1978. Microbial Methods for
Monitoring the Environment, Water and Wastes, EPA-600/8-78-017 (Edited by R.
Bordner and J. Winter)
7 U.S. Environmental Protection Agency, ERT, 1992. Performance Evaluation (PE)
Samples, Quality Assurance Technical Information Bulletin (May 1992)
8. U.S. Environmental Protection Agency, ERT, 1992. Quality Assurance/Quality
Control Samples, Quality Assurance Technical Information Bulletin (May 1992)
9. U.S. Environmental Protection Agency, ERT, 1995. Superfund Program,
Representative Sampling Guidance, Volume 5: Water and Sediment, Part I; Surface
Water and Sediment, December 1995
CHEERS QAPP-1
- 48 -
July 2008
10. U.S. Environmental Protection Agency, NRMRL, 2007. EPA 625/R02/017: Time
Relevant Beach/Recreational Water Quality Monitoring and Modeling, January 19th,
2007
11. APHA-AWWA-WPCF: Standard Methods for the Examination of Water and
Wastewater, 21st Edition, 2005: Part 9000: Microbiological Examination
12. U.S. Environmental Protection Agency, OWOW, 1997. Monitoring and Assessing
Water Quality, Volunteer Stream Monitoring: A Methods Manual EPA 841-B-97-003
13. U.S. Geological Survey. 2003: National Field Manual for the Collection of Water-
Quality Data: Chapter A7: Biological Indicators (7.1.2A: Surface Water collection
11/2003)
14. MWRDGC R&D Department (Report No. 05-15) Interim Report, Fecal Coliform
Densities in Chicago area Waterways during dry and Wet Weather 2004
15. MWRDGC: Interim Phase I Dry Weather Risk Assessment of Human Health Impacts
of Disinfection vs. No Disinfection of the Chicago Area Waterways System (Project No.
CHE8188) 2006 November
16. Prepared by Research Triangle Institute, U.S.EPA Contract 68-D4-0091 (August 24,
1999): Data Quality Objectives and Statistical Design Support for Development of a
Monitoring Protocol for Recreational Waters, Final Report.
Table of Contents
A. PROJECT MANAGEMENT
1
1. Distribution List
1
2. Project/Task Organization
1
3. Problem Definition/Background
4
4. Project Task/Description
5
5. Quality Objectives and Criteria
6
6. Special Training/Certification
6
7. Documents and Records
8
B. DATA GENERATION AND ACQUISITION
11
1. Sampling Process Design (Experimental Design)
11
2. Sampling Methods
11
3. Sample Handling and Custody
31
4. Analytical Methods
31
5. Quality Control
33
6. Instrument/Equipment Testing, Inspection, and Maintenance
37
7. Instrument/Equipment Calibration and Frequency
37
8. Inspection/Acceptance of Supplies and Consumables
37
9. Non-direct Measurements
39
10. Data Management
40
C. ASSESSMENT AND OVERSIGHT
42
1. Assessments and Response Actions
42
2. Reports to Management
43
D. DATA VALIDATION AND USABILITY
44
References
45
List of Tables
ii
List of Figures
ii
List of Appendices
iii
List of Tables
CHEERS QAPP 2
i
July, 2008
Table
Description
Page
Table 1 Index of Documents and Records, Storage and Distribution
9
Table 2 Composition of Field teams
20
List of Figures
Figure
Description
Page
Figure 1
Overall Project Management Structure for Survey Methods
3
Figure 2
Field Team Management Structure
20
CHEERS QAPP 2
ii
July, 2008
List of Appendices
Appendix
Description
1A-P
CHEERS Survey Training Manual
2
EPA/CDC NEEAR Study Beach Survey
3
EPA/CDC NEEAR Telephone Interview
4A-B
Eligibility Screener & Refusal Tally
5
Field Survey A
6
Field Survey B
7
Follow-up Telephone Interview
8A-E
CHEERS Pilot Study protocol and IRB approval
letter
9
Use Survey Data Sheet
10
IRB Exemption for user survey
11
IRB approval for CHEERS Survey Methods
Protocol
12
Thermal wristband bar coding technology
13
CHEERS Survey Methods Field Report
14A-U
Maps of recruitment areas
15
CHEERS Location Specific Flyer
16
Adult Consent Form
17
Parental Consent Form
18
Assent Form
19
Questionnaire Variables for Data Analysis
CHEERS QAPP 2
iii
July, 2008
A. Project Management
1. Distribution List
UIC: S. Dorevitch, P. Scheff, M. Javor, P. Pratap, S. Wuellner, A. DeLaquil, J.
Wuellner, T. Schoonover and all other CHEERS staff.
MWRDGC: T. Granato
2. Project/ task organization
Figure 1 outlines the lines of authority linking key members of the CHEERS study
involved in the questionnaire development and project implementation. The study director will
have overall authority in the development and implementation of the study questionnaires and
hiring of project managers involved in recruiting and survey administration. The quality
manager will establish overall data quality objectives for the CHEERS study and will be in
charge of reviewing the quality of the data and the questionnaire data collection process. The
detailed organizational structure is provided in the Study Overview.
The Survey Project Manager (SPM), Preethi Pratap, PhD, has primary responsibility for
the development and implementation of the study survey questionnaires, hiring and monitoring
of field interviewers and recruiters, communicating with the Survey Research Laboratory (SRL),
and maintaining the overall quality of the field data collection and management process. The
Survey Project Manager, with assistance from the Assistant Survey Project Manager (A-SPM), is
responsible for the following project related tasks:
2.1. Develop the study questionnaires and conduct necessary pilot tests and reviews in
order to improve and ensure quality of these questionnaires and the field data
collection methods.
2.2. Obtain Institutional Research Board (IRB) approval for the use of the study
questionnaires in the field and data collection methods.
CHEERS QAPP 2
1
July, 2008
2.3. Work with the University of Illinois at Chicago (UIC) Survey Research Laboratory
(SRL) to program and review the study questionnaires.
2.4. Work with the project manager to recruit and hire data managers and interviewer-
recruiters.
2.5. Develop, implement, and evaluate training programs for field data managers and
interview-recruiters.
2.6. Ensure all field team members involved in recruiting and survey administration are
certified (and maintain certification) by the UIC IRB for Human Subjects Research.
2.7. Work with the SRL to develop and conduct the appropriate training methods to
administer the questionnaires.
2.8. Ensure all field team members involved in recruiting and survey administration are
trained in questionnaire administration and interviewing techniques before they
begin recruiting in the field.
2.9. Implement adequate quality control methods during field events to ensure accurate
compilation of consent documents, assignment of Case IDs for study participants,
and the smooth flow of participants through the study process.
2.10. Meet regularly with survey data managers to review and, when possible, improve
the field work conducted by the interviewer/recruiters.
2.11. Securely transfer data to SRL after each field event.
2.12. Assist the Project Manager to staff the field events. Assist the Clinical Manager,
when necessary, to ensure timely clinical specimen collection or pickup.
2.13. On a weekly basis track study enrollment and data collection of the CHEERS
study, including study completion by participants, and attrition from the study.
2.14. Provide timely feedback and reports to the CHEERS study quality manager and
study director throughout the data collection process.
CHEERS QAPP 2
2
July, 2008
Study Director
S. Dorevitch, MD, MPH
Quality Manager
P. Scheff, PhD
Survey Project Manager
P. Pratap, PhD
Survey Research Lab
Project Manager
I. Farrar
- Questionnaire design
- QRC Reviews
- IRB
- Training
- QC methods
- CHEERS call center
Assistant Survey
Project Manager
J. McGowan
Survey Research Lab
Data Manager
V. Parker
- Tablet programming
- Hardware selection
- Training
- Data transfers
Interviewer/recruiters
(Approximately 50)
Field Data Managers
(Approximately 12)
Participant
Recruitment Manager
A. DeLaquil, BS
Figure 1: Overall Project Management Structure for Survey Methods.
CHEERS QAPP 2
3
July, 2008
3. Problem Definition/Background
The overall scientific background and goals of the CHEERS study are outlined in the
Study Overview document. The following sections will focus on the development,
implementation and use of the CHEERS questionnaire data collection methods.
In order to meet the following study objectives:
3.1 To determine rates of acute gastrointestinal and non-gastrointestinal illness
attributable to recreation on the Chicago Area Waterways System (CAWS)
3.2 To define the relationship between concentrations of microbes in the water and
rates of water illness among recreators
It is necessary to:
•
define participant demographics, non-water-related risk factors for illness, other
water exposures, and health end-points
•
ensure that the survey questionnaires are easy to follow and complete
•
characterize usage at CAWS recruiting locations
•
use available technology for reliable recording of responses in a timely fashion
•
develop a system for assigning unique participant ID and be able to track their
progress throughout the study
•
develop a secure and reliable method for transferring data from the field to SRL
•
have a system in place for tracking participant completion of study elements and
attrition rates
•
construct a database of the survey data
CHEERS QAPP 2
4
July, 2008
4. Project/Task Description
The overall project objectives are:
4.1. To develop CHEERS study questionnaires
4.2. Work with SRL to program the questionnaires into portable computers and desktop
computers
4.3. Ensure all project staff are IRB certified, and trained in survey administration and
interviewing techniques
4.4. Conduct a pilot study in order to evaluate and refine the study questionnaires prior to
use in the field
4.5. Conduct a user survey to identify potential sampling locations for CHEERS study
recruitment
4.6. Obtain IRB approval (pilot and final epidemiologic study) for use of study
questionnaires and the data collection methods involving human subjects
4.7. Establish the field study base of operations on day of event
4.8. Provide advance and day-of-event publicity
4.9. Screen interested individuals for eligibility
4.10. Enroll those who are eligible through the informed consent process
4.11. Administer survey questionnaires prior to and after recreation
4.12. Transfer and receive data through secure channels to/from SRL at the end of each event
4.13. Conduct follow-up telephone interviews
4.14. Coordinate the above with clinical specimen collection and collection of water samples
4.15. Implement QA/QC methods throughout the data collection and management process
4.16. Provide field reports and quality reports to the study director and quality manager on a
regular basis
4.17. Track progress of participant enrollment and completion of study
4.18. Analyze data to improve and refine the data collection process and recruiting methods
The proposed dates and locations of various recruiting events are outlined in Study Overview.
CHEERS QAPP 2
5
July, 2008
5. Quality Objectives and Criteria
The overall quality objectives are:
5.1. To develop appropriate data collection methods to gather high quality data that can be
used to test the hypotheses of the study described in the Study Overview
5.2. To ensure all CHEERS personnel will receive proper training and oversight
5.3. To maintain integrity of data at all times during data collection and data transfer from
field/phone to final dataset
5.4. To ensure that 100% of the participants who complete the field surveys will receive a
follow-up telephone call on days 2, 5 and 21 of the study
6. Special Training/Certification
As per the University of Illinois at Chicago (UIC) Institutional Review Board (IRB) all
UIC Investigators and key research personnel in the CHEERS study are required to meet the
training requirements in human subjects protections before their involvement in any research
involving interactions with human subjects (including the publicity and recruitment process,
consent process, administration of surveys and telephone interviews). No CHEERS study
personnel will be allowed to participate in research activities involving human subjects until they
have, and maintain, IRB certification.
All CHEERS personnel involved in recruiting and survey administration or any human
subject interaction in the field will be required to obtain IRB certification in Biomedical
Research at UIC. The SPM is responsible for maintaining a log of the IRB certification status of
all CHEERS study personnel at all times during the study. Study personnel will be reminded
about the expiration of their IRB certification status at least 2 months in advance, and if
necessary, provide information as to how to take the online training. Any study personnel with
an expired IRB certification status will not be allowed to participate in field activities involving
human subjects.
CHEERS QAPP 2
6
July, 2008
In order to be eligible to recruit participants, administer survey questionnaires, and
conduct telephone interviews all CHEERS study personnel will also be required to complete an
in class training session designed and conducted by the SPM with assistance from SRL.
The training will cover all documents included in the training manual (Appendix 1).
Many training documents from the EPA/CDC NEEAR study were adopted and revised. In
addition CHEERS study personnel will also complete the following:
•
An on-line general recruiting and interviewing training session designed by SRL
•
A power-point presentation of the CHEERS study overview
•
A power-point presentation outlining a day in the field, including:
o
An introduction to survey questionnaires
o
An introduction to interviewing techniques, “Do’s” and “Don’ts”
o
How to approach participants and recruit them
o
How to check participant eligibility
o
How to conduct the consent process
o
How to administer the CHEERS survey questionnaires (including use of
hand-held computers in the field and telephone interviews on a desktop
computer)
o
How to hand out incentives in the field
•
Mock interviews - These are conducted in groups or one-on-one. The SPM, SRL
staff, and/or A-SPM will conduct these mock interviews. We will go through each of
the surveys question by question. The mock interviews will focus on:
o
Probing methods
o
Recording the answers correctly
o
What to do if problems arise
The SRL Project Manager and the SPM will plan and conduct an in-class training session
prior to each recreation season.
CHEERS QAPP 2
7
July, 2008
All new CHEERS personnel hired for the study will complete an in-class training session
(including the mock interviews) under the guidance of the SPM or any other staff member
identified by the SPM. When the new hire is conducting a mock interview, the SPM or other
designated staff member will note any problems with the administration of the questionnaire, as
well as any misclassification of responses.
7. Documents and Records
The development and use of all CHEERS documents and records related to Survey
Methods has been outlined in Section B of this document. The following Table 1 identifies these
documents and records, the personnel responsible for record keeping and the study personnel
who will receive copies of these documents.
Only the study director, the SPM, and SRL project staff will have access to raw data.
Study personnel will be IRB trained to treat data confidentially. Several levels of protection
minimize risks of disclosure to others in order to protect confidentiality of participants. The
consent forms which will contain both the name and unique Case ID for each participant will be
kept in a locked cabinet in a pre-assigned CHEERS office. De-identified data (only with Case
ID) will be stored in a computer at the study director’s office, SPM office, and SRL. Only de-
identified datasets will be made available to CHEERS staff (such as the study biostatisticians)
involved in data analysis. All computer data files will be password protected during transfer.
Only consent documents and contact-tracing forms will contain participants' names and
Case ID. All other study-related forms/data will contain the Case IDs only. A master file linking
the Case ID and participant names will be stored in a password protected computer file. Nothing
in this file will indicate participant characteristics, responses or results.
All data without personal identifiers will be retained for an indefinite period. The master
files linking names and Case IDs will be destroyed 6 years after completion of the study. No
audio or video tapes will be made of research subjects.
CHEERS QAPP 2
8
July, 2008
Table 1. Index of Documents and Records, Storage and Distribution
CHEERS Study Documents
and Records
Personnel Responsible
for developing and
storage
Storage Type
Distribution List
QAPP # 2 with appendices.
SPM
Computer file
Hard copy
-
All CHEERS study personnel
-
MWRDGC liaison
IRB certification/Training
completion status for
personnel
SPM
Computer file
Log book
-
SPM
-
Study director
CHEERS survey training
manual
SPM
Computer file
Hard copy
-
SPM
-
A- SPM
-
Study director
-
Data managers
-
Interviewer/recruiters
SRL phone/ call center
training manual
SRL/ SPM
Computer file
Hard copy
-
SPM
-
SRL
-
Study director
-
Call center staff
BLAISE technical manual
SRL / SPM
Computer file
-
SPM
Hard copy
-
A-SPM
-
All CHEERS Field personnel
Field Supply Checklists
SPM/Project Supply
Manager
Hard copy will be
stored in a
-
Project Supply Manager
file folder
-
SPM
-
All Field event coordinators
Equipment Maintenance
SPM/ SRL
SRL record
-
SPM
-
Assistant SPM
-
Study director
-
Data managers
-
SRL
-
Supply managers
Field Report
SPM/A-SPM
Hard copy will be
stored in a file folder
-
Quality Manager
-
Study Director
Call center reports
SPM/SRL
Computer file
Hard copy of reports
will be stored in a file
folder
-
SPM
-
A-SPM
-
SRL
-
Study Director
CHEERS QAPP 2
9
July, 2008
-
Quality manager
Field Recruitment/Attrition
reports
SPM/SRL
Computer File
Hard copy reports
stored in a file folder
-
SRL
-
SPM
-
A-SPM
-
Study Director
Paper-based forms (consents)
SPM/A-SPM
Copied and stored in a
secure file folder or
locked cabinet
-
SPM
-
Study Director
Gift card receipt book/ T-shirt
checklist
SPM/ Project Manager/
Project Supply Manager
Stored in the Program
Coordinators Office
-
Project Manager
-
SPM
-
Project Supply Manager
-
Supply manager
-
Field Supervisors
-
Data Managers
-
Incentives staff
CHEERS QAPP 2
10
July, 2008
B. Data Generation and Acquisition
1. Sampling Process Design
The study design, sample size calculations, sampling locations and rationale for design
are outlined in the Study Overview document. The following sections will focus on the
development, implementation and use of the CHEERS questionnaire data collection methods.
2. Sampling Methods
2.1. Development of CHEERS study questionnaires
The CHEERS study questionnaires developed for this study are derived from those used
in the National Epidemiological and Environmental Assessment of Recreational Water
(NEEAR) study, conducted by the US EPA and CDC (NEEAR Study Beach Survey and
Telephone Interview, Appendix 2 and 3). Like the NEEAR study, we will use surveys to
conduct pre-exposure health assessments, post-recreation exposure assessments, and post
recreation health follow-up by telephone. Modifications to the NEEAR approach are: 1) the unit
of recruitment (and interviewing) will be individuals, rather than family groups, and 2) exposure
questions specific to secondary contact recreational activities have been added.
A total of five CHEERS study questionnaires will be administered:
2.1.1 Eligibility screener and refusal tally sheet (Appendix 4)
2.1.2 Field Survey A (Appendix 5)
2.1.3 Field Survey B (Appendix 6)
2.1.4 Follow-up Telephone Interview (Appendix 7)
2.1.5 Home Clinical Evaluation Form (Described in QAPP# 3, Clinical Evaluation)
CHEERS QAPP 2
11
July, 2008
2.2 Survey Research Laboratory Computer Assisted Interviewing System
The CHEERS study questionnaires have been developed in conjunction with the
University of Illinois at Chicago (UIC) Survey Research Laboratory (SRL), a national leader in
survey development, administration, and analysis. Field Surveys A and B will be administered as
face-to-face interviews, with the exception of the telephone follow-up interview, which will be
administered by telephone. The questionnaires will be administered using computer assisted
interviewing (CAI) methods, with the exception of the eligibility screener and the home clinical
evaluation, which will use paper forms. The CAIs conducted in the field will be administered
using computer assisted personal interviewing (CAPI) methods, while the telephone follow-up
questionnaire will be administered using computer assisted telephone interview (CATI) methods.
Field interviews will be conducted using tablet computers and the telephone interview will be
conducted in a call center at UIC SRL using desktop computers.
We have chosen to use CAI methods for administering the questionnaires rather than
traditional paper and pencil techniques as CAI methods are known to enhance the quality of
survey data in a number of ways:
•
Routing problems (or skip patterns) within the questionnaire are eliminated.
•
Interviewers cannot miss questions or ask the wrong questions.
•
Questions are 'customized' correctly for each individual. For example, if a
participant’s recreational activity is “fishing,” questions will be asked about number
of fish caught, type of bait used, etc.
•
Information from one question can be carried out within the program or dates can be
populated for one question using information from a previous question.
•
The computer checks for inadmissible or inconsistent responses.
•
Data is entered one time, rather than two (in the field on paper and manually entered
into a into a computer site at the study center). This elimination of a data entry step
prevents errors.
•
Facilitate the fast turn around of data for the follow-up telephone interviews and
home visits.
CHEERS QAPP 2
12
July, 2008
SRL uses the Blaise CAI system developed by the Statistics Netherlands. Version 4.7
will be used for this study. This system functions on a Novell Network and is based on a
shareable storage device. It also works on notebooks and tablet computers for on-site
interviewing needs. Computing power and random access memory are located at each
“intelligent” interviewing station. The CAI software (a) simplifies the sign-off or start-up
procedures between the interviewing station and the network; (b) provides temporary back-up
storage for the data produced by an interview, in the event that the connection to the network
fails, is busy, or malfunctions or in the event of power and operating system errors on notebooks;
and (c) stores and executes the questionnaire text and administration logic per programmed
specifications.
The system also facilitates recoding of closed- or open-end text answers, execution of
real-time and post data collection consistency and range checks, complex branching and
calculations, and production of data files formatted for use with several popular statistical
analysis packages.
SRL’s Office of Survey Systems (OSS) works with the computer-related aspects of
interviewing. OSS contributes to the design and programming of software to schedule, screen,
track, and conduct computer assisted interviews. After the data collection period, staff members
will produce and run cleaning programs on closed-ended variables and produce composite
variables as necessary. They will also produce a final dataset in SAS format.
CHEERS QAPP 2
13
July, 2008
2.3 Evaluation of study questionnaires
The overall objective is to evaluate and refine the field and telephone questionnaires to
be used in the CHEERS Study. Specific aims are to:
- Evaluate question wording for problems of comprehension and sensitivity
- Estimate the time and difficulty of completing the questionnaires
- Evaluate question order to minimize potential satiation and or non-response
- Identify potential problems with the administration of the questionnaires
- Identify improved methods for recruiting study participants
- Identify improved methods for evaluating water contact for several water recreational
activities
2.3.1. SRL Questionnaire Review Committee
In order to produce high quality data it is necessary to make sure that the questionnaires
ask the right questions in the right way, and are user friendly. The CHEERS study questionnaires
were reviewed by an infectious disease epidemiologist, an environmental epidemiologist, and an
industrial hygienist at the UIC School of Public Health.
Following questionnaire development, each questionnaire was reviewed by members of
SRL’s Questionnaire Review Committee (QRC). This committee, composed of technical experts
within SRL, reviews all survey instruments at their pretest and final stages to ensure that
approved ethical practices are maintained and that basic principles of questionnaire construction
are followed. No instrument is administered to respondents before approval is obtained from this
committee.
CHEERS QAPP 2
14
July, 2008
The QRC members have extensive experience related to questionnaire construction. The
QRC review ensures that this expertise is applied to each SRL instrument regardless of which
staff member has drafted it. To this end the committee reviews each instrument with the
following kinds of concerns in mind:
•
Is the ordering of the questions appropriate?
•
Does the ordering present any possible problems of context effect?
•
Does the ordering meet the needs of the science while minimizing respondent burden
and optimizing cooperation?
•
Are all of the questions necessary? Should any be added?
•
Are the response options appropriate?
•
If response categories are given, are they consistent with the question, understandable
to the respondent, and least likely to invoke socially desirable answers?
•
Are the vocabulary and constructs in the questions comprehensible to the
respondents?
•
Is it reasonable to expect that the respondents have the knowledge or memory
required to answer the questions?
•
Are sensitive questions constructed to limit, as much as possible, less-than-truthful
responses because of social desirability?
•
Are there design or format questions remaining that might require the use of
cognitive methodologies, such as focus groups or think-aloud interviews prior to
typical pretesting?
Before instruments are used in the field, they are tested in-house. In the case of
computer-assisted telephone interviews like the proposed study, the CHEERS field staff will
conduct extensive testing of the computer programming to test the questionnaire logic during the
SRL training session and pilot study. This allows for evaluation of all skip patterns and
contingencies that are programmed into the questionnaire and avoids the awkward situation that
occurs when an interviewer encounters a problem while trying to keep a respondent on the
telephone.
CHEERS QAPP 2
15
July, 2008
2.3.2. Pilot Field Testing
In addition to expert review of the study questionnaires, pilot field testing is highly
recommended to maximize the ability to refine the questionnaires for validity. Although the
CHEERS questionnaires were developed using the EPA/CDC NEEAR study survey, the
modifications had not been tested in the field. Additionally, the study wants to avoid asking
potentially troubling or uncomfortable questions.
Prior to the field launch of the epidemiologic study, a pilot study was conducted to solicit
reactions from respondents on the questionnaire length, difficulty in comprehension, sensitivity
of questions asked, any wording problems, gauge the appropriateness of the questions and the
validity of the responses. In addition, the pilot work also assisted in debugging the questionnaire
administration process. The pilot data helped refine the final version of the questionnaires to be
used in a larger field study.
The CHEERS Pilot study protocol was submitted to the UIC IRB, along with a copy of
all the study questionnaires. The IRB approved the CHEERS pilot study. The field pilot study
was conducted from July 21- 30, 2007. A detailed description of the pilot study methods, IRB
approval letter, and evaluation forms used is found in the 2007 QAPP 2 document (Appendix 8).
2.4 Use Survey
This section will describe the use survey that we will undertake to characterize usage so
that the activities and locations of recreation among participants enrolled in the CHEERS study
can be compared with actual usage patterns. If users who enrolled in the study tend to engage in
low-exposure activities, or recreated in areas of relatively low microbe densities, the true risk
among users in general may be underestimated. By working to ensure the comparability of the
study subjects to the larger population of users, findings about risks of illness due to recreational
exposure on the waterways will provide a more accurate estimate of risk among the population
of users.
CHEERS QAPP 2
16
July, 2008
Specific aims of the use survey are to determine:
- Usage, in terms of numbers of users, and types of recreational activities at various
access points
- To identify differences in usage between locations
- To identify differences in usage at the same location at different times of day, and
different days of the week
- To identify use at informal access points
The procedure of the usage survey will be as follows:
a. At CAWS recruiting events staff will select a clear view of the access point of
interest, preferably in a shaded location. They will fill out the top half of the Use
Survey Data Sheet (Appendix 9).
b. Using the datasheet, staff will tally the number of individuals who begin recreation,
by recreational activity. The tally will be done in 10-minute intervals. The first
interval will begin at 0, 10, 20, 30, 40, or 50 minutes past the hour. Staff will write
into the chart the hour (clock time) for each interval.
c. In order to avoid counting the same individual more than once, individuals will be
counted only when they begin a recreational activity. Those recreating at the time
that counting begins will be identified by circling the tally marks for those
individuals.
d. After a datasheet has been completed, staff will begin a second sheet, taking care to
again complete the top portion, including the page number.
e. Safety of the researchers will be maintained at all times. The field study will not be
done without adequate light and will be terminated in the event of inclement weather.
Researchers will also be instructed to leave the area and seek a safe location in the
event that the feel that their safety is threatened in any way.
f. At the completion of data collection, data sheets will be brought to the UIC School of
Pubic Health and photocopied. One copy will be placed in the mailbox of the SPM,
and the other in the mailbox of the study director.
g. Data will be entered into an excel file.
CHEERS QAPP 2
17
July, 2008
h. On two dates per month during the summer months, while the team is at the primary
access points at which the study will take place, another pair of staff members will
follow the same protocol for one-hour intervals at public access points that are not
designated as recruitment sites for the CHEERS study.
i. Two surveys by boat will be conducted, one on the North Shore Channel / North
Branch of the Chicago River and a second survey on the Little Calumet River/ Cal-
Sag Channel to identify use at areas not identified on the Public Access Inventory
compiled by the MWRDGC.
j. Schedule and locations: When field teams are conducting the CHEERS study at
various locations, one staff member will monitor use.
k. Consent: Because this is a study of the behavior of anonymous individuals in a public
space this study has been granted exempt status by the UIC IRB for the protection of
research subjects (Appendix 10). Thus no consent process for the recreational use
study is necessary and none will be performed.
l. Only field staff trained in this protocol will conduct this use survey. When the
survey is conducted in isolation (i.e., on days or at locations that participants are not
being enrolled in the CHEERS study), staff members will work in pairs. When the
survey is conducted at the time and place of the CHEERS study, staff may conduct
the survey without a partner.
m. Data management: Following the field survey, copies of the datasheet will be used to
enter the tallies by category into an Excel spreadsheet, which will be converted to
SAS format for analyses. These analyses will include:
1. Frequency of each activity
2. Frequency of use by location
3. Frequency of use by activity, by location
4. Differences in use within a location, weekend vs. weekday
5. Differences in use within a location, morning vs. afternoon
These analyses will be conducted by a CHEERS staff member, and data will be
stored in a computer file on a desktop computer in the study director or SPM’s office.
CHEERS QAPP 2
18
July, 2008
2.5 Field Health Measures Protocol
The pre-event study publicity methods and organizations involved have been outlined in
the Study Overview. The CHEERS survey methods protocol, outlined below, was approved by
the UIC IRB, (this includes all the study questionnaires, recruitment materials) (Appendix 11).
The following section outlines the steps involved in day of event staffing, publicity, recruitment
and data collection using the study questionnaires.
2.5.1 Day of Event Staffing
The expanded recruiting schedule will require significant increases in staff, including
managers of the field teams. The proposed management structure for the field work is presented
in Figure 2. At each location (A-D), a morning team and an afternoon team (teams 1 and 2) will
operate. The composition of each team is described in Table 2. While each member of the field
team will have a primary responsibility, they will be cross-trained, and data managers and
recruiters will be able to function as interviewers as need. Likewise, interviewers will be able to
recruit potential participants. The structure of teams for large events will be similar, though 2-4
teams may be in the same location.
Responsibilities of field team managers are:
•
Project Manager (Sara Wuellner, MS): Responsible for staffing and scheduling
field events.
•
Survey Project Manager (Preethi Pratap, PhD): Responsible for recruiting and
training survey staff, developing and using questionnaires, ensuring the transfer of
field survey data to the call center, working with SRL to track completion rates at
all phases of the study, and ensuring the collection of high-quality survey data.
•
Field supervisors: Assist the SPM in monitoring field work, training field
personnel, tracking quality of field data collection, and serve as a resource to field
team. Supervisors are responsible for transferring data to SRL at the end of each
field event.
CHEERS QAPP 2
19
July, 2008
Team A2
Team A1
Team B1
Team B2
Team C1
Team C2
Team D1
Team D2
Daily field supervisor A/B
Project Manager
Daily field supervisor C/D
Figure 2. Field team management structure
Table 2. Composition of field teams
Position
Primary responsibilities
Number per team
Data manager Consent form tracking,
ID/wrist band assignment,
tracks return for survey B,
downloading data
1
Recruiter
Approaches recreators, passes
out fliers, evaluates eligibility
1-2
Interviewer
Conducts field interviews A
and B; distributes T-shirts, gift
cards, obtains signature on cash
receipt, provides stool
collection instructions.
1-4
Note:
Detailed job descriptions of the above positions are provided in the CHEERS survey
training manual (Appendix 1)
Use survey
Records the number of
waterways users
1 (CAWS
only)
CHEERS QAPP 2
20
July, 2008
On the day of the event study personnel will wear CHEERS T-shirts and identification
badges. Teams of CHEERS study staff members will work together in a coordinated fashion to
conduct the water sampling and questionnaire administration. The field base of operations will
be based around a tent prominently displaying the CHEERS study banner. With the cooperation
of the MWRDGC, UIC will work with local municipalities and other government entities to
arrange all necessary permits for this field work.
The publicity and screening will take place outside of the tent, with study personnel
speaking with potential participants nearby. The consent process and administration of surveys
will generally take place in the tent. A typical day in the field can range from 8-12 hours.
CHEERS staff will work in shifts, at multiple sites. In addition to conducting interviews in the
CHEERS tent, we may conduct some mobile recruiting and interviewing. The composition and
responsibilities of the field teams will be as follows:
a. A field manager, who will have overall responsibility for establishing the sites;
coordinating the field logistics; allocating vehicles; and the collection and transport
of water samples.
b. A supply manager, who will ensure that all supplies and equipment necessary for
recruiting and interviewing study participants arrive on time to each location of field
work.
c. A field supervisor, who will be responsible for the overall administration of surveys,
management and security of consent forms and other field documents. The field
supervisor is the final authority in making decisions regarding questionnaire
administration and assigning interviewer duties. The field supervisor will have
responsibility for teams working over two shifts (AM/PM) at two locations. The field
supervisor will be in cell phone contact with data managers as needed. The field
supervisor will also assist in recruiting, interviewing and managing data as needed.
d. A data manager, who will receive the paper eligibility and consent forms. The data
manager will maintain a log of study participants, assign and record their Case ID
(unique identifiers), and place a wristband with that ID number and a bar code
version of that ID number, printed in waterproof ink (Appendix 12). The data
CHEERS QAPP 2
21
July, 2008
manager will also track participant progress through interview process, and will
ensure the efficient flow of participants through the process. The data manager will
ensure that all computer data files are consolidated and downloaded onto a flash
drive following each day of field data collection. The flash drive will be transferred
to the supervisor at the end of the day. The data manager will also ensure the proper
flow of research participants through each phase of the data collection process. The
data manager will work under the direction of the field supervisor and report any
unusual events or staff issues to the supervisor as and when they come up. The data
manager is responsible to download the data and transfer the files to the supervisor at
the end of the day. The data manager will also complete a field report (Appendix 11)
at the end of each day. The data manager will hold a briefing prior to each field work
shift during which time the recruitment area for the site will be defined and displayed
on a map. Interviewer-recruiters, both stationary and mobile (where applicable) will
be reminded to approach all individuals recreating within the sampling area.
e. The field supervisor will collect all the flash drives from the data mangers at the end
of each day. The data from these flash drives will be secured in a password protected
zipped file and emailed to the SRL staff. In addition an email with the details of
number of participants recruited for the day will also be sent to SRL.
f. Two to four interviewers, who will perform event day publicity, evaluate eligibility,
take potential study participants through the consent process, and administer Field
Surveys A and B. The interviewers may take turns to recruit and interview. In
addition some interviewers may work in mobile teams of 2 to recruit participants
away from the CHEERS tent. This increases our radius for recruitment.
g. Water sampling personnel, as described in QAPP # 1.
h. Many staff members will be cross-trained so that survey staff will be able to
participate in recruitment and consent. Similarly, some water sampling staff will be
able to conduct surveys. At the beginning of each field session, all staff trained in
recruitment and consenting will conduct such activities, and will then assume their
other roles (such as survey administration) as participants enroll. Likewise, when a
staff member assigned to administer surveys has no participant to interview, they will
conduct recruiting.
CHEERS QAPP 2
22
July, 2008
i. An incentive provider will be assigned to hand out the T-shirts and gifts cards at each
site. All participants will have to sign a receipt book as and when they receive their
gift cards. In addition a checklist will be used to record t-shirt distribution. It is the
responsibility of the incentive provider to report the final number of receipts to the
supervisor on site at the end of each day.
2.5.2 Recruitment areas
Recruiting locations are chosen with the goal of efficiently recruiting 9,330 study
participants distributed approximately equally among the three groups (CAWS, GUW and
unexposed) at locations that provide a range of water quality measures. At a given field site,
however, interviewer-recruiters are to approach all recreators within the recruitment area. This
is to avoid any bias, conscious or otherwise, that recruiters may have. Within a recruitment area,
all recreators (regardless of age, gender, ethnicity, or race) are approached, engaged by study
personnel, and if interested, screened for eligibility. Maps of the recruitment areas for specific
sites are included in Appendix 14.
2.5.3 Day of event publicity
Study personnel will approach individuals within the recruitment area of each site, and
work to interest them in participating in this research using a specified recruiting script and a
FAQ sheet outlined in the CHEERS survey training manual (See Appendix 1) outlining
responses to commonly asked questions. CHEERS team members will approach individuals
within the pre-defined recruitment area at the launch sites at access points, piers, harbors,
beaches (for lake kayakers), and at nearby running/biking trails, tennis courts, and sports fields.
Individuals will be provided with a location specific CHEERS flyer (See example in Appendix
15) before they begin their recreational activity and those interested in learning about the
research will be told more about the purpose of the study and what their participation in this
research would involve. They will also be given an opportunity to ask any questions. Any
CHEERS QAPP 2
23
July, 2008
vendors (water-activities or food/water) on site will be requested to pass along the flyers to
customers.
2.5.4 Eligibility
Individuals interested in participating in the CHEERS study will answer a series of
questions from the paper-based Eligibility Screener, which will determine their inclusion in to or
exclusion from the study.
Inclusion criteria are:
•
Will be engaging in outdoor recreational activities and will be available for
telephone follow-up over the next three weeks
•
Intend to return for interview B prior to that day’s departure of CHEERS
personnel, the time of which will be specified (generally before 8 PM)
•
Prior participant who has completed the 21- day telephone survey
Exclusion criteria are:
•
Recreational activity on day of enrollment will be swimming, water skiing or
tubing
•
Are currently participating in the CHEERS study (have not completed day-21
telephone survey)
•
Have participated in water recreation in the past 48 hours (not including
swimming pools, but including wading in beaches)
•
Are not able to complete Survey B in the field, or not willing to participate in
telephone follow-up
After the eligibility screener has been completed, individuals who are interested in
participating but are not eligible will be notified. Those who are eligible will begin the consent
process. Any reasons for ineligibility of participants or refusals will be recorded in the refusal
tally sheet (Appendix 4) section of the eligibility screener.
CHEERS QAPP 2
24
July, 2008
2.5.5 Consent
Study personnel will explain to eligible participants 1) what would be asked of them if
they agree to participate, 2) that participation is voluntary, and 3) the potential benefits and risks
of participation. They will provide a consent document which provides this information in
greater detail. After reading the consent document, participants will be given an opportunity to
ask any questions they may have about the study. All eligible participants 18 years or older who
choose to enroll in this research will sign the consent form (Appendix 16). A parent or guardian
will complete a parental consent form on the child’s behalf for all participants under the age of
18 years (Appendix 17). All children 7-17 years of age will also be given an assent form
(Appendix 18) to complete. All consent forms will be paper-based. Participants in group events
organized by Friends of the Chicago River who express interest in participating in the CHEERS
study in advance of the event will receive study information and consent documents by e-mail
prior to the event.
Eligible participants can enroll in this research as often as every 21 days. Each time they
would receive the same set of financial incentives (gift cards and checks) as well as a CHEERS
T-shirt. This research study will seek to recruit members of rowing teams and other sports
team/clubs several times during the 2008 season. If a child is on a team/club, the parent can
check a box on the signature page of the parental consent form that would allow the child to
enroll and re-enroll into the study even if a parent is not present. Parents can withdraw their
permission at any point by contacting the CHEERS team.
All CHEERS team members involved in recruiting will be certified by the UIC
Institutional Review Board (IRB) and all human-subjects research will take place with the
approval of the IRB (Appendix 11).
CHEERS QAPP 2
25
July, 2008
2.5.6 Unique identifiers (“Case ID”)
Upon completing the consent process participants will be directed to the field data
coordinator, who will take the consent forms, and apply a bar-coded wrist band with a unique
Case ID to the wrist of each participant. A second copy will be attached to that particular
participant’s consent form. In addition the participants case ID will be logged into a field book.
At each survey station a CHEERS staff member will scan the bar-code on the wrist band into a
laptop computer (the scanner will be connected through a USB port) and enter the participants
name into the form before beginning the surveys. At the end of the data download process each
day, SRL will be able to link the data from individual forms by Case ID and name for each
participant.
Bar-coding, a form of keyless data entry will allow automatic identification and data
collection is commonly referred to as Auto ID (Appendix 12). The motivation to use bar coding
is to improve data management and reduce errors related to data entry and tracking. The benefits
of bar coding for our study include:
•
Improved data accuracy: Scanning a bar-code rather than typing a number is over
99% accurate. With data entry playing such a critical role in this study, it is
absolutely necessary for each participant to have a unique ID and for us to be able to
link their data from individual forms in the field.
•
In addition, it is easier for a participant to have a bar-coded wrist band with their
unique case ID than to have a participant remember a case ID number, or to carry a
piece of paper with their Case ID when they are recreating.
•
The wristbands are durable and water proof and will have to be cut in order to be
released. In addition they will serve as a colorful reminder to the participant to return
to the tent to complete the survey after their activity.
•
Bar-coding reduces the impact of human error and enables users to work faster,
without sacrificing accuracy.
CHEERS QAPP 2
26
July, 2008
2.5.7 Field Survey A
Field Survey A (Appendix 5) will be administered in a face-to-face interview by trained
personnel. This will take approximately 2-3 minutes.
2.5.8 Field Clinical Evaluation
The 2007 QAPP included a plan for conducting a clinical evaluation of study participants
in the field on the day of enrollment. After conducting the field clinical evaluation on first 130
participants in August, 2007, little useful information was gleaned (findings were limited to bug
bites and allergic conjunctivitis). Given the low yield of this component of the study and the
recommendations of the WERF peer review panel, the field clinical exam was deleted from the
CHEERS protocol in September, 2007.
2.5.9 Field Survey B and exit protocol
a. After participants finish their recreational activity and return to the CHEERS
tent, study personnel will administer Field Survey B (Appendix 6). This will take
approximately 8-10 minutes.
b. Subjects will be reminded that in the three weeks following the field interviews,
they may be asked to provide a stool sample. They will be told that if they are
asked to provide a stool sample, they will receive all necessary materials by
FedEx delivery.
c. Participants who swam, jet-skied, went tubing or intentionally fell into the water
as part of their recreational activity will be disqualified from participating in the
follow-up phone surveys. However, participants who accidently fell into water
will still be allowed to complete Survey B and the phone follow-ups.
CHEERS QAPP 2
27
July, 2008
2.5.10 Incentives
After completing Field Survey B participants will be given a T-shirt that displays the
CHEERS study logo and a $15 Target gift card. They will be reminded that they will receive a
check for $35 after they complete all three follow-up telephone surveys. They will be told that if
they are selected for a home visit or provide a stool sample they will receive an additional $75.
2.5.11 Special Events
As noted in the Overview document, participants will be recruited using one of three
general approaches: intercept interviews, planning recruiting of teams and clubs, and special
events. In 2007 recruiting participants in two special events was arranged: the Chicago
Shoreline Marathon and the Chicago River Flatwater Classic. In 2008, Friends of the Chicago
River will again hold the Chicago River Flatwater Classic. Canoeists and kayakers will begin on
the North Branch of the Chicago River, and paddle downstream approximately 7.5 miles. This
will be an opportunity to enroll as many as several hundred research participants.
At the Flatwater Classic and other large events, approximately 30 CHEERS personnel
will staff the event to maximize participant enrollment. In addition to the larger number of staff
performing the various functions listed above, two individuals will be “team captains” who will
help ensure the smooth flow of participants through the enrollment and evaluation process.
Additionally extra supply management staff will ensure that all necessary supplies are readily
available to field staff. For events such as the Flatwater Classic and the Des Plaines River Canoe
Marathon, which have separate put-in and take-out locations, the bulk of the research team will
relocate from the site of initial recruiting/Survey A (put-in location) to the site where Survey B
will be administered (take-out location).
CHEERS QAPP 2
28
July, 2008
2.6 Telephone interview protocol
2.6.1 Location and facilities
The SRL call center will be used for the CHEERS follow-up telephone surveys. All call
center telephone follow-up instruments will be programmed in the SRL Blaise software for
CATI (Computer-Assisted-Telephone-Interviewing) administration. The call center computers
will be networked and have access to the call management software in order to track and
complete the telephone follow-ups in a timely manner. Staffing of the call center at a given time
will depend on the number of participants in the sample list. The call center staff will be
supervised by the SRL call center supervisor, and the SPM.
2.6.2 Methods
Once the field data is received by SRL they will send an email confirming the number of
cases they received that day. SRL will then set up a file including a list of individuals to follow-
up, their case IDs, and their telephone numbers in the call management system at the call center.
This turnaround of data will require roughly 24 hours (or one SRL day). Using the sample list
the call center staff will contact participants by telephone on days 2, 5, and 21 following
recreation. Participants who could not be reached as scheduled will be called later that day and
again over the following days. Participants will also be given a contact phone number to call
back if necessary. On establishing phone contact with the participant the Follow-up Telephone
Interview (Appendix 7) will be administered. This interview addresses the development of
symptoms of AGI and NGI during the interval since the date of recreation, or last phone contact.
Other questions address water recreation subsequent to the date of enrollment, and non-water
related risk factors for illness. The telephone interview process may take about 10
minutes
depending on the participant symptoms.
CHEERS QAPP 2
29
July, 2008
2.6.3 Triggers for the collection of clinical specimens
At the end of the telephone interview a list of all study participants, who on telephone
follow-up interview indicate that they have had, in the last 24 hours, symptoms of AGI,
conjunctivitis or skin infections, will be generated. The criteria for clinical specimen collection
will be:
a.
Any one or more of the following gastrointestinal symptoms:
o
Diarrhea
o
Vomiting
o
Abdominal pain or cramps ( that for adult female participants is reported as
being different than menstrual cramps)
o
Nausea accompanied by Fever
b.
The following eye symptoms
o
Eye drainage or crusting
c.
Skin symptoms
o
Any draining area on the skin
d.
Acute respiratory symptoms will not prompt a home visit, given the limited value
that such a visit would have in either identifying pathogens or establishing
objectively the presence of infection.
e.
At the completion of the third and final telephone interview, participants will be
thanked and notified that they will receive a check for $35 in the mail.
The protocol for specimen collection is described in QAPP # 3.
CHEERS QAPP 2
30
July, 2008
3. Sample Handling and Custody
Described in sections B 2 (sampling methods), and section B 9 (data management) of this
document.
4. Analytical Methods
4.1. Health end-points, predictors, and confounders/effect modifiers for overall study
Health end-points of interest in this study are based on previous research studies of
water-related GI and Non-GI illnesses. A review of literature is presented in the Study Overview.
Therefore, results of our study can be compared to other studies that have used similar end-points
or definitions for GI illness (Wade, 2006; Cabelli, 1982) and non-GI illnesses (Colford 2007).
The data analysis methods for the CHEERS study have been outlined in the Biostatistics section.
This includes the data collected from QAPP # 1 AND # 3.
Responses to questions from the CHEERS study questionnaires (QAPP #2) will be
categorized as described in Appendix 19.
4.2. Interim data analysis
Apart from the overall data analysis, the study director, biostatistician and SPM will also
create interim reports of process measures (recruitment by site, attrition through the phases of the
study) that will be used to modify the recruitment strategy, staffing and training involved in the
study.
CHEERS QAPP 2
31
July, 2008
4.2.1. Activities
Following the 2007 season we will review the kind of recreators (by activity) that we
have been able to recruit in the GUW and CAWS groups of participants. For example, if we have
recruited more fishers along the CAWS, compared to the GUW group, we will increase the
frequency with which recruiting teams are stationed at locations where GUW fishing occurs.
4.2.2. Demographics
All through the 2008 season we will also review the demographic similarities between
the exposed and unexposed groups, and by activity. We want all three recreator groups to be as
similar as possible in age and race/ethnicity. The 2007 data will assist in modifying the
recruitment strategy for the 2008 season to ensure equal demographic representation. For
example, if a large number of rowers are high school and college aged, we will seek to work with
high school and college sports teams that engage in non-water recreational activities.
In addition, enrollment and attrition rates will also be followed to make necessary
modifications to the study recruitment or data collection protocol.
4.2.3. Interviewer bias
All forms will include the name of the interviewer. We will identify interviewers that
have above average rates of incomplete “refused” or “don’t know” responses, as well as very
brief or prolonged average duration of interviews. Retraining of an interviewer maybe required,
based on the results.
4.2.4. Health end-points
We will also analyze the data to see if there is a discrepancy between reports of
symptoms and findings in clinical examination and culture. We do expect a number of
participants to have negative stool cultures in spite of having symptoms.
CHEERS QAPP 2
32
July, 2008
5. Quality control
Quality assurance methods have been employed throughout the questionnaire design,
development, programming and administration. These have been outlined in section 2. The main
goal of the questionnaire implementation process is to ensure that all data collected in the field
follows a systematic process, and all data is securely and accurately transferred to SRL. This
involves smooth operating of the equipment and tracking of participant progress through each
station, so that at the end of the day the data for each participant is complete and all this is
correctly transferred to SRL. This will lead to the compilation of a complete and usable dataset
of the study analysis. The following section describes the appropriate quality control methods
that will be employed before, during and after a field event.
Prior to each field event all equipment and supplies will be checked by the SPM, A-SPM,
Project Supply Manager and/or the field supervisor. Any monitoring will be more heavily
concentrated during the early phases of data collection and for newly-hired interviewers so that
any problems in performance can be detected immediately. The monitor (who could be the field
supervisor, SPM, quality manager, study director, or other designated CHEERS staff) will be
instructed regarding whom and when to monitor based on random selection of interviewing
stations and times during each field shift, or as a result of problems noted from previous
monitoring sessions.
The ongoing monitoring of field and CATI interview permits interviewer skills to be
evaluated in a number of key areas, including the ability to follow instructions correctly and the
ability to probe for clear and complete answers. Through monitoring, additional factors,
including the interviewer’s skills in eliciting cooperation and maintaining neutrality, are
routinely evaluated. Errors detected during these reviews will be recorded by the SPM to help
give feedback to interviewers and to identify the need to retrain as necessary. Positive as well as
negative feedback is given to ensure that the interviewers maintain a high level of commitment
to quality.
CHEERS QAPP 2
33
July, 2008
The quality control checks will include (but not be limited to):
a.
Check field event supplies (outlined in Section B7)
b.
Check all study equipment- tablets, flash drives, bar-code scanners, printers
etc. (outlined in Section B 6)
c.
Availability of paper-based forms (this includes the eligibility screeners, and
consent forms). Any paper-based materials with participant names or Case
IDs will be stored in a locking file cabinet. Only the SPM, field supervisor
and data manager will have access to this box/folder. All consent forms will
be counted periodically to make sure the numbers tally with the log book.
d.
Once the consent form is complete and a Case ID has been assigned (that is a
bar-coded wrist band is placed on the participant’s wrist) a second wrist band
with the participants ID number is stapled to their consent form. This creates
a paper-based record of the CaseIDs assigned to each participant. The
consent forms will be stored in a secure folder on site.
e.
The data manager will record details of each Case ID and the surveys
completed in field data log book.
f.
In order to assess interviewer performance, a “mock subject” will be assigned
to an event during different times of the data collection process. The location,
date and time will be selected randomly by the quality manager/study
director. This “mock subject” will behave and participate, throughout the
field event, just like any other participant, and neither field nor telephone
survey staff will be aware that this individual represents a “performance
evaluation” interview. The “mock subject” would have already been told to
answer certain questions in a certain way. The data collected for this “mock
subject” will be reviewed after the event to check if the interviewer recorded
the responses correctly i.e., if the responses were coded correctly. Based on
this review, the SPM will sit down with the “mock subject” and the
interviewer to discuss any issues with the survey administration.
CHEERS QAPP 2
34
July, 2008
g.
At the end of each field data collection day all the tablets and files will be
checked and secured by the field supervisor. The data manager will also
ensure that all computer data files are consolidated and downloaded onto a
flash drive following each day of field data collection. The flash drive will be
transferred to the supervisor at the end of the day. The field supervisor will
collect all the flash drives from the data mangers at the end of each day. The
data from these flash drives will be secured in a password protected zipped
file and emailed to the SRL staff. In addition, an email including the details
of number of participants recruited for the day will be sent to SRL.
h.
The downloaded raw data from the field will be stored in a password
protected files at UIC as a back-up to the data transferred at SRL.
i.
We will track all data transmissions to and from SRL with an email. SRL
staff will send an email acknowledging the number of files they received.
Any discrepancies in the number of files uploaded or downloaded will be
resolved immediately.
j.
A field report (Appendix 13) will be completed (See Section C) after each
field data collection event.
k.
Upon receiving the files at the end of each field event SRL will create a
sample list of all participant Case IDs, name and telephone numbers by date
of telephone follow-up. SRL will upload the sample list to programmed call
management software that will be used to track the progress of the telephone
follow-up. Telephone interviewers at the call center will have access to the
sample list, and can pick a Case ID to call. Once this Case ID is picked by
one caller it is removed from the list until it is complete. After completion of
the telephone interview, the interviewer will answer a set of pre-programmed
questions that will track disconnected numbers, appointments, unreachable
participants etc. After completion of the call the Case ID gets released to the
call management document, that will update the status and display the status
of the call, including the date and time for the next call. This assists in
tracking the telephone follow-up which is critical for this study. Disposition
codes will be created by SRL for use with the call management document.
CHEERS QAPP 2
35
July, 2008
The SPM will receive the call management reports at various points during
the telephone interview process to track the progress of the telephone follow-
ups.
l.
To receive effective feedback on the performance of telephone interviewers,
it is standard SRL practice to monitor approximately ten percent of each
telephone interviewer’s work. Interviewers will not know when they are
being monitored, since telephone monitoring is done using specially
equipped extension telephones. Monitoring will be done by Interviewer
Supervisors or the SPM. In addition we may use “mock participants” like we
did in the field.
m.
Data integrity/ Field devices: data collected on notebooks are password
protected. In addition we will use full disk encryption software to further
secure all data and programs stored on the notebooks’ hard disk.
n.
SRL network: The network servers use Microsoft Windows 2000, Windows
2003, and Novell IntraNetWare operating systems. These servers provide
data storage, dialup data transfer, e-mail, Web services, and data backups.
The Backup EXEC from Veritas is used to perform nightly backups on all
network servers on the network.
o.
Weekly quality meetings will review any issues with data collection/transfer.
Each week a different quality assurance procedure will be reviewed with the
interviewer-recruiters.
CHEERS QAPP 2
36
July, 2008
6. Instrument/Equipment Testing, Inspection, and Maintenance
SRL will develop a BLAISE CHEERS study technical manual that will address how to
run/troubleshoot the field and telephone interviewing programs, how to evaluate tablet
performance and conduct maintenance (including battery and key board checks), and perform
bar code scanner maintenance. This manual will be made available to all CHEERS survey staff
at the initial training (Appendix 1).
•
Prior to a field event the Project Supply Manager and/or the assistants will check each
laptop to make sure they are powered up and have 2 additional battery packs ready for
use in the field. Check the tablet key boards and screens as well.
•
External battery charging stations will be used to charge additional batteries.
•
It will be ensured that the bar-code printer and scanners are working and the bar-coded
wrist bands are prepared for the event.
•
At the end of each field event (after the data is downloaded at UIC), the Project Supply
Manager and/or the assistants will securely store all the tablets, printers and scanners in
a locked room at UIC.
7. Instrument/Equipment Calibration and Frequency
Not Applicable
8. Inspection/Acceptance of Supplies and Consumables
The SPM, Project Supply Manager and/ or the assistants are responsible for making sure
that all supplies outlined in the following sections are available during any field event and the
telephone interviews. A supply checklist will be used to record this information before and after
each event.
CHEERS QAPP 2
37
July, 2008
8.1 Supplies for use survey
Supplies for the use survey field study will be prepared at least one day prior to the
planned activity, and stored in plastic crates in the CHEERS storage space at the UIC SPH.
Supplies to be brought to the field are:
¾
Clipboards
¾
Sunblock
¾
Pens
¾
Watches or equivalent
¾
Folders
¾
Stapler
¾
Identification Badges
¾
Use survey forms
¾
Cell phone
¾
CHEERS telephone contact directory
¾
Cooler with beverages
¾
First Aid Kits
8.2 Supplies for day of event
Supplies for the day of event field study will be prepared at least one day prior to the
planned activity, and stored in plastic crates in the CHEERS storage space at the UIC SPH.
Supplies to be brought to the field, in addition to those listed as part of the water sampling
protocol are included (Described in QAPP# 1).
¾
Tent
¾
Banners
¾
Folding tables
¾
Chairs
¾
Laptop computers
¾
Bar code scanners
CHEERS QAPP 2
38
July, 2008
¾
Spare laptop batteries
¾
Publicity flyers (250)
¾
Consent Document-adult (100)
¾
Consent Document-parental, for child (100)
¾
Consent Document-assent for child (100)
¾
Field data log book
¾
Bar-coded wrist bands with Case IDs
¾
Bar-code scanner with USB ports
¾
Clipboards
¾
Pens
¾
Markers
¾
Accordion files for signed consent documents
¾
USB flash memory drives
¾
CHEERS telephone contact directory
¾
T-shirts (50 XL, 50 L, 50M, 50 S, 20 kids-L, 20 kids-S, 20 kids junior)
¾
Target Gift cards
¾
Exit packets: contact information
¾
Cooler and beverages for study staff
9. Non-direct Measurement
Not Applicable
CHEERS QAPP 2
39
July, 2008
10. Data Management
10.1. Transfer of field data
10.1.1.
Electronic data copies
In the field, data obtained from Field Surveys A and B will be copied from each laptop
onto a flash memory device, which will used by the field data coordinator.
10.1.2.
Data file transmission
These data files will be transmitted to UIC Survey Research Laboratory (SRL) via email
using password protected zip files. Once the files are transmitted, we will send the SRL staff an
email with the number of cases that we transmitted, including the data transmittal form.
10.2.
Survey Research Laboratory data handling
SRL personnel will follow this process in reverse to send to the SPM an email
confirming the number of files they received. SRL will create a list of individuals to contact,
their case IDs, and their telephone numbers for the call center. This turnaround of data will
require roughly 24 hours (or one SRL day). For data that is transmitted over the weekend, SRL
call center supervisors will run the necessary programs to create the phone list. These sample
files will be loaded onto a desktop with the call management software, to use as our telephone
follow-up sample list. SRL will create a field report after each event. This will be reviewed by
the SPM or A-SPM to track recruitment/attrition rates on a weekly basis.
CHEERS QAPP 2
40
July, 2008
10.3. Transfer of telephone data
At the end of each day all telephone interview forms will be saved on the desktop and
uploaded to the SRL server. SRL will create reports through the call management system. These
reports will be monitored on a weekly basis by the SPM, or the A-SPM.
10.4. Paper forms
Consent documents, any paper based forms and Case ID logs will be placed into locked
boxes/ folders on site. The originals will be stored in a locked file cabinet in an assigned
CHEERS office.
10.5. CHEERS study datasets
The SRL Office of Survey Systems (OSS) will be responsible for creating the final
datasets for the CHEERS study. Because all responses are entered directly into the computer at
the point of interview, the time consuming and potentially error prone process of converting raw
data responses to machine readable form is largely eliminated. To create an output file ready for
analysis, the data processing component creates a blank rectangular file and fills in codes from
closed-ended questions (and open-ended questions that have been coded), using the skip logic
programmed into the survey questionnaire. This process can be executed concurrently with data
collection (and is done at regular intervals as another quality control point).
The processing component is also used to test and edit the data while producing clean
computer files. When clean files are available, the system also produces documentation. This
includes code books, record layouts, and SAS, SPSS or STATA control cards (with both variable
and value labels). These code books can be used to produce absolute and percentage frequencies
and/or cross-tabulations for every coded variable at any time during the study and routinely used
as part of the quality control procedures.
CHEERS QAPP 2
41
July, 2008
SRL will deliver one preliminary and one final dataset at the end of each season for each
time-point/instrument prepared in SAS. These deliveries will contain lists of open-ended
responses, too. The SPM will be responsible for all coding and back coding of data at that point.
A final methodological report that summarizes the study design, questionnaire development
process, and programming and data processing procedures will be submitted by SRL.
All datasets will be saved in password- protected computer files. The datasets will not
contain any participant names or contact information. Access to the datasets will be restricted to
the study director, SPM, biostatistician and other personnel involved in the data analysis and
report writing process.
C. ASSESSMENTS AND OVERSIGHT
1. Assessments and Response Actions
a. IRB certification tracking: CHEERS staff will not be allowed to participate in any
field work involving human subjects without current IRB certification.
b. All CHEERS staff must complete the CHEERS survey training on interviewing
techniques that will be conducted by the SPM. This training will also include the
mock interview. Only after the SPM has observed and signed off on the mock
interview and training process and verified the IRB certification status, will a
CHEERS staff member be allowed to recruit participants in the field.
c. The SPM, A-SPM, or field supervisor will be in charge of observing the process in
the field on any given day. The field report completed at the end of each field event
will include any information on unusual occurrences, staffing problems, staff
concerns, and equipment issues. In the event of any trouble that cannot be resolved in
the field, it is the responsibility of the SPM to submit the field report to the Study
Director and the Quality Manager, who will have the final authority to make any
decisions regarding staffing, equipment changes or other participant issues.
CHEERS QAPP 2
42
July, 2008
d. The SRL call management document will track the telephone follow-up process (See
Section B5).
e. Any field and telephone interviewing issues specific to an interviewer in the field or
during the telephone interview process will be identified using “mock subjects” and
CATI interview screening methods. Steps will be taken to assist/retrain the
interviewer, or the interviewer may be reassigned to other appropriate tasks (See
Section B5).
f. Any equipment that does not work will be fixed before the next field event. For
example, if we have trouble with a tablet key, the SRL- CHEERS study technical
manual will be used to resolve the issue, failing which, SRL will be contacted to
service the tablet before taking it to the field. All equipment failure will also be
reported to the Study Director and Quality Manager (see Section B6).
2. Reports to management
All CHEERS questionnaire related documents described in the preceding sections (See
List in Section A9) will be stored by the SPM. It is the responsibility of the SPM to make these
available to the CHEERS Quality Manager and study director at all times. Any unusual
occurrence reports will be submitted within 24 hours to the quality manager. Any recruitment
issues, staff related issues or equipment trouble will be reported within 24 hours to the Study
Director.
In addition the interim data analysis reports (as described in Section 4) will be submitted
to the study director as outlined in Project Quality Management Plan document, or as and when
requested.
CHEERS QAPP 2
43
July, 2008
D. DATA VALIDATION AND USABILITY
Data review, verification, and validation methods have been employed during the
development, programming and implementation of the survey methods. These are outlined in
Section B and include:
•
Questionnaire review and validation process (including internal/external reviews,
IRB clearance, and pilot evaluations of survey questions)
•
Use of CAI (Computer Assisted Interviewing Techniques) and limited open-ended
questions to ensure accurate data entry. The questionnaires have been developed with
pre-determined acceptable response options. This prevents the entry of unacceptable
or out of range responses.
•
Data will be deemed unusable if the transmission of data files introduces errors in the
dataset. There are control in place to prevent this (back up data files on desktops,
transmission reports etc), and an error could be identified by a mismatch in the
encrypted emails (generated at the time of uploading data to SRL) and the
number/type of files received by SRL. Any mismatch will prompt a review of the
status of that particular Case ID awaiting upload, and/or the Case ID’s forms received
by SRL.
CHEERS QAPP 2
44
July, 2008
References
Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. 1982. Swimming-associated gastroenteritis and
water quality. American journal of epidemiology 115(4): 606-616.
Wade TJ, Calderon RL, Sams E, Beach M, Brenner KP, Williams AH, et al. 2006. Rapidly
measured indicators of recreational water quality are predictive of swimming-associated
gastrointestinal illness. Environmental health perspectives 114(1): 24-28.
Colford JM, Jr., Wade TJ, Schiff KC, Wright CC, Griffith JF, Sandhu SK, et al. 2007. Water
quality indicators and the risk of illness at beaches with nonpoint sources of fecal
contamination. Epidemiology 18(1): 27-35.
CHEERS QAPP 2
45
July, 2008
QAPP 2
Appendix 1: CHEERS Survey Training Manual
QAPP 2
Appendix 1A
CHEERS Survey Training Manual
Gaining Cooperation
GAINING COOPERATION
Let’s start out by talking about the role of the interviewer. This part of the
discussion will be brief because we will talk about each of the points in more detail later. The
following discussion will highlight the role of the interviewer.
1. It is important for interviewers to gain cooperation from recreators.
The percentage of people who answer our questions is critical. A high response rate
means greater confidence in the results of the study. You all know about response rates from
watching the nightly news. How many times have you heard a report of the outcome of a poll
that states 57% surveyed support something (+/-3%)? That means that between 54% and 60%
support the issue. The (+/- 3%) is the confidence interval.
A low response rate means a greater chance of error. We need to avoid getting a low
response rate.
2. It is critical for interviewers to communicate that we are doing scientific
surveys.
You are now all professional interviewers and researchers.
The key to
communicating that is to practice good techniques.
Know the answers to the CHEERS FAQ Sheet inside and out, backwards and
forwards.
Use your voice. Don’t try to sound like someone else.
Be confident and professional.
3. It is important to practice your delivery.
Throughout this training use every opportunity you get to read as a chance to
improve your ability to communicate that you are a professional interviewer.
4. True or False: People don’t want to do surveys?
This is a myth. People don’t want to talk with a telemarketer. We do scientific
research. People will answer our questions because they are posed by researchers, and it gives
them a chance to be heard.
How we are different from telemarketers:
We are not selling anything.
We are not asking for any money.
We will provide written information about the survey.
5. It is important to be prepared to answer respondents’ questions.
Respondents are likely to ask questions before you even get started with an
interview. For each survey, we anticipate these questions and provide you with answers. You
each have a copy of the CHEERS FAQ Sheet, which you should always keep available for quick
reference. Practice the answers to these questions. It is necessary for you to be able to answer
them with correct information in a confident and natural manner.
6. Turning a “No” into a “Yes!”
Listen carefully to the respondent's question, answer briefly and to the point, and
then continue with gaining their cooperation.
Your interest in the survey is communicated to the respondent by your voice, body
language, and appearance. There should be a pleasant and professional tone to your voice. You
should display open or neutral body language. Do not stand too close to the respondent.
You need to listen to the respondent's tone and watch his/her face and body
language. At what point did s/he become hesitant or uncomfortable? Is it possible s/he
misunderstood something you said? You must be prepared at all times to answer his/her
questions and you need to speak to him/her at his/her level of comprehension, without sounding
condescending.
Most respondents do not refuse outright; rather they express some hesitancy,
reservation or initial hostility. You will soon become sensitive to the firmness of the "no"
conveyed by the respondent's tone of voice and the wording of his/her comments. You will also
learn to sense the reasons behind a respondent's hesitancy and develop ways of dealing with
those "hidden" concerns. Listen very carefully to what the respondent has to say, and then
address your remarks to the respondent's concerns.
7. Common Reasons Given for Refusing
The reasons why respondents refuse are varied, and the approaches for handling
them will vary from respondent to respondent and Interviewer to Interviewer. There will be
times when a refusal cannot be avoided.
Listed below are some common reasons respondents give for refusing:
Too busy; don't have the time;
Not interested in the survey;
If I participate, you'll ask me to do more interviews later.
Don't want to be bothered/ don't want to be involved.
Afraid of being involved;
Waste of time and money;
Government interference;
"Nothing in it for me."
Too ill; don't feel well enough;
I don't give out that type of information, and
How do I know who you are/this survey is legitimate?
These reasons reflect the broad types of concerns respondents may have about the
time you are asking him/her to give and/or about surveys in general, as well as specific concerns
about the survey itself. When a respondent refuses, it is important that you listen carefully and if
possible, clarify the reason for the refusal.
Your responses should emphasize the importance of the survey and assure the
respondents that we appreciate their contribution to the project.
REMEMBER: Remind them that their participation is really important to the Cheers
Study and all information they provide to us will be kept confidential.
Factors Affecting Respondents' Participation
There are factors, both positive and negative, that may affect a respondent's decision
to participate in any survey. Let's look at some of the negative factors involved and how they
can be used to gain completed interviews.
Fear of the survey, the interviewer or the use of the data;
Hostility toward the interviewer or the sponsor;
Perceived invasion of privacy;
Assumed threatening subject matter; and
Cost to them in terms of time and energy.
A knowledgeable interviewer who is LISTENING to the respondent and answering
the respondent's objections directly can counter all of these factors.
The following are some of the problems you may encounter as you start doing interviews. Let's
look at the negative factor involved in each problem and talk about the ways to avoid or lessen
the problem.
1.
Respondent is suspicious or expresses doubt about the legitimacy of the survey
and your role.
This factor is sometimes expressed by the respondent by asking, "What are you
selling?" "Why did you pick me?" What are you going to do with the data?" or "If I participate,
you'll keep coming back to me for more information."
To counter the concern, you can:
Let your respondent know you are not selling anything.
Show him/her your badge.
Restate confidentiality.
Restate the purpose of the survey.
Let the respondent know the information will only be released in statistical
form--that we will take everyone’s' results and average them together, and that
no one outside of the survey will be able to put his/her responses with his/her
name.
Tell him/her that his/her name will be separated from his/her data before it is
analyzed and his/her data can be traced back to him/her; or
Offer to let him/her speak to your Field Manager/Data Manager/Supervisor.
2.
Respondent refuses outright without listening to any information about the
project or without listening to your entire introduction.
You may hear, "I'm not interested," or "I can't be bothered." You can:
In response to "I'm not interested" or "I can't be bothered," your first response
should always be, "May I ask why not?" You want to be very careful how you
say this and to not sound rude, abrupt, or unsure of yourself. If the question is
asked sincerely and there is a look of concern on your face, the respondent
should not object to you asking. This question can open up a dialogue with the
respondent, so that you will be able to provide him with more information and
help him become more comfortable with the survey. In addition, this is
something you can record on your tracking log.
Be sure to ask him/her if there is any more information you can give him/her,
and restate that you are not selling anything.
3.
Respondent expresses concern about the survey or the use of the data.
You may hear, "I don't give out personal information" or "I don't want to give out my
information because of what you may do with it." You can:
Let your respondent know what will happen to the information/data s/he gives
us;
Reassure him/her of the confidentiality of the data;
Restate the purpose of the survey;
Let him/her know s/he can refuse to answer any question that makes him/her
uncomfortable; or
Offer to let him speak to your Field Manager/Data Manager/Supervisor.
4.
Respondent is concerned about costs in terms of time and energy.
You may hear, "I'm too busy," or "Are you going to pay me for my time?" or maybe
you will hear, "What's in it for me?"
You need to let the respondent know that each questionnaire will take about 15
minutes. Also inform them that they will receive a $15 gift card and a t-shirt in
the field, followed by a $35 check in the mail after the 3 telephone interviews.
Restate the purpose of the survey.
5.
Respondent expresses distrust of government studies.
This may be expressed as "The government never listens," "This is a waste of
taxpayer's money," or "The decisions have already been made."
These objections often just need to be heard--then the respondent is willing to
cooperate. You should just listen and accept his/her feelings in a neutral
manner.
Assure him/her that his/her views and experiences are important.
Tell him/her that we don't want his/her experiences to be overlooked.
Restate the purpose of the survey. Also tell them- we are the UIC School of
Public Health and this is a scientific study.
6.
Other Questions and Concerns Respondents May Have About the Survey
How was I selected for the survey?
Explain that anyone who recreates in or
around these waterways is eligible to complete the survey.
How will the results be used?
The results of this survey will be used to help
us learn more about water quality and health of people who recreate in these
waterways.
Other Ways to Avoid Refusals
1.
Listen carefully to what the respondent is saying.
What is the reason for his objection? If it is not clear to you, ask the
respondent to explain further. Probe as necessary but not to the point of
annoyance. Then you should respond to his/her stated reason (s).
2.
Acknowledge that you have heard and understood the respondent's objections.
Be sympathetic, using phrases like, "I understand how you might feel that
way," or "That's very interesting." These phrases express in a neutral way that
you have heard the respondent's objections. You want to avoid too brief an
acknowledgement like "Yes, but…" that may be seen by the respondent as a
dismissal of his feelings.
Be direct, answer only what is asked and get right back to the business at hand-
-getting the respondent to complete the questionnaire. You can tell the
respondent s/he can start the questionnaire and see how it goes, and that s/he
can skip any questions s/he does not want to answer.
Do not volunteer additional information--which will only lead to more
questions on the respondent's part.
If you do not know the answer to a question, be honest and tell the respondent
that you do not know, but will find out. Then see your Field Manager/Data
Manager/Supervisor on site and get the answer for the respondent. This shows
the respondent that your actions match your words and will help convince
him/her that the other information you are giving him/her (the survey sponsor,
use of the data, confidentiality, etc.) is true.
Tips for Gaining Participant Cooperation
Carefully prepare for your interviewing day. A knowledgeable and enthusiastic
interviewer is the best defense against a refusal. Review your manual as often
as necessary, so you can answer respondents' questions without hesitation.
Be thoroughly familiar with all the survey materials so you can readily answer
a respondent's questions about the survey.
Make sure the respondent knows exactly who you are and why you are asking
him/her to be in the survey.
Make sure your respondent feels s/he is valuable to the survey and knows how
important his/her participation is.
Approach the respondent confidently and calmly. If you are not confident in
what you are doing, the respondent will quickly pick up on your nervousness.
Listen very carefully to the respondent's voice and watch his/her face and body
language. Knowing how to put the respondent at ease is one of the most
important refusal avoidance tools you can utilize in your efforts to gain
cooperation. Regardless of your respondent's mood or attitude, you must
always remain calm, confident, and pleasant.
Try to establish a good rapport as quickly as you possibly can. Getting the
respondent to start (and finish) the survey is the purpose of your efforts as an
Interviewer. Avoid unnecessary conversation with respondents.
Never take a refusal personally. No matter how good an interviewer you are,
you are going to get refusals.
Results of Your Efforts
1.
What will happen when you are successful in answering respondents'
questions?
Your respondent will cooperate and the respondent will complete the survey.
2.
What happens if you are not successful at convincing them to continue with the
survey?
It is important that you know when to stop. If you feel your respondent is becoming
annoyed instead of just hesitant you should discontinue your efforts.
If you get a couple of refusals, and it is starting to get you down, speak with your
Field Manager/Data Manager/Supervisor. They can help get you “back on track.”
QAPP 2
Appendix 1B
CHEERS Survey Training Manual
Probing
Section 11. Probing
When you finish this section, you should:
9
Know how to probe initial ‘don’t knows’ and refusals
9
Know how to probe for amounts, dates, and numbers
9
Understand how to probe on precoded and open-ended questions
and the difference between them
9
Know the correct way to record probes on open-ended questions
9
Know what it means to probe to the point of the question
9
Understand how to probe for additional ideas
9
Be able to successfully probe for occupation
Ideally, respondents would answer most or all questions using the response
categories provided. In reality, that doesn’t always happen.
Probing
is an interviewing
technique used to obtain specific, complete and relevant answers from a respondent. It is
one of the most difficult skills used in interviewing, but can also be one of the most
rewarding.
An interviewer who probes well is natural and friendly and shows a real interest
in the respondent while maintaining his or her neutrality regarding the respondent's
answers. Without being overly aggressive or seeming to cross-examine the respondent,
the interviewer works
with
the respondent to obtain specific information for the
researcher. Remember that the purpose of many studies is to help researchers make
decisions about programs or services for the public. This cannot be done unless specific
likes, dislikes, problems or experiences are known. You act as a "reporter" for the people
you interview, trying to get all the details possible for the story.
11.1 Probing Initial "Don't Knows" and Refusals
When a respondent answers "I don't know" to a question, it may mean one of
several things:
1.
He is in a hurry to get the interview over with and
doesn't want to take the time to think about an answer;
2.
The subject of the question is something the
respondent has never thought about before;
3. The respondent does not feel he is expert or
knowledgeable enough to give the "correct" answer; or
4. The respondent really has no idea of the answer.
In the first two situations, a remark such as, "I wonder if you could take a moment
to think about it" is appropriate. In the third situation, reassure the respondent that there
are no right or wrong answers and that we are only interested in his opinions. In the
fourth situation, encourage the respondent to give his impressions or, if the question asks
for a number, help him or her arrive at an estimate.
When a respondent refuses to answer a particular question, remind him of the
confidentiality of the information and opinions he gives.
Probe initial "don't know" responses and refusals
once
in an effort to obtain an
answer. Do not, however, badger the respondent to answer or convey the impression that
you are annoyed by his inability or refusal to answer.
11.2 Probing for Amounts, Dates and Numbers
When a question requires a numerical answer, the respondent may answer with a
range rather than a single number, or in units different than those asked for, or he may
initially say, "I don't know." In each of these situations, it is necessary to probe for the
specific information required in the question.
If the question asks for a dollar amount and
1. the answer is given as a range, probe with "I need (or would like to
record) an exact figure if possible." This tells the respondent that you
are interested in exact amounts. The same probe should be used for
responses such as "Oh, around $200."
2. the respondent says, "I don't know," probe with "Just your best estimate
is fine."
If the question asks for a date and
1.
the respondent answers with a time reference, such as "a year or so
ago," probe by asking the calendar information that you need: "What
was the actual date?" or "What month and year would that be?"
2.
the respondent gives only part of the information you need to record,
for instance, only the month or only the year. Again, probe by asking
for the missing piece(s) of information.
3.
the respondent says "I don't know," begin by asking the respondent to
try to remember the time of year or the season. If he can't remember,
ask him to think about anything else that may have been happening at
that time which would help him pinpoint the date more exactly.
If the question asks for a number and
1. the respondent gives a range such as "two or three," probe with "I need to
record a single number; which would you say is correct?" or "Which
would you say would be most likely; 2 or 3?"
2.
the respondent answers in units that are different from those required by
the question (hours instead of minutes, weeks instead of months), probe
by asking for the answer in the units needed: "How many months would
that be?" The same method should be used if he replies with a time
reference instead of a number: "Once a week," when you have asked for
the number of times per month.
3. the respondent says, "It depends," probe with "On the average" or
"Usually . . ."
4.
the respondent says, "I don't know," probe with "Just your best
estimate."
11.3 Probing on Precoded Questions
Remember that precoded questions are used when researchers want the
respondent to place himself or his opinions in one of several predetermined categories.
Let's look at our examples of precoded questions again:
Would you say your health is excellent, very good, good, fair or poor?
<1>
Excellent
<2>
Very good
<3>
Good
<4>
Fair
<5>
Poor
<8>
DON'T KNOW
Are you now . . .
<1>
Married,
<2>
Widowed,
<3>
Divorced,
<4>
Separated, or
<5>
Have you never been married?
<9>
REFUSED
In precoded questions, probing is used to focus the respondent's attention on the
question and/or to direct the respondent's attention to the existing code categories. You
must indicate to the respondent that his job is to choose one of the existing code
categories
without
biasing his response or leading him to a particular category. You
should repeat all categories to the respondent without narrowing down his choices for
him. Always preface a repetition of the code categories with an appropriate
acknowledging remark. If the respondent answers in his own words instead of using an
answer category, a prefacing remark such as, "Well then, would you say . . . ?" or "Would
that be . . . ?" is appropriate. If the respondent says, "It depends," a remark such as, "In
general, would you say . . . " or "Overall, . . . " will let him know that we want him to
generalize on the subject.
If, after probing, the respondent does not choose an existing category, record his
answer verbatim and go on to the next question. On PAPI questionnaires, record
verbatims that do not fit into existing code categories in the left-hand margin. On CAPI
studies, record this type of verbatim using the NOTE command before entering the code
for "No coded response applicable."
11.4 Probing on Open-Ended Questions
On open-ended questions there are no precoded answer categories. The
respondent is allowed to answer in a free-flowing manner, in his own words. The
interviewer records the responses(s) as the respondent speaks, in his own words. Let's
look at our examples of open-ended questions again:
Why did you choose to live in this location?
What do you like about your HMO?
Because open-ended questions tend to be very general, respondents tend to
answer in a general way and to use general adjectives when describing situations and
opinions. Respondents also tend to give brief or single answers without going into much
detail. For this reason, two types of probing are used in conjunction with open-ended
questions--
probing for clarification,
which is always used, and
probing for additional
ideas
, used when you are instructed to do so in the questionnaire.
11.5 Probing for Clarification
Probing for clarification
is often a matter of asking for specifics or an
explanation. Never assume that a respondent is using a vague or general word or phrase
in the same way you would. The same general word can mean many different things to
different people.
There are many general words or phrases used in everyday conversation that
should automatically suggest to an interviewer that probing for clarification is needed.
Below is a list of some of the more common words that should always be probed.
Positive
Negative
like
dislike, don't like
good
not good, poor, bad
better, better off
worse, worse off
best
worst
(good) quality
(poor) quality
(high) quality
(good) reputation
(poor, bad) reputation
(good) appearance
(poor, bad) appearance
appealing
unappealing
satisfying
dissatisfying
satisfactory
unsatisfactory
convenient
inconvenient
effective
not effective, ineffective
works
doesn't work
Can Be Positive or Negative
different
interesting
okay
all right
average
cost
cheap
expensive
Probing for clarification should encourage the respondent to speak
without
influencing his answer
. The potential for introducing bias while probing is great. Under
pressure of the interview situation, an interviewer may imply quite unintentionally that
some responses are more acceptable than others or that a respondent may want to
consider certain things in formulating his answer. Many respondents wish to be helpful; if
they can tell how you as an interviewer feel about the subject, they may answer in a way
they think would please you. Be careful not to encourage the respondent with your
reaction to his answers. Avoid saying, "That's good," "That's true," or "That's right." Also
be careful not to suggest possible meanings or specific connotations to the respondent.
Remember that you must remain impartial during every moment of the interview.
In short, the best probes for clarification are those that tell the respondent exactly
what kind of information or response you want from him without leading him toward a
specific possible response. Some examples of neutral probes that can be used are:
What, specifically, do you mean by (__________)?
What, exactly, was (__________)?
In what way was it (__________)?
What about the (__________) do you (like/dislike)?
Would you explain that please?
Would you describe that for me?
Tell me a little more about that.
Often a respondent will volunteer more than one general word or idea that needs
to be clarified,
or
he may answer a probe with just another general word. You are
expected to
probe each word or idea that needs clarifying
and to
probe each as many
times as necessary
until it is clarified. If the respondent is unable to clarify or be more
specific in response to your probe, move on.
QAPP 2
Appendix 1C
CHEERS Survey Training Manual
A Typical Day for the CHEERS Staff
A TYPICAL DAY FOR THE CHEERS RECRUITER
This is a general overview of a typical data collection day for a CHEERS
recruiter/ interviewer/incentive person. Please note you can be assigned to do one or all of
these jobs on any given day.
Arrive at the pre-designated site at the given time. Please inform the on-
site Data Manager or the CHEERS Field Supervisor (for that
day/location) if there are any unforeseeable delays.
Wear your CHEERS study T-shirt; and
Wear appropriate pants and shoes.
Report to the on-site Data Manager. He/She will record your arrival on a
time sheet.
Listen to changes/location specific details/ additional instructions and for
any adjustments; and
Ask any questions, if necessary. There will always be copies of
CHEERS Study FAQ sheets and Water quality info. sheets available to
help you prepare for the day.
Please discuss your lunch time/break times before you start work
Pick-up recruiting material and place on clip-board. This includes:
Location specific flyers
Eligibility Screener (Make sure you know what time the CHEERS
study will wrap up that day)
We are interested in water recreators before they start their activity
of the day.
We are interested in non-water recreators at any time
before/during/after their activity.
Participants must be able to return to the CHEERS tent to complete
Survey B before we end the CHEERS event for that day.
Refusal Tally sheet
Commence recruiting.
Check Recruiting script, CHEERS FAQ Sheet,
and slides for more help
.
Return all recruiting material at the end of the day.
De-brief with the on-site Data Manager.
Ask questions/get clarification, if necessary
Discuss any problems you encountered during the day;
Discuss any positive elements of the day; and
Listen for general instructions.
Checkout for the day. Data Manger will complete your time sheet.
A TYPICAL DAY FOR THE CHEERS INTERVIEWER
Arrive at the pre-designated site at the given time. Please inform the on-
site Data Manager or the CHEERS Field Supervisor (for that
day/location) if there are any unforeseeable delays.
Wear your CHEERS study T-shirt; and
Wear appropriate pants and shoes.
Report to the on-site Data Manager. He/She will record your arrival on a
time sheet.
Listen to changes/location specific details/ additional instructions and for
any adjustments; and
Ask any questions, if necessary. There will always be copies of
CHEERS Study FAQ sheets and Water quality info. sheets available to
help you prepare for the day.
Please discuss your lunch time/break times before you start work
Pick-up your laptop and scanner. Commence interviewing.
See the
slides for more help
. The following items are of most importance:
Don’t Be in A Hurry: Slow down, be patient, and be observant.
Record the CASE ID number very carefully. Double check Case ID
entries.
Be Accurate: Listen intensely to what the respondent is saying to
you. Capture the respondent responses exactly as they give them
to you.
Repeat: Repeat back to the respondent all names, addresses and
phone numbers. This is the method that is used to accurately
match the two parts of the survey.
Report any data/laptop troubles to the on-site Data Manager
immediately.
Check to make sure each participant has completed Survey A
before they do Survey B.
All water recreators will receive Survey A before their activity
and Survey B after their activity.
All non-water recreators will receive Survey A and B at the
same time.
If you enter the wrong case IDs or you start the wrong survey,
or you lose power and restart the survey on a new laptop-
PLEASE ENTER ALL THIS IN A REMARK and inform the DM.
Check the laptop battery power every now and then. If power is 25%
or less then please swap the battery and inform the on-site data
manager.
Always close all surveys before placing laptops on stand-by or
powering off. We need to protect the data and save battery power
in the field.
Protect laptops from sun/rain/physical damage. They are your
responsibility.
Return all laptops and scanners at the end of the day. The on-site
Data Manager will download all the data before you pack them in
their bags.
De-brief with the on-site Data Manager.
Ask questions/get clarification, if necessary
Discuss any problems you encountered during the day;
Discuss any positive elements of the day; and
Listen for general instructions.
Checkout for the day. Data Manger will complete your time sheet.
Please note: You may be asked to go out and do some mobile
interviewing- if so, you will be a recruiter/mini- data manager/
interviewer. This means:
You will approach participants and recruit them
You will complete the consent process and assign a Case ID.
You will do the surveys.
You will record this data on a log and return to the tent for
incentives.
You will report all data to the on-site Data Manager.
A TYPICAL DAY FOR THE CHEERS INCENTIVE PERSON
Arrive at the pre-designated site at the given time. Please inform the on-
site Data Manager or the CHEERS Field Supervisor (for that
day/location) if there are any unforeseeable delays.
Wear your CHEERS study T-shirt; and
Wear appropriate pants and shoes.
Report to the on-site Data Manager. He/She will record your arrival on a
time sheet.
Listen to changes/location specific details/ additional instructions and for
any adjustments; and
Ask any questions, if necessary. There will always be copies of
CHEERS Study FAQ sheets and Water quality info. sheets available to
help you prepare for the day.
Please discuss your lunch time/break times before you start work
Pick-up the incentive material. This includes:
The incentive check list on a clipboard.
The gift-cards/receipt book.
Stool flyers.
T-shirts
Commence incentive distribution.
See slides for more help
. Please be
aware:
Count all gift cards at the beginning of your shift and enter the
number on the white form provided in the gift-card box.
Record the date/location and start/end gift card count on the
incentive sheet before you start your day and at the end of the day
again.
You will be stationed at a table close to the on-site Data Manager.
Please make sure all participants have completed Survey A and
Survey B- before they take their incentives
.
Take their wrist bands before they leave. Please collect as trash.
Participants can choose to donate their cash ($50) incentive. They
will sign the consent form. The on-site Data Manager can help you
track this. If someone says they are donating their cash incentive
to any club/team/group then-
DO NOT GIVE THEM A GIFT CARD.
Enter “
DONATE
” next to their
case ID. This data is very important as we track donations every
week.
If you run out of t-shirt sizes you can record details of the participant
NAME, ADDRESS AND T-SHIRT SIZE. We can mail it out to
them. Please give details to the on-site Data Manager.
Once again, count all gift- cards and enter the number on the white
form provided in the gift-card box at the end of your shift/day. You
are responsible for an accurate account of the gift cards. This is as
good as cash.
De-brief with the on-site Data Manager.
Ask questions/get clarification, if necessary
Discuss any problems you encountered during the day;
Discuss any positive elements of the day; and
Listen for general instructions.
Checkout for the day. Data Manger will complete your time sheet.
1
QAPP 2
Appendix 1D
CHEERS Survey Training Manual
BLAISE Field Interviewer Training
2
Blaise Manual for study 1027 CHEERS
(7/19/07)
Logging in to your laptop
After you turn the computer on, you will be prompted for your user name and password.
Select the user name “user” (not “admin”), and enter the password you were given to use
during training. If you bypass the sign-in dialog by closing the window or pressing the
Escape key, the programs will not work.
Password: survey
Checking the time and date
After you’ve logged in, you will be presented with a time/date window. You should
verify that both are correct, and fix them if not. (The time and date are used by the
instrument in various calculations.) After you are done, press the “OK” button to
continue.
Figure 2
Verifying that you are running off of a wall plug rather than the battery
It’s very important that interviews be carried out while the laptop is plugged in, if at all
possible. The battery is intended as backup, to prevent the loss of data in the case of a
power outage; it’s unlikely that you will be able to complete an entire interview on
battery power alone. PLEASE CONDUCT A BATTERY CHECK- IF YOU HAVE 25%
OR LESS POER THEN YOU NEED TO SWAP THE BATTERY.
There are two kinds of laptops used for the 1057 study: IBM and ACER. The laptops
have been set up so that an icon in the system tray (at the bottom of the screen, on the
right) shows you whether you are operating on “full” or “battery” power, and, if you are
operating on battery power, tells you what percentage battery power you are operating
on. The icons for the two kinds of laptops are different.
3
For the laptops:
1) An icon that looks like a plug with a blue lightning bolt over it indicates you are
running on full power. If you rest your mouse on the icon, it will give you a
message like “92% remaining (charging)”, indicating that your battery is “92%”
charged and that the laptop is in the process of further charging the battery. You
should double-click on the icon to get a display like the following, confirming you
are plugged in and giving you additional details.
Figure 5
4
2) If the icon appears as a blue battery, with a red plus and minus sign on it, this
indicates that you are running on battery power. If you rest your mouse on the
icon, it will give you a message like “96% remaining”, indicating that your
battery is “96%” charged. You should double-click on the icon to get a display
like the following, confirming you are running off the battery and giving you
additional details.
Figure 6
3) You can also tell that the laptop is running on full power from a small indicator
light on the laptop itself: above the left-hand corner of the keyboard. If the laptop
is plugged in to a working outlet, the light under the image of a plug will be on,
otherwise not.
5
6
“CHEERS” desktop folder
Icons for data collection
1) “Run Part A”, “Run Part B” : used for data collection with actual respondents; for
further instructions see the section on “Accessing the Blaise instrument for data
collection”, below.
2) “Salvage”: used to recover if your case locks or you get other error messages
indicating a problem with the database; for further instructions see the section on
“Salvaging a case after data problems”, below.
“Training” directory (for training/review)
1) “Joint” sub-directory:
a) “Run Training” will allow you to go through the entire questionnaire using
a training case. (Do
not
use this icon for actual data collection.)
b) “Salvage Training” is a training version of the “Salvage” icon.
c) “Training Sample.xls” = copy of test sample file, which you will need in
order to run through a case in the “Joint” directory; it lists the CaseID and
associated Randno values which you will need to type in when prompted
by the program.
2) “Section1” – “Section9” and “Post” sub-directories:
Each of these sub-directories has a “Run Training” icon which will allow
you to practice on just that particular section of the questionnaire. (Click
to start.) You will not need to enter a CaseID and Randno to go through
these.
7
Accessing the Blaise instrument for data collection
1) Click onto desktop folder CHEERS.
2) Click on the appropriate icon for the phase of the study or training you are
working on.
3) Click on the “Main Laptop” folder.
4) Click on the “Part A. bat” or Part B. bat” file icon- This will bring up the study
instrument in the Blaise data entry program.
5) To access a particular case, see instructions under the “Accessing a case (‘form’)”
section below.
Troubleshooting
You click on your “Run” icon and nothing happens
– Try clicking on it several
times. All icons should be working but sometimes you have to click on them
several times before they will kick in. (NOTE: the “Salvage” icon discussed
below will run a program very quickly in the background; you may see only a
flash on your screen, and you should test before clicking on “Salvage” again.)
Salvaging a case after data problems
1) You may need to salvage a case if it locks (freezes on a particular screen and will
not let you go backwards or forwards), or if you get an error message that refers
to the data or database being corrupt or unusable.
2) The very first thing you should do is to write down the error message (if you get
an error message), and a description of what went wrong.
3) Then exit Blaise and attempt to re-enter the case regularly (without using
“Salvage”). Only if that fails should you proceed to salvage.
4) To salvage start by clicking onto desktop folder ST1057 Patient and then click on
the “Salvage” icon (right beside the “Run Production” icon). This will cause the
salvage program to run very quickly in the back-ground (you may see just a flash
on your screen).
5) Now attempt to re-enter the case you were in.
6) If you still cannot access the case, run salvage one more time and attempt to re-
enter the case.
7) If you still cannot re-enter the case, you can call one of the SRL staff members
listed in your manual, and we will attempt to address the problem over the phone
with you.
8) If none of the above works, you will need to reschedule your appointment with
the respondent and return as soon as possible to SRL so that we can take a look at
your computer.
Blaise screen layout
Each Blaise screen consists of 5 basic sections:
Figure 7
1) The menu bar – The bar consists of 5 drop down menus; Forms, Answer,
Navigate, Options, and Help. Clicking on any one of these titles will display a list
of options that you may need/want to use during the interview session. Any option
grayed-out is not activated for use on that particular screen. Active options may
change from screen to screen (for example, the DK/RF options, which are
available on most but not all screens).
2) The speed buttons – The second bar consists of several speed buttons that you
may need/want to use during the interview session. Resting your mouse pointer
on the speed button will display a description of what it does. Buttons include
Get
form
,
Don’t know answer
,
Refuse answer
,
Make Remark
,
Goto previous
page
,
Goto next page
,
Goto first page
,
Goto last page, Search tag,
and
Form
Language
.
8
3) The info pane – This is the portion of the screen shaded pale yellow. It will
display the question text, interviewer instructions (in blue, and usually in all
9
capital letters), and answer choices. The text should be read/used as instructed in
general and study specific training.
4) The form pane – This is the pale gray area at the bottom of the window. It
contains one or more field panes or fields where the data is entered. Each field has
a field name or variable name. On fields which display numbered response
options, after you enter an answer a variable label will be displayed to the right of
the entry for that field for reference as you view a page. (Numeric and string
fields don’t have such variable labels.) These labels can be useful when checking
previous answers without having to scroll back through all fields one by one.
Each form pane also corresponds to one page. You can use the mouse pointer or
arrow keys to navigate one field at a time or use the “previous page” and “next
page” speed buttons or the “page up” and “page down” keys to move backward
and forward one screen/page at a time. NOTE: You will not be allowed to move
past fields that are required to be answered (and that have not been answered yet).
5) The status bar – This is the last line on the screen that contains some information
about the case. The three sections of particular interest are the second, which
displays the current page/total pages involved in the interview (ex = “6/259) and
the last two, which display the current CaseID number (ex = “100001) and the
name of the field that the cursor is currently on (ex = “Section1.PD1_intro”).
Accessing a case (“form”)
1) After entering the Blaise system you will be brought to a screen that asks for
CaseID number.
2) Enter the 6-digit CaseID number to access the form.
Figure 8
10
11
Entering answers
You can use the keyboard to enter the answer to any question in the form. Simply enter
the appropriate number(s) or letter(s) into the field pane and press the “Enter” key. This
will take you to the next appropriate question.
Alternatively, questions such as those with radio buttons or check boxes, can be entered
by clicking on the appropriate response. Clicking on the text of the response itself is the
best way to ensure that the correct response is selected.
Whether using the keyboard or using the mouse to select answers it is always advisable to
quickly glance at the screen to ensure that the correct response was selected.
Closed ended (numbered response) questions:
For those with radio buttons you use the keyboard to enter the number of the response
and press the “Enter” key to move on to the next question. Or you can click once on the
response to select it and press the “Enter” key to move on. Or you can double-click on
the response to both select it and move to the next screen; but this is dangerous because
it’s hard to catch any mistakes caused by double-clicking on a different response than the
one you were aiming at.
Figure 12
CAUTION: if there are 2 or 3 columns of responses presented onscreen, and you use the
mouse to select an option in the 2
nd
or 3
rd
column, you have to be careful not to click to
the
left
of the radio button, or you may end up selecting the answer to the left of the one
that you intended. This can be a hard error to catch if you are double-clicking in order to
both select the response and move to the next screen. Clicking on the answer text just to
the
right
of the radio button (that is, on the response number or label itself) may be the
best way to avoid this error.
12
For those close ended questions with check all that apply boxes you can check the boxes
in any order by clicking on each box in the order the response is given by the respondent.
Or you can use the keyboard to enter each response separated by a dash. For example,
1-3-2-10
would indicate that the first, third, second and tenth answer categories from the list were
named by the respondent in that order.
CAUTION: If you are using the keyboard instead of the mouse to enter responses the
program will allow you to enter the same option twice (for example “1-3
-1
”, entering
option “1” twice), but you will get an error message when you try to proceed.
Figure 13
13
CAUTION: For “check all that apply” questions a “don’t know” response or a “refusal”
response is intended to be used as the only answer to the question. It may not be used in
conjunction with checking other responses shown on the screen. If other responses have
been entered followed by the don’t know or refusal option, the previous entries will be
erased, so if you want to preserve those other answers you should enter the don’t know or
refusal answer as a “SOMETHING ELSE” of “OTHER” answer with an appropriate
message in the follow-up field (“Refused to say what the other illness was”, or whatever).
14
CAUTION: For “check all that apply” questions be careful when backing up to edit or
append to an answer. When you back up the entire answer field will be highlighted.
1-3-2-10
Typing in any answer while the field is highlighted will overwrite the previous answer(s).
To edit or append to the field you can press the “Insert” key or click with the mouse on
the answer field and then edit as desired.
CAUTION: as with radio button responses, check boxes that are arrayed in 2 or 3
columns can result in data entry errors when you click to the
left
of the item you intended
to select. Clicking on the answer text just to the
right
of the radio button may be the best
way to avoid this error.
“Don’t know” answers:
“Don’t know” is not displayed on-screen as a potential answer category but it is available
for use on most questions. It can be selected by entering the key combination “Ctrl” +
“k”, or by clicking the “Don’t know answer” speed button, or by choosing “Don’t Know”
from the “Answer” menu on the menu bar. The answer is displayed on-screen in the field
pane as a yellow circle with a question mark in the middle.
If “don’t know” is not available for a particular item, you will be notified by an onscreen
interviewer instruction indicating “(NODK)”. If you try to use the Ctrl-K shortcut, or the
speed button, on such items, you won’t get an error message; instead nothing will
happen. You can also tell that the option is unavailable because both the “Answer” menu
option and the speed button will be grayed out.
If “don’t know” is not available for an item, you can assume that this was intentional and
that you should probe further to get the R to choose one of the other precodes.
If the R gives an answer that doesn’t fit into one of the existing codes, and when probed,
can’t pick one of those pre-codes, do not code this as a “don’t know” answer but instead
select a precode and leave a remark.
You can review all of your “don’t know” answers by selecting the “Show DontKnow and
Refusal” option on the “Navigate” menu. (It displays both “don’t know” and “refusal”
answers by default, but you can uncheck the “Show refusal” check-box.) To go to an
item answered with a “don’t know” (if for example the respondent now has an answer),
select that item in the left screen of the dialog box and then click on the “Goto” button.
Figure 14
Figure 15
15
“Refusal” answers:
“Refusal” is not displayed on-screen as a potential answer category but it is available for
use on most questions. It can be selected by entering the key combination “Ctrl” + “r”, or
by clicking the “Refuse answer” speed button, or by choosing “Refuse” from the
“Answer” menu on the menu bar. The answer is displayed on-screen in the field pane as a
blue circle with an exclamation point in the middle.
If “refusal” is not available for a particular item, you will be notified by an onscreen
interviewer instruction indicating “(NORF)”. If you try to use the Ctrl-R shortcut on
such items, you won’t get an error message; instead nothing will happen. You can also
tell that the option is unavailable because both the “Answer” menu option and the speed
button will be grayed out.
If “refusal” is not available for an item, you can assume that this was intentional and that
you should probe further to get the R to choose one of the other precodes, emphasizing
the confidentiality of the information as appropriate.
If the R gives an answer that doesn’t fit into one of the existing codes, and when probed,
refuses to pick one of those pre-codes, do not code this as a “refusal” answer but instead
select a precode and leave a remark.
You can review all of your “refusal” answers by selecting the “Show DontKnow and
Refusal” option on the “Navigate” menu. (It displays both “don’t know” and “refusal”
answers by default, but you can uncheck the “Show don’t know” check-box.) To go to
an item answered with a “refusal” (if for example the respondent now has an answer),
select that item in the left screen of the dialog box and then click on the “Goto” button.
Entering a note/remark:
You can enter an interviewer note on any item by entering the key combination “Ctrl” +
“m”, or by clicking the “Make remark” speed button, or by choosing the “Make Remark”
option from the “Answer” menu. This will bring up a “Remark” box for you to type into.
Figure 16
CAUTION: Be sure to click on the “save” button to save your text. If you press the
“cancel” button, or otherwise close the Remark box without saving (by, for example,
16
clicking on the “X” in the corner), any text you just entered will be erased.
17
Once you have saved the remark a paper clip symbol will appear on the screen to the left
of the field pane. To view and edit your saved answer, you can open the remark the same
way you did before (from shortcut keys, speed button, or menu), or you can double-click
on the paper clip icon beside the question. To delete a remark you have to delete all of
the remark’s text (open the remark, delete all of the text
1
, then click on the “Save”
button); once the remark has been deleted the paper clip symbol for that remark will
disappear.
You can review all of your remarks by selecting the “Show All Remarks” option on the
“Navigate” menu. To go to an item that has a remarks attached to it (if for example the
respondent’s current answer requires editing a previous note), select that item in the left
screen of the dialog box and then click on the “Goto” button. Once you’re at the item,
open the remark in order to view and edit it.
Please note: It may be appropriate at times to make a remark in conjunction with
precodes other than “NO CODED RESPONSE APPLICABLE”. A remark can be left on
any screen where you feel a remark is needed based on instruction you received in
general or study specific training.
String questions:
These are limited-text-entry questions (unlike the “open ended” questions mentioned
often used for names and addresses, and usually provide between 40
nd 80 characters for data entry.
r than 80 characters on an 80-character string question).
those cases you can enter the rest of the data in a remark attached to the string
dress in
below). They are
a
You may occasionally run out of space to record your answer on a string question (if for
example you get an address longe
In
question. For something like an address, you might want to start the remark by indicating
that you ran out of space to record it; then copy the text already entered for the string
question and paste it into the remark
2
, before continuing to enter the rest of the ad
1
You can delete the text in any of several different ways (and the list below is not exhaustive):
a) From the end of the text (arrived at by pressing the “Ctrl” + “End” keys), press the
“BkSp” key
ted text by pressing the
“Del” key
.
Del”
e)
ng, left-clicking, and then, with the mouse left pad held down, moving the mouse pointer to
2
To copy
a)
until you’ve deleted the text all the way to the beginning.
b) Or, from the start of the text (arrived at by pressing the “Ctrl” + “Home” keys), press the
“Del”
key
until you’ve deleted the text all the way to the end.
c) Or, from the start of the text (press “Ctrl” + “Home”), select all of the text to the end by pressing
the
“Shift” + “Ctrl” + “End” keys
, and delete the selec
d) Or, from the end of the text (press “Ctrl” + “End”), select all of the text to the beginning by
pressing the
“Shift” + “Ctrl” + “Home” keys
, and delete the selected text by pressing the
“
key
.
Or, use the mouse
to select text starting from either the beginning or the end of the text by
pointi
the other end of the text. Then delete the selected text by pressing the
“Del” key
.
and paste the string answer into the remark:
Select the text already entered for the string question, in any of the following ways…
18
se
ote that the entry of “don’t know” and “refusal” answers for string questions is done in
pen ended (memo) questions:
ese are
unlimited
-text-entry questions (unlike the “string” questions mentioned
hey
o enter data, you can either just begin typing, or double-click, or press the “Insert” key.
s.)
igure 17
the remark. (Do
not
manually re-type the string answer into the remark, since any
differences between the two will create a puzzle as to which is the correct version; u
copy-paste as described in the footnote.)
N
the same way as for close-ended questions.
O
Th
above), although they look just like a string question until you start to enter data. T
are used in places where the respondent might give us an entire story (for example, when
we ask “what did the doctor tell you to do next?”).
T
The system will bring up a memo box that allows you to enter a virtually unlimited
amount of text. (The box looks and acts much like the one that comes up for remark
F
1) Back up to previous item (using up arrow key), then go forward again to string question
(using down arrow key), which will automatically highlight (select) all of the text you’ve
entered for that string question.
2) Or, from from the start of the string question text (arrived at by pressing the “Home”
key), select all of the text to the end in one step by pressing the
“Shift” + “End” keys
.
3) Or, from the end of the text (arrived at by pressing the “End” key), select all of the text to
the beginning in one step by pressing the
“Shift” + “Home” keys
.
4) Or, from the start of the text (press “Home”), select all of the text to the end, character by
character, by pressing the
“Shift” key and
continuing to press the
right arrow key
till
you’ve arrived at the end.
5) Or, from the end of the text (press “End”), select all of the text to the beginning, character
by character, by pressing the
“Shift” key
and continuing to press the
left arrow key
till
you’ve arrived at the beginning.
6) Or,
use the mouse
to select text starting from either the beginning or the end of the text
by pointing, left-clicking, and then, with the mouse left button held down, moving the
mouse pointer to the other end of the text.
b) After selecting the text, copy it to the clipboard by right-clicking and selecting “Copy” from the
menu, or by pressing the “Ctrl” + ”C” shortcut keys.
c) To paste the selected text, open the Remark and either right-click and select “Paste” from the
menu, or press the “Ctrl” + “V” shortcut keys.
19
CAUTION: Be sure to click on the “save” button to save your text. If you press the
“cancel” button, or otherwise close the Memo box without saving (by, for example,
clicking on the “X” in the corner), any text you just entered will be erased.
You can back up to this open ended question at any time to view and edit your saved
answer (but see the “CAUTION” below). You can also review all of your answers to
open ended questions by selecting the “Show All Open Answers” option on the
“Navigate” menu. To go to one of these open ended questions (if for example the
respondent’s current answer requires editing a previous answer), select that item in the
left screen of the dialog box and then click on the “Goto” button.
CAUTION: To edit an existing Memo-type (open ended) answer, you need to double
click on it or press the “Insert” key after your cursor is on the answer. If instead you
typing, or click
once
in the answer and start typing, you will end up deleting the text
as previously there.
-
start
that
pondent, and you are
w
Note that the entry of “don’t know” and “refusal” answers for open ended questions is
done in the same way as for close-ended questions.
Introduction screens:
These are screens on which some introductory text is read to the res
instructed to “PRESS ENTER TO CONTINUE” after you are done, without having to
enter any data. The screens will
accept
a single character of data if you enter it, but this
will not be stored in the final dataset, so it’s not a problem.
Edit check screens:
Edit check screens wil
hard” error which req
l pop up either when a data entry error has clearly been made (a
uires a change to an answer entered), or when an error is suspected
r last live birth must have
een at a later age than her first live birth, and this is therefore an example of a hard error.
he edit check programmed to catch such an error would bring up a popup window like
“
(a “signal” which can be suppressed by the interviewer).
For example, an interviewer may record that the respondent’s first live birth was at age
“18” (DM21c1=18), and that her last live birth was at age “17” (DM21c2 = 17)…perhaps
because of a typo of “17” when “27” was meant. Clearly he
b
T
the following:
Figure 24
Notice that the “Suppress” button is greyed out. This popup will only go away when the
interviewer returns to one of the two questions and changes the answer so that the
inconsistency is eliminated. (For example, by selecting the “Section9.DM21c2” item,
clicking on the “Goto” button, and changing that answer from “17” to “27” before
proceeding to the next question.)
20
On the other hand, an interviewer
can
suppress a “signal” generated by an edit check of a
suspicious answer. Suppressing a “signal” confirms that the suspicious answer is in fact
correct; the “signal” popup window therefore goes away and the interviewer is allowed to
proceed to the next question.
For example, an interviewer may record that the respondent spends “0” hours awake with
her spouse on an average weekday (NT5a=0). This is an unusual answer, but not
impossible (since some spouses live apart for work reasons during the week, and get
together only on weekends). The edit check programmed to query interviewers about
such an unusual but possible answer would bring up a popup window like the following:
Figure 25
Note that such an interviewer “signal” can be suppressed if the answer entered was
indeed the correct one (by clicking on the “Suppress” button). If on the other hand the
answer entered turns out to be incorrect (due perhaps to a typo of “0” when “10” was
meant), the interviewer can return to the question by clicking on the “Goto” or the
“Close” button, and then change the answer before proceeding to the next question.
21
22
Exiting and saving a form
You can exit a form in several ways at any point in the interview for a partial interview or
at the end.
After the last question in the questionnaire is asked and answered you will be asked if
you want to save the form or cancel. If you click on the “cancel” button you will be put
back in the questionnaire form. If you click on the “Yes” button your form will be saved
and closed.
You can also exit a form at any point by selecting the “Forms” menu option “Close” (to
close the form but remain in Blaise) or “Exit” (to both close the form and exit Blaise).
Clicking on the “x” box in the upper corner of the Blaise window has the same effect as
choosing “Exit” (it closes the form and exits Blaise). In all cases, you will be prompted
with the same options as when you complete a form: to either save and exit the form or
cancel the exit procedure and return to the form.
CAUTION:
You must enter a “1” on the last “End_All” item (as it tells you to) before exiting. If
instead you get to the “End_All” item and just exit the program, the case will not be
treated as a completion but as a partial. So the safest way to exit a completed case is to
answer all of the questions, including “End_All”, and press enter so that the program
prompts you to save the form.
QAPP 2
Appendix 1E
CHEERS Survey Training Manual
How to do a CAWs Use Survey
HOW TO DO A CAWs USE SURVEY?
Specific aims of the CAWs usage survey are to determine:
•
CAWs usage, in terms of numbers of users and types of recreational activities at
various access points.
•
To identify differences in usage between locations
•
To identify differences in usage at the same location at different times of day, and
different days of the week.
•
To identify use at informal access points.
The procedure of the CAWs usage survey will be as follows:
a. The CHEERS Field Manager/Supervisor or the on-site Data Manager will have
overall responsibility for identifying sites and times to start sampling as well as
for conducting user counts. Only field staff trained in this protocol will conduct
this CAWs use survey.
b. Staff will select a clear view of the access point of interest, preferably in a shaded
location. They will fill out the top half of the CAWs Use Survey Data Sheet.
c. Using the datasheet, staff will tally the number of individuals who begin
recreation, by recreational activity. The tally will be done in 10-minute intervals.
The first interval will begin at 0, 10, 20, 30, 40, or 50 minutes past the hour. Staff
will write into the chart the hour (clock time) for each interval.
d. In order to avoid counting the same individual more than once, individuals will
be counted only when they begin a recreational activity. Those recreating at the
time that counting begins will be identified by circling the tally marks for those
individuals.
e. After a datasheet has been completed, staff will begin a second sheet, taking care
to again complete the top portion, including the page number.
f. Consent: Because this is a study of the behavior of anonymous individuals in a
public space this study has been granted exempt status by the UIC Institutional
Review Board (IRB) for the protection of research subjects. Thus no consent
process for the CAWs recreational use study is necessary and none will be
performed.
g. Safety of the researchers will be maintained at all times. The field study will not
be done without adequate light and will be terminated in the event of inclement
weather. Researchers will also be instructed to leave the area and seek a safe
location in the event that the feel that their safety is threatened in anyway.
h. At the completion of data collection, data sheets will be given to the on-site Data
Manager.
QAPP 2
Appendix 1F
CHEERS Survey Training Manual
Know Your Job
KNOW YOUR JOB
Scheduling
Your schedule is planned around your availability, classes and activities and within the
framework of our study. Some schedules may stay the same during the entire season, while
others may change on a regular basis. You will be scheduled to work Fri/Sat or Sun. We
require you to sign up for a minimum of 12 hours of work each week. You may work as many
hours as allowed by the university and at the discretion of the study coordinators. Please ask
your supervisor for further details. If you have a conflict due to vacation, classes, activities or
any other reason, please see Sara and let her know in advance. We will assist you in
resolving conflicts with your work schedule. Due to the nature of this study you will probably
be required to work holidays and during exam periods. Every effort will be made to
accommodate course work and other activities, however a definite commitment in time and
availability will be required during the periods of active sampling in the study.
Pay Day
All employees are paid on a bi-monthly basis. Earnings are directly deposited into the bank
account you designated on the NESSIE new hire forms.
Employees are responsible for filling out a time card after each shift which will be signed by
the immediate supervisor. If you believe there is an error in your paycheck, please bring it to
the attention of the project coordinator immediately.
Evaluations
Your work performance will be evaluated periodically throughout the season. You will also be
given an opportunity to give us feedback.
Resignations
In the unfortunate event that you have to leave your position, please see Amelia/Preethi or
Sara BEFORE you resign. We may be able to help you work out the conflict. If you are sure
that you must resign, however, we ask that you give us at least two (2) weeks advance notice.
This will allow you to remain eligible for future employment. In addition, this courtesy will give
supervisors and coworkers time to replace your shifts.
YOUR WORK DAY
Rest Periods
You can ask for two (15) minute rest periods when you work at least six consecutive hours.
This includes a meal/rest room/phone call break (and smoking breaks too). Your
supervisor/field manager/data manager will determine when your breaks may be taken. We
encourage you to eat prior to, or at the end of your shifts. Also note, many of the recruiting
sites are in relatively remote areas and do not have restaurants or food establishments
nearby. Plan to bring food if you need to eat during your workday.
Training and Supervision
You will be trained in all aspects of your job prior to be sent into the field. Our supervisors/field
managers/data managers will then provide further assistance when in the field. We will teach
you the proper techniques and safety procedures to ensure that you will be successful in your
job. If you are not sure of something during the course of your work day, please see your
supervisor/field manager/data manager.
Reporting for your shift
1. You are expected to report to work on time as scheduled. Please do not arrive late.
2. Arrive at work wearing your CHEERS T-shirt and ready to work.
3. Please be prepared to work your entire shift
4. Complete your time card and submit to the immediate supervisor at the end of each
shift. Over reporting of time worked will require disciplinary action.
EMPLOYMENT POLICIES
Every job is important and yours is no exception. It is necessary that you report to work
according to your schedule.
• If you are scheduled to work and are unable to do so (for example: you become ill or there is
a schedule conflict), you are responsible for finding a substitute who is capable of performing
your job. A list of coworkers’ phone numbers is posted on the CHEERS website (blackboard).
• If you fail to report to work without finding a substitute, the absence will be unexcused.
Unexcused absences may result in termination and a negative evaluation.
• If you are ill and unable to work, we may require you to provide verification from your doctor.
• If you might be late for work, inform your supervisor/field manager/data manager on the
schedule for that day. Failure to do so may result in disciplinary action.
• Major emergencies or illnesses will be taken into consideration on an individual basis.
Discuss such situation with us.
• Remember, we try to be FLEXIBLE. Keep us informed and you will remain in good standing.
• You will be provided with two CHEERS T-shirts which should be worn during every shift. We
expect you to look clean, neat and professional at all times. You can always ask for
replacement T-shirts and for any questions about what is appropriate to keep yourself looking
great!
• It is unlawful to manufacture, distribute or possess a controlled substance while working for
UIC. Working while under the influence of drugs or alcohol is prohibited and will result in
termination. All employees are prohibited from carrying out any box, package or container
from their workplace without prior consent. Those employees who do not follow this policy
will be subject to appropriate discipline, which may include termination. Lost and abandoned
items should be reported to your supervisor.
• As a member of the CHEERS study team, your success during the course of your
employment is important to us. Work rules are established and are expected to be followed.
Your unit will also express additional expectations to you.
Safety Rules / Regulations
As an employee, you share in the responsibility for the health and safety of yourself, your
coworkers, study participants, and other members of the community.
• Follow the Cheers study safety plan at all times.
• Wash your hands or use hand sanitizer after using the rest room, coughing, sneezing,
touching your hair, eating or wiping your face.
• Report unsafe working conditions to a supervisor/field manager/data manager.
• If you are injured on the job, regardless of the degree of severity, notify us immediately. Your
supervisor will see that you get medical attention if necessary.
Quality Control
A lot of information will be presented during the training period. All this information is
designed to provide to you the tools, knowledge and experience necessary to be the best
interviewer that you can be. The study relies on the accuracy of the data collected during all
parts of the survey. It is very important that data collected is not biased or accidentally
misrepresented at any time.
The Assistant Study Director- Surveys, CHEERS Assistant Research Specialists, Study
Director, Program Coordinator and field supervisors will monitor each interviewer in the field
to receive effective feedback on the performance. Interviewers may not know when or how
they are being monitored. Monitoring is done in the field while an interviewer is recruiting
participants and administering questionnaires or through the data collected in a survey. The
CHEERS surveys have certain built-in checks that help us monitor the data quality by
interviewer.
Monitoring is more heavily concentrated during the early phases of data collection and for
newly-hired interviewers so that any problems in performance can be detected immediately.
The monitor is instructed regarding whom and when to monitor based on random selection of
interviewing stations and times during each field shift or as a result of problems noted from
previous monitoring sessions.
This ongoing monitoring of CATI interview files permits interviewer skills to be evaluated in a
number of key areas, including the ability to follow instructions correctly and the ability to
probe for clear and complete answers. Through monitoring, additional factors, including the
interviewer’s skills in eliciting cooperation and maintaining neutrality, are routinely evaluated.
Errors detected during these reviews are recorded on a standardized form to help supervisors
give feedback to interviewers and retrain as necessary. Positive as well as negative feedback
is given to ensure that the interviewers maintain a high level of commitment to quality.
Falsifying Data Collection
Intentionally falsifying data collection is illegal and unethical and will compromise the reliability
of the study. All of your work is to be performed in a faithful, industrious, and professional
manner in accordance with the code of conduct specifically established for the CHEERS
study. Your work must be authentic. CHEERS may validate your work by contacting
respondents and you are expected to cooperate with these validation efforts. CHEERS used
internal and external checks to validate data collection in 2007 and will continue to do so in
2008.
It is unethical and fraudulent to submit any work that you represent as data that you have
collected from sample respondents, when you have not, in fact, done so. Any violation may
result in termination of employment and may violate IRB rules regarding scientific integrity. A
violation may also lead to other actions by CHEERS, such as criminal court action and claims
for money damages.
Interacting with the Media Press
We are not the experts on this survey. We are their means of acquiring data and are not the
spokespersons of CHEERS. The CHEERS representatives are the scientists involved with
this study. Dr. Samuel Dorevitch (Study Director), Dr. Preethi Pratap (Assistant Study Director
- Surveys), Sara Wuellner (Program Coordinator), Amelia DeLaquil (Assistant Research
Specialist) and Todd Schoonover (Field Logistics Coordinator) are the CHEERS staff
authorized and prepared to answer any and all questions any representative of the media
should have.
Tips for Handling the Press
Don’t Panic: The media are people too. Be calm, if you’re in the middle of an
interview finish the interview and politely speak with the media person.
Be Polite: Politely explain to the media person that you’re job is to conduct
interviews and that the principal investigator or a supervisor will be able to
more accurately answer their questions. Direct the media person to Sam or to
the supervisor available at your location.
Be Proactive: If you see a media person looking for someone to interview,
direct them to either Sam or to a supervisor.
QAPP 2
Appendix 1G
CHEERS Survey Training Manual
Professionalism Policy
CHEERS Professionalism Policy
:
It is the study’s expectation that all employees will conduct themselves according to high
standards of conduct and performance. Our work takes place in the public arena, and
unprofessional behavior can impair our ability to collect high quality data, damage to the
study’s image in the eyes of public, and promote discord within the project team. When
employees do not behave professionally, it is the immediate supervisor's responsibility to
act in a timely manner and initiate a program of disciplinary steps to address the problem.
This policy presents the basic principles and procedures of a system of progressive
discipline which is intended to ensure that all employees are treated as consistently and
fairly as possible. The disciplinary program has four major purposes:
¾
To ensure that the employee knows what the problem is
¾
To communicate what the supervisor's expectations are in order for the employee
to correct the problem
¾
To provide appropriate penalties for improper work conduct
¾
To provide a record of corrective action taken by supervisors in such problem
situations.
Progressive Discipline
Progressive discipline is a formal process which includes several steps or levels of
discipline, each of which provides the employee with the opportunity to correct the
problem or inadequacy.
A. Preliminary Actions. Prior to moving to formal discipline the supervisor should do the
following:
1. Do a thorough fact-finding which includes collection of all information and
applicable records. This will be done by the Field supervisor and Survey
Managers.
2.
Hold a discussion in private with the employee. During the discussion the
supervisor should state the problem clearly and allow the employee to respond.
3.
Follow up with the employee after the meeting and after all information has been
gathered, to report the findings. If the supervisor intends to move to formal
discipline, the employee should be told at the conclusion of the follow-up meeting
or as soon after as possible. It should be made clear to the employee which level
or step of the discipline process is being applied.
4. Provide a follow up letter as soon after the meeting as possible. The letter should
include the date and time of the follow-up meeting, a brief statement of the
problem, the supervisor's expectations, and the conclusion reached in the meeting.
The stage of discipline must be clearly noted and a statement made that lack of
improvement will result in further discipline.
B.
The Steps of Progressive Discipline. There are
four steps
in the progressive discipline
process; however, in cases of misconduct or repeated infractions, the process may be
shortened and the supervisor, in consultation with the Principal investigator, may move
directly to a later step in the process, including termination.
All disciplinary action should be taken within a reasonable time frame it is recommended
that no more than two days elapse between the time the supervisor learns or has
knowledge of the offense and the action is taken.
The standards of professionalism differentiate between minor and significant violations.
Examples of minor violations of standards of professionalism include:
•
Not wearing the CHEERS t-shirt while working
•
Speaking on a cell phone while working (meaning, while not on a break
scheduled by a supervisor)
•
Arriving more than 15 minutes late to the study location
•
Smoking at or adjacent to the study location
Examples of significant violations of standards of professionalisms include:
•
Failure to show-up to work without arranging a shift swap or notifying the
supervisor the day before
•
Arguing with or making inappropriate comment to study participants
•
Insulting study participants
•
Theft
•
Refusal to perform work
•
Falsifying data collection
1. Oral Warning
Oral warnings are appropriate for minor first offenses. It is important that supervisors not
overuse the oral warning for the same type of offense; no more than two oral warnings
should be given.
The supervisor should have a full discussion with the employee before giving the
warning to ensure that the employee has the opportunity to respond or to give additional
information. If the supervisor believes that an oral warning is appropriate, it should be
made clear to the employee that the oral warning is the first step in the progressive
discipline process. The oral warning should be
documented
for the supervisor's record
and it is recommended that a note summarizing the warning be given to the employee.
The record and note should record the date, time and reason for the warning. This will be
done by the Field Supervisor or Survey Manager.
Note: The oral warning remains in effect for 2 months
.
2. Written Warning
After an employee has received an oral warning, a subsequent minor offense should be
addressed by a written reprimand as appropriate. Arriving more than 30 minutes late
will also results in a written warning. When a field supervisor notes such an offence,
his/her manager (survey manager, field manager) must review the draft of the written
reprimand with the Principal Investigator. The supervisor and employee first meet to
discuss the problem. In the discussion, the supervisor must review the incident or
performance problem which requires the reprimand and the supervisor and employee
should exchange ideas and information regarding solution(s) to the problem. The written
reprimand should be given to the employee directly following the discussion. Employees
can provide a written response to the warning within two (2) days of receiving the
warning and both documents will be placed in the employee's official personnel file.
The written warning should:
¾
Be identified as a disciplinary warning
¾
Describe as specifically as possible the situation which prompted the warning.
including day, date, time, location, and what the supervisor saw or heard
¾
Indicate why the behavior or performance is unacceptable
¾
Review the decisions that were reached during the discussion regarding how the
employee would correct the problem
¾
State that if the behavior continues or other problems occur, additional corrective
measures may be taken, which may result in termination of employment.
¾
The supervisor should discuss the matter with the employee when giving the
employee the warning.
Note: The written warning can be given without a prior discussion regarding the incident
between the supervisor and employee.
Written warnings are retained in the employee's formal record.
3. Suspension
Suspension is the third step of the disciplinary procedure. It is intended to indicate to the
employee the seriousness of the infraction and that the employee can reasonably expect
that the next step is termination of employment. Suspension is an appropriate response
to arguing with study participants, engaging in unsafe or illegal activities, intoxication, or
more than one failure to show up for work.
Before determining if an employee should be suspended,
the supervisor must meet with
the employee
to discuss the incident or problem and consult with the Principal
Investigator.
The employee should be notified in writing of the suspension as soon as
possible and given two (2) days to respond. The letter should outline the reason for the
suspension and the dates of the suspension. Suspensions are normally for three (3) to five
(5) consecutive work days and the dates are determined by the supervisor in consultation
with the Principal investigator and program coordinator. Longer suspensions because of
severe infractions may be given and scheduled at the convenience of management. The
employee should be warned that continuation of the behavior may result in termination of
employment.
Suspensions are without pay. There may be instances when a final written warning may
be more appropriate, and may, upon consultation with the Principal Investigator,
substitute for a suspension (for example when discipline is for a pattern of absenteeism).
4.Termination
Termination of employment is the culmination of the progressive discipline process or
the penalty for very serious offenses such as theft or violence. Whenever possible, the
Principal Investigator, Field Manager, Project Manager, Survey Manager, or Quality
Control Manager should conduct a
pre-termination
hearing. The purpose of the hearing is
to review with the employee's supervisor and the employee, the past record and any new
circumstances leading to the supervisor's request to terminate.
Termination Procedure:
The Supervisor or Survey Manager will provide the pre-termination panel and the
employee with any oral warning(s), any written warning(s), and a written statement
outlining the reason for termination. A Minimum of two (2) days should be given to the
employee to provide a written response to the panel. The panel may then either schedule
a meeting or provide an immediate decision as to the appropriate course of action. In
either case an action should be taken within fourteen (5) days of the initiation of the
procedure.
Note: Typically the employee will be suspended without pay during the time of this review
QAPP 2
Appendix 1H
CHEERS Survey Training Manual
Survey Training Manual
CHEERS Survey Training Manual Documents (2008)
1. CHEERS Survey Methods power-point presentation
2. Professionalism Policy
3. Know your job
4. Standards and Ethics in research
5. Survey Research Lab (SRL) CD
6. Recruiting:
a. Script,
b. FAQ sheet
c. Water Quality info. sheet
d. Mobile recruiting
7. Eligibility screener and Refusal Tally
8. Informed Consent:
a. Consent/Assent/Parental Consent
b. How to consent document
9. CAWs Use Survey:
a. How to conduct a use survey
10. Field surveys:
a. Refusal Aversion Exercise
b. Probing
c. Gaining Cooperation
11. BLAISE Manual
12. Mock Interviews (using laptops)
13. CHEERS job descriptions:
a. Recruiter
b. Interviewer
c. Incentive Person
d. Data Manager
QAPP 2
Appendix 1I
CHEERS Survey Training Manual
Consent Process
HOW TO CONSENT A CHEERS STUDY PARTICIPANT?
Please do take some time to read the consent/ assent and parental consent forms. If a
participant is eligible to enroll in the study then you take them through the consent
process.
1. All adults
18 years or older
will need to sign and date a CONSENT FORM.
2. All children
8-17 years old
will need a signed/dated PARENTAL CONSENT and
also need to sign and date an ASSENT FORM.
3. All children
7 years and below
will need a PARENTAL CONSENT ONLY.
4. Please highlight the 5 main points in the consent form-
a. CHEERS is a voluntary research study. You can choose to quit any time
you want. This will not affect your relationship with UIC at anytime.
b.
You will complete a 3 minute Survey A, and a 5-7 minute Survey B. For
this you will receive a $15 Target gift card and a T-shirt. (Note: Water-
recreators will need to return (mention time) before we leave for the day
and complete Survey B. Only then will they receive the incentives). Tell
them about the follow-up phone surveys 2, 5 and 21 days later. The phone
surveys take about 6-8 minutes. We will ask them about their health. They
will receive a $35 check in the mail after the final phone call.
PARTICIPANTS CAN CHOOSE TO DONATE THE $50( $15 gift card
+ $ 35 check) CASH INCENTIVE TO ANY CLUB/ORGANIZATION
THAT HAS AN MoU WITH UIC. IF WE ARE NOT WORKING WITH
A CLUB OR TEAM THEN PARTICIPANTS CAN ONLY DONATE
TO: CHEERS STUDY OR FRIENDS OF THE CHICAGO RIVER.
c. Participants may require a home visit if they do experience any skin, eye
symptoms. A clinician will visit them at home and we may request a skin
or eye swab. OR if participant experiences certain GI symptoms (stomach
cramps, vomiting, diarrhea) they may be requested to provide a stool
sample. If this happens they will receive an additional $75.
d. The only risk to them is that we ask for their name, phone number and
address. We will not share this information with anyone else. This
information is used to contact them for the phone surveys and to send
them their check. Once their participation is complete- we will only use
the case id number (from the wristband) for the data analysis. Show them
the wristband.
e. Finally, tell them page 4 of the consent form has a number where they can
contact us with any questions or concerns about this study.
5. Participants will need to sign and date the consent form. Ensure you sign it and
date it and store it in the black folder.
6.
Strap one copy of the wrist band on their wrist and staple the other to the consent
form.
Participants who lose their wrist bands cannot receive any incentives.
7. Ensure you give them the first 4 pages of the consent forms.
Troining
moteriol:
Not
for
distribufion
CHEERS
FAQ SHEET 2OO8
DY PURPOSE/STUDY
QUE
1.
What is CHEERS?
-
CHEERS
is
the
"Chicago
Health and Environment
Exposure
Recreation
Study"
STARTsAPPROVAL
eidne$
FiB012003T0JUN20?008
unlK$',+[[85{A[H[?lEfiLH'$[qr
2.
"What's
the
purpose
of this survey?"
"l
need
more information."
-
Researchers
at the University
of lllinois
Chicago, School of Public Health,
want
to better understand
the
connection
between
water
quality
and the
health
of
people
like
you
who
are active in
and around
these
waterways.
-
This
is
a
multi-year
research
project
evaluating
the health of
people
like
you
who
use recreational
waters
such
as Lake
Michigan,
the
Chicago River and other waterways,
for kayaking,
boating, fishing
and other non-water
activities
such as
running, biking
,
golfing
etc.
-
Results from this study
could be used for developing
better
environmental
water
quality
standards
to
improve
public
health.
-This
study
is being funded by the
Metropolitan
Water
Reclamation
District
of Greater Chicago
3.
Why can't swimmers
be in this study?
-
Many research studies have
evaluated
the link
between
water
quality
and swimming.
This study addresses
other forms of
water recreation.(
Such as-
mention
participants
proposed
activitv for
the dav)
4.
"What
questions
will
you
ask?"
The
questions
asked are
primarily
about
your
activity for
the
day,
other
watenruays
you
have recently
used,
your
current health status,
and some
questions
about
food
and
pets.
During
the follow-up
telephone survey we will ask
you
about
any symptoms
or illnesses
you
have
experienced
after
your
activity today.
3.
"And,
what am
I
going
to
get
out
of this?"
-
lt
is
your
opportunity
to
have
your
input included in an important
effort
influencing
policies
and
programs
that
may reduce
pollution
in recreationalwaters
across the country and to
protect
recreators
from
illness.
-
You can impact environmental
conditions
in
your
community and
protect
the future health
of
your
family
and
other
lake/river users.
-
You
will receive a t-shirt and
a
$15
gift
card today,
followed
by a
$35
check in the mail when
you
finish
the
final
phone
survey.
5.
"Why
has River/Lake
been singled out
for
these
interyiews?"
-
This study
is being conducted
for different waterways
in
the
Chicago Area. We
are
recruiting
people
at Lake
Michigan,
Chicago
River, Skokie
Lagoons,
Crystal Lake, Fox River,
Desplaines
River etc... .,..
6.
"What
about beach
water
quality?'
What
results
are
you
seeing?
-
The data
is
yet
to be analyzed,
but
you
can
get
more information
about the water
quality
from these
websites
in
this
information
sheet.
Please hand out the
Water
quality
Information
sheet,
7.
"How
long
willthe study
last?"
-
The
research study
will
last about 3
years.
lf
you
enroll in the research,
your participation
will last 3 weeks.
Please
note
this
does
not mean every
day. You will answer a
few
questions
today and
2,5
and
21
days from
today.
CHEERS FAQ
Sheet,
Version 2,
December 2007
Troining
moteriol: Not
for
distribution
8.
"Can
I
participate
later?"
-
Yes,
you
can reach us at this number on the flyer. We will be at different
locations
on different days.
9.
"lf
I
volunteer to
be
in this study,
what will I
be asked to do?"
We
will
explain
the study to
you
in detail
and any
questions
you
have will
be answered. Then,
we will ask
you
a few
questions
about
your
recent health and
recreational
activities
to see if
you
are
eligible to be in this
study. lf
you
still
want to be
in
the study,
we will ask
you
to sign a consent form to
participate
in
this study for the next
3
weeks.
Then-
.
We will ask
you
a
few
questions
before
your
activity
today.
This will
take about 3 minutes.
.
We
will
ask
a few
questions
after
your
recreational
activity and
your
current
health. This will take
about 7
minutes.
.
We
will
call
you
2,5 and 21 days
from
today to ask
you
about how
you
feel. This will
take about 6-8 minutes.
In addition,
if
you
feel ill
you qILE
selected for a home visit by a clinician in next
21 days or we may
request a
stool sample
from
you.
Note: lf
participant
needs more details
you
can direct
them to a senior research
staff member.
lf
you
decide to
participate,
we will
give you
a
T-shirt
and a
$15
gift
card after
you
have finished
the surveys
today.
At the end
of 21 days of
participation
we will send
you
a
$35
check for
your
time
and effort. We will also
give you
a
check
if
you qualify
for a
home visit
by
a nurse or
provide
a stool sample.
CHEERS
FAQ Sheet,
Version
2, December 2OO7
Troining
moteriol:
Not
for
distribution
10.
"What
is
going
to
happen to
the information
I
give you?"
S
"l
don't want to do this study and
get
on
some
mailing
list."
$
"ln
this
computer
age, I
just
don't trust
who
can
gain
access to my
personal
business."
-
By
law, all
information
you
give
is kept strictly confidential.
-
The
information
you
provide
is released
only in summary
form as statistical
totals.
(Your
responses
are added to
the
responses
of others
and
published
as combined
information
only.)
-
No
individual
responses
or information
that
would
permit
the identification
of any individual
will be released or
published.
-
All data
is stored
in
password
protected
files or
in locked
cabinets.
-
All
personal
identifying
information
is removed and
will not be disclosed or
released
to anyone
for
any
purpose
other than
persons
directly
involved
with
the
study, and
we
are all
required to sign a statement of confidentiality
regarding
all
information
provided.
-
You
will not be added
to any
mailing
list.
11.
"l
don't
know anyone
who
gets
sick
from the water, and this
is
something
being blown out of
proportion
for
you
and the
government
people
to
have a
job."
-
Research
studies
such as this
always
welcome a cross-section
of answers and opinions.
-
We don't
know
if anybody
gets
sick
from the water.
This is what we are trying to
find
out.
[BEAR
WITH
ARGUMENTATIVE
RESPONDENTS
WHO WISH
TO
"VENT.'
THEY HAVE NOT REFUSED
IF
THEY
ARE
TALKING.
DO
NOT ARGUE; SIMPLY
MAKE SHORT,
NEUTRAL COMMENTS SO
THEY KNOW
YOU ARE
LTSTENING.I
'tr2.
"No
thanks.
l've had
a bad experience
doing
a
phone
survey."
l'm sorry
that
you've
had
a bad experience.
I would certainly
hope
that
your
experience
with
me would be a
pleasant
one.
This
is a special
research
effort and by
participating
in
the study,
you
will help
protect
the
future
health
of
your
family and
other
recreators.
CHEERS
FAQ Sheet,
Version
2,
December
2OO7
Troining
moteriol:
Not
for
distribution
ME
QUESTIONNAI
13.
"How
long willthis
take?"
-
The amount
of time
varies from
person
to
person,
but
it
typically takes around
10 minutes.
14.
"l'|la
be happy
to do this
if
you
send
the
questions
to
me in the mail."
-
There
is no
paper questionnaire
available
because
people
are asked different
questions
based on the answers
they
give
to
previous
questions.
The computer
selects the appropriate
questions,
which
saves time.
PONSORS
(CLIENTS)/INFORMATIONAL
REQUES
15.
"Who
do
you
work tot?"
I work for the
University
of lllinois
Chicago, School of
Public Health. The School of Public Health conducts
studies and
evaluations
on
many different topics
related to the
health
and safety of the
public.
16.
"Do
you
have a
website?"
"Can
I
get
a copy
of the study?"
-
You can
contact
Dr.Sam
Dorevitch
(Study
Director),
or Sara
Wuellner
(Program
Coordinator)
at 312-996-
2094. Mention
that
you
are calling about
the CHEERS study.
-
CHEERS
website
address
is :
17.
IF
ASKED
A PERSONAL
QUESTION:
-
I'm unable
to discuss
my own
personal
habits
or
opinions because they
may
bias
your
response and the
study
results.
My
job
requires
that I only ask
the study
questions.
18.
"What
if I
get
sick after
going
in the
water today?"
-
We will
want to know
about that.
We'll call
you
in 2,5, and 21 days
from
today.
We'd want
you
to call us as
well.
-
We won't be
able to say
if
your
water contact today
made
you
sick, but
by
enrolling
thousands of
people
,
we
willfind out
if in
general
,
water contact
is hazardous.
19.
"
Who
will
pay
for my treatment,
if I
get
sick?"
-
No injury
is expected
to occur
as a
result of
your
participation
in
this research.
However, in the event of any
illness that
you
develop
after
your
water activity
today we encourage
you
to follow
your
usual
way of
managing
sickness
in
your
family. UIC
will not be
responsible
for diagnosis
or treatment of
illness
that
occurs
in
your
family
during
participation
in this study.
CHEERS
FAQ
Sheet,
Version
2, December
2007
QAPP 2
Appendix 1K
CHEERS Survey Training Manual
For Mobile Recruiting
For mobile recruiting:
1. Organize the mobile folders-
a. Consents ( All adults 18+)
b. Assents +Parental Consents (All children between 8-17 years)
c. Parental Consents only (All children 0-7 years)
d. Location flyers
e. Case IDs
f. Laptop and scanner
g. Stapler
2. Clipboard-
a. Eligibility screener and Refusal Tally sheet
b. Mobile Log (just a sheet of paper)- to track Case IDs with survey A and B
3. What to do?
a. Approach people (make sure fishermen are yet to start fishing)
i. Recruit
ii. Eligibility screener
iii. Record in refusal tally if ineligible or refused to participate
b. If eligible to participate
i. Consent participant
ii. Give Case ID number- one on the wrist and one on the consent form.
Make sure participant gets first 4 pages of consent form. Secure
signed consent with ID in the folder.
iii. Record Case ID given out in a log (JUST A SHEET OF PAPER)
iv. Administer survey
1. Non-water recreators get both survey A and B
2. Water recreators will get only survey A and will be asked to
return to the CHEERS tent for Survey B.
c. Incentives- please note that all participants will have to return to the
CHEERS tent to collect their incentives. Incentive person will have to make
sure that participant has done Survey A and B before they hand out the gift
card/t-shirt and stool flyer.
d. DM- You can send them out in groups of 2-3 (please monitor the process
the first time). These are new interviewers and have minimal interview
experience.
e. DM- Please review the consent process with them.
f. DM- Make sure all interviewers return the log (sheet of paper) with case IDs
given out and the corresponding consent forms.
QAPP 2
Appendix 1L
CHEERS Survey Training Manual
Instructions for Data Managers
Instructions for data manager
DM responsibilities:
You will receive your assignment by time/location and date from Sara. There will be an
option for you to sign-up as DM for each event.
Remember you are the immediate field supervisor in the field for the CHEERS staff.
•
Arrive at the location at least 15 minutes before your schedule.
•
Receive the supplies from the CHEERS Supply Manager/Driver for that
day/location.
•
Use the supplies checklist to ensure you have everything you need for that
day/location.
•
Fill out the time sheets for all staff.
•
Each member is allowed 2 fifteen minute breaks in a 6-hour shift. Staff doing a
12-hour shift may take one 30 minute break and 2 fifteen minute breaks. Please
read the ‘CHEERS professionalism’ and ‘know-your-job’ document to be familiar
with the field rules for staff.
•
Ensure all supplies are in place and safe.
•
Count and account for the signed consent forms.
•
Monitor the incentive process- count and account for the gift cards.
•
Monitor the recruiting/interview process.
•
Assign the CAWs use survey task to a CHEERS staff each time we visit a CAWS
location.
•
Ensure safe and accurate transfer/download of the data from the laptop to the
flash drive.
•
Complete the CHEERS field report form (see the sample form)
•
Complete the DM task checklist (see sample checklist)
•
Hand-over the following to the CHEERS Supply Manager/Drive at the end of the
shift:
o
laptops,
o
flash drive,
o
used refusal tally sheets,
o
used incentive forms,
o
signed consents,
o
completed field report form
o
completed DM task checklist
o
completed CAWS use survey
•
You can always call the CHEERS on-call senior staff if you have any questions or
concerns.
Data Manager Survey Supplies List:
The CHEERS Supplies Driver/Manager will give you the following supplies-
1. A paper rack
2. Data Manager Accordion file
a. Permits
b. CAWs Use Survey Forms
c. CHEERS Field Report form
d. Time sheets
e. FAQ sheet and Recruiting script of interviewers
f. CHEERS staff job description document
3. Black plastic “Signed consents” folder for storing consents and other documents.
4. A Log book to record Case ID, and status of Survey A/ B
5. A box of scanners
6. A box of pens, staplers/pins, bungee cords
7. A box of UNUSED back-up batteries for the laptops
8. An empty box to store DEAD/USED batteries.
9. Set of laptops
10. One flash drive
11. A roll of wrist band case IDs.
12. A box of gift cards
13. Boxes of t-shirts
14. Clipboards
15. Plastics file folders
a. Consents ( All adults 18+)
b. Assents +Parental Consents (All children between 8-17 years)
c. Parental Consents only (All children 0-7 years)
d. Water Quality Information sheets
e. Location specific flyers
f. Eligibility/Refusal Tally sheet
g. Incentive sheets
h. Stool flyers
16. Mobile recruiting folders (if necessary for that event or location)
In addition- each location will have a set of tables, chairs, tents, water cooler, and a
CHEERS Study banner.
When you reach the site:
1. Set up the tent and supplies
a. Arrange the papers and laptops on the table
b. Put up the CHEERS banner
2. Fill-out time sheets.
3. Announce the location to all CHEERS staff: for example, tell them ‘we are at the
CAWs location: Alsip or General Use Waterway location: Crystal Lake today’.
This will ensure everybody enters the correct location in survey A and B.
4. Offer CHEERS staff a copy of the recruiting script/FAQ sheet, if they want to
brush up on what to say.
5. Offer the CHEERS staff job description documents, if they want to better
understand their task for the day.
6. You can also brief the staff about any previous experiences at this location.
7. Assign tasks to CHEERS staff (you can rotate these assignments)
a.
Recruiters- Each recruiter will have a clipboard with flyers, eligibility and
refusal tally sheet. Recruiters will use eligibility and refusal tally sheet as
and when they approach people. Participants must be able to return to
finish Survey B before the CHEERS event for the day ends. They will
then direct the participants to the CHEERS tent.
We do not need
recruiters when we work with clubs/teams.
b. DMs will consent participants and assign Case IDs. Please ensure all case
IDs are in duplicate.
You will have rolls of Case IDs in your survey
supplies. Please start from where the previous DM ended.
Read the
instructions on how to consent.
c. DMs will record location, date of event, and participant progress by case
IDs in the field data log book. Store signed consents in the black plastic
file. Please count them on a regular basis.
d. DMs can direct the participants to the interviewers. You can also advice
the interviewers as to whether the participant is a water or non-water
recreator.
e. Participants returning to complete Survey B must check-in with the
DM first. If participant has lost/misplaced their wrist band- then find
their consent form so that the correct Case ID can be used to finish
the survey B.
f. Interviewers- Each interviewer will have a laptop and scanner.
Interviewers doing surveys must check with the participant if they are
water recreators or non-water recreators before they start the surveys.
g. Interviewers will always open the CHEERS folder on the desktop.
Followed by the “MAIN LAPTOP-X” folder. Please use “Part A.bat” file
for survey A and “Part B.bat” file for survey B.
h. Interviewers close out all interviews – answer all questions
(
including
proxy question). If you enter the wrong case ID or start a wrong
survey- then please leave a “Remark” before you start a new survey.
This is also the case when you lose power and start a survey on a new
laptop. The remarks you leave helps the SRL staff reconcile the data
for the phone surveys.
i. DM and Interviewers will check laptops batteries every 2 hours and swap
batteries, if necessary. Place USED batteries in the USED/DEAD battery
box.
j. Incentive person- Please position yourself close to the DM. You can help
the DM when he/she gets swamped. Always direct the participant to the
DM when they return for their Survey B and incentives.
k. Incentive person will always ask participants:
i. Have you completed both surveys today?
ii. Have you consented to donate incentives today?
l. Incentive person will use the incentive sheets to record the date/location
and start/end count of gift cards along with the participant’s incentive
status by case ID.
m. Each participant will receive a t-shirt, gift card and stool flyer. Record
“DONATE” if they donated incentives.
Please inform the DM if you run
out of t-shirts or gift cards. You will need to record the participants
cased ID, name and address so that we can mail it to them
.
n. Monitor the CAWs use survey (see instructions for use survey).
o. DM back-up the files onto the flash drive at the end of the shift. Copy the
“
Main Laptop- X
” folder from each laptop.
p. Complete CHEERS field report Note any problems in the field, including
interviewer issues.
q. Return all laptops, signed consents (in the black file), refusal tally sheets,
and incentive sheets, time sheets, FLASH DRIVES WITH DATA, CAWs
use survey and field report to the CHEERS supply manager/driver. The
black log book can stay in the same box. Please count consents again.
For mobile recruiting:
1. Organize the mobile folders-
a. Consents ( All adults 18+)
b. Assents +Parental Consents (All children between 8-17 years)
c. Parental Consents only (All children 0-7 years)
d. Location flyers
e. Case IDs
f. Laptop and scanner
g. Stapler
2. Clipboard-
a. Eligibility screener and Refusal Tally sheet
b. Mobile Log (just a sheet of paper)- to track Case IDs with survey A and B
3. What to do?
a. Approach people (make sure fishermen are yet to start fishing)
i. Recruit
ii. Eligibility screener
iii. Record in refusal tally if ineligible or refused to participate
b. If eligible to participate
i. Consent participant
ii. Give Case ID number- one on the wrist and one on the consent
form. Make sure participant gets first 4 pages of consent form.
Secure signed consent with ID in the folder.
iii. Record Case ID given out in a log (JUST A SHEET OF PAPER)
iv. Administer survey
1. Non-water recreators get both survey A and B
2. Water recreators will get only survey A and will be asked
to return to the CHEERS tent for Survey B.
c. Incentives- please note that all participants will have to return to the
CHEERS tent to collect their incentives. Make sure they check-in with the
DM and then follow the same incentive procedures.
d. DM- You can send them out in groups of 2-3 (please monitor the process
the first time). These are new interviewers and have minimal interview
experience.
e. DM- Please review the consent process with all mobile interviewers.
f. DM- Make sure all interviewers return the log (sheet of paper) with case
IDs given out and the corresponding consent forms.
QAPP 2
Appendix 1M
CHEERS Survey Training Manual
Mobile Recruiting Log
For mobile recruiting:
CASE ID
Survey A
Survey B
QAPP 2
Appendix 1N
CHEERS Survey Training Manual
Recruiting Script
srARrsApmOVA[
exgRes
rrR
0
1,
??ni
I
0
,jul,{
?_
a
2{d0g
Troinins
Moterior:
Nor
for Disrriburion
unlYt$Tf+IT'8[li.S?iE6!6X,,?d"o
CHEERS
2008
Field
Recruiting
Script
Greeting:
Good morning,
good
offernoon
or
good
evening.....
I
om
(mention
full
nome)
from the
University
of lllinois
Chicogo
(UlC)
School
of
public
Heolth. We
ore conducting
o reseorch
study.
Hove
you
heord
obout
the
CHEERS
study?
OR
Greeting:
Good morning,
good
ofternoon
or
good
evening.....
I
om
(mention
full nome)
from
the
University
of lllinois
Chicogo
(UtC)
School
of
pubtic
Heolth. We
ore
conducting
o reseorch
sludy. lf
you
hove
q
few
minutes
we
would
like
to tellyou
obout the
Chicogo
Heolth
ond
Environment
Exposure
Reseorch
Study,
olso
known
os CHEERS.
OR
Greeting:
Good morning,
good
ofiernoon
or
good
evening.....
I
om
(mention
full nome)
from
the
University
of lllinois
Chicogo
(UlC)
School
of
public
Heqlth. We
ore
conducting
o reseorch
study.
Did
someone
hond
you
o CHEERS
flyer
(show
poriicipont
o
flyer)?
OR
Greeting:
Good morning,
good
ofternoon
or
good
evening.....
I
om
(mention
full
nome) from
the University
of lllinois
Chicogo
(UlC)
School
of
pubtic
Heolth.
(Hond
out o flyer)
We
ore conducting
o reseorch
study. lf
you
hove
o few
minutes
pleose
stop by the
CHEERS
tent
(point
the
direction
or mention
locotion)-
we
would
like to tell
you
obout the
Chicogo
Heolth
ond Environment
Exposure
Reseorch
Study,
olso
known
os CHEERS.
OR
Greeting: Good morning,
good
ofternoon or
good
evening.....
I
om
(mention
full nome) from the
University
of
lllinois
Chicogo
(UlC)
School
of Public
Heolth. We
ore conducting
q
reseorch
study.
CHEERS
is on interesting
reseorch
study for
people
who
use
vorious
Chicogo
woterwoys
for octivities.
You will
receive
o free t-shirt
ond
$50
for
your porticipotion
in
our study todoy.
CHEERS
Recruiting
Script,
Version
l,
Jonuory 2008
QAPP 2
Appendix 1-O
CHEERS Survey Training Manual
Standard Ethics
1
Standards and Ethics
in Survey Research
CHEERS Survey Training
Handout
2008 Season
2
STANDARDS AND ETHICS IN SURVEY RESEARCH
CHEERS is committed to the collection of high-quality, independent and unbiased
data. We are also committed to following ethical principles and practices in the collection of
these data. This commitment assures our clients, researchers, educators, business leaders, and
policymakers that they can have confidence in the data we collect.
The material in this handout will help you to:
1.
Understand the importance of ethical principles and practices in survey research.
2.
Provide you with historical information related to the development of ethical practices in
research.
3.
Raise your awareness of the process of informed consent and the importance of
confidentiality.
4.
Help you understand your ethical responsibilities during the performance of your job as a
data collector.
5.
How to apply the training to field work.
You WILL fill out this survey!
You WILL do it NOW!
You have NO choice!
3
1)
What is at the core of ethical principles and practices in survey research?
At the core of ethics in data collection is that researchers have responsibilities to the
public, to their clients, to study participants, and to you—data collectors.
Researchers have a responsibility to the public to ensure that the survey
findings released/published are an accurate portrayal of survey data. This
includes conducting mandatory checks on the accuracy of the information
collected.
Researchers have a responsibility to clients to conduct work as agreed upon (in
their contracts) and to maintain all proprietary information in a confidential
manner.
Researchers have a responsibility to study participants (1) to inform them
about the basic elements of participation; (2) to maintain participant data
confidentiality; and (3) to avoid using practices or methods that may harm,
humiliate, or seriously mislead participants.
Researchers have a responsibility to you—data collectors. Researchers cannot
ask you to engage in any activity during the performance of your job as a data
collector that does not follow the general principles specified above with
regard to the public, our clients, and study participants.
2)
What are the key international and national events surrounding the development of
standards and ethics in research?
Following World War II, the allies convened the Nuremberg Court to try Nazi war
criminals and expose Nazi medical war crimes. These crimes included medical testing on
unsuspecting human subjects.
In 1949, the court published the
Nuremberg Code
that:
Formed the basis of global ethics codes for medical researchers.
Emphasized the importance of voluntary consent for research participants.
Stated that risks to research participants should be minimized.
Stressed that participants must be allowed to withdraw from research.
4
During the years following the publication of the Nuremberg Code, the World
Medical Association continued to work on ethics in research, and in 1964 they issued a landmark
document called the
Declaration of Helsinki
. This document expanded on the principles laid
out in the Nuremberg Code and, most importantly, made “informed consent” a requirement for
medical research. It stipulated that study participants must “formally” consent to participation in
a study after being fully informed about the basic elements of the study.
At the same time, ethical problems were being uncovered in medical experiments
and testing in U.S. research.
Three of the most famous or infamous examples of a lack of oversight were the:
Tuskegee Alabama Syphilis Study.
Jewish Chronic Disease Hospital Study.
Willowbrook Study.
The
Tuskegee Alabama Syphilis Study
tracked the progression of syphilis in
African American males. Study researchers “knowingly” did not treat study participants who
had syphilis with penicillin even though it was known to be an effective treatment for syphilis.
In the
Jewish Chronic Disease Hospital Study
of 1963, live cancer cells were
injected into chronically ill patients without informed consent. The researchers believed that the
cancer cells would be rejected. They rationalized their actions by stating that they didn’t want to
“alarm” the patients.
The
Willowbrook Study
ran from 1963 through 1966. In this study, live hepatitis
was injected into children labeled mentally impaired. The researchers rationalized that the
majority of the children would have acquired the infection in the future anyway. In this study,
the parents of the children did give consent.
These and other studies raised important questions about ethical practices in our
country. The result was that, beginning in 1966, Congress and Federal agencies implemented
legislation and developed regulations to protect human subjects—also referred to as study
participants.
5
In
1966
, the U.S. Surgeon General and the head of the National Institutes of Health
(NIH) decreed that to conduct research sponsored or subsidized by the Federal Government,
researchers would have to submit their research plans for prior review and approval to an
independent committee. Today these committees are called
Institutional Review Boards or
IRBs
. All Westat studies dealing with human subjects must be reviewed and approved by
Westat and client IRBs.
In
1974
, Congress passed the
Privacy Act
. This act focused on restricting the
disclosure of personally identifiable records by establishing a code of “fair information
practices” to protect study participants.
In
1979
, the Federal Government published the
Belmont Report
, which is
considered to be the foundation of ethical research in the United States. It stipulates that study
participants must be treated with respect, with beneficence (i.e., protection of participant well-
being), and with justice (i.e., in a fair manner).
In
1981
, the
Code of Federal Regulation of Research with Human Subjects
was
issued and a Federal office to monitor and support the code was established. By 1991 17 Federal
departments and agencies had adopted these regulations—often referred to as the
“Common
Rule.”
In
1995
, another important piece of legislation was passed—the
Paper Work
Reduction Act
. It mandated that each Federal agency must review the collection of information
in terms of its need, the burden on the respondent, and the plans for using the information
collected. It also reinforced the informed consent process and stipulated that authorized studies
cannot be fielded or implemented without a valid control number assigned by the Office of
Management and Budget displayed on printed material.
3)
What is informed consent?
Informed consent
is a process where the researchers explain both the risks and the
benefits of the research study to the potential human subjects and obtain the subject's
permission/consent, whether verbal or written, for them to participate in the study.
6
Participants must agree or consent to take part in a study before the start of their
participation. Their agreement or consent to participate must be an informed one. “Informed
participants” are those knowledgeable about the basic elements of study participation.
3a)
What are the basic elements of informed consent that participants must know?
Study participants must:
Know who the sponsor is and what the purpose and duration of the research
are.
Understand the procedures of the study—the tasks they will be asked to
participate in.
Know that their participation is voluntary.
Understand the expected risks and benefits of participating in the study.
Know if and what compensation exists.
Know that the information they provide will be maintained following the rules
and laws pertaining to confidentiality.
Understand that they can withdraw from the study at any time.
Know who to contact if they have questions.
Every study has an informed consent process. The process can vary.
At a minimum, the study must include a
verbal introduction
which specifies all of
the basic elements of informed consent we just reviewed. Some studies have
prepared
materials
such as advance letters and brochures that must be given to the participant and again
include the basic elements of informed consent.
Other studies have an actual
consent form
that must be read and signed by the
participant. Studies can have some or all of the above procedures. Data collectors must follow
the informed consent procedures of the study they are working on “to the letter.”
7
3b)
What is confidentiality?
Confidentiality involves:
The appropriate treatment of information disclosed in a relationship of trust.
Meeting the expectation that such information will not be disclosed to others
without permission.
Confidentiality includes information collected during an interview and information
gleaned from observation, such as observations of the interview setting, the condition of a
respondent’s home, interpersonal communications observed among family members, etc.
3c)
Why is confidentiality important to survey participants?
Survey participants must be assured:
That information provided in confidence will not be used outside the stated
purposes of the study.
That they cannot be uniquely identified based on any study information
distributed for public and/or private use.
3d)
Why is confidentiality important to CHEERS?
CHEERS staff are ethically bound to follow the confidentiality requirements
set forth by our UIC IRB.
A major breach in data confidentiality procedures could affect our ability to
obtain contracts and/or gain respondent cooperation in the future.
3e)
Who must maintain study data confidentiality?
All project staff must maintain study data confidentiality. This includes:
CHEERS interviewers
8
CHEERS water staff
CHEERS home visit staff
CHEERS consultants and subcontractors
Field supervisors and data collectors
Field translators, interpreters, and escorts (if used)
Study Director, Assistant Directors, Coordinators, etc.
3f)
What are your responsibilities with regard to confidentiality?
You are the front line of the data collection process—data confidentiality in the
field begins with you.
You cannot disclose anything learned during data collection to anyone except
project team members or supervisors.
You cannot discuss information collected or observed with anyone outside the
project staff (i.e., other data collectors not on the project, family members, or
friends).
Unless a special exception is made by project managers, you should not
interview anyone you know.
All data you collect must be submitted through secure electronic transmission
and/or other hard-copy submission procedures established for the project.
4)
CHEERS Data Security
CHEERS has established a quality assurance plan to ensure data security
confidentiality. Our plan is based on the following principles:
Study data must be protected from unauthorized access;
Every step of data collection, from design to data release and distribution, must
be reviewed.
9
Study protocols for maintaining confidentiality and data security must be based
on documented and proven procedures. This is outlined in the Quality
Assurance Protocol.
All project staff, even volunteer staff and/or short-term staff (e.g., interpreters,
escorts), must make every effort to protect data and ensure quality.
All project staff must be trained to follow the quality assurance plan and
maintain confidentiality.
The quality assurance plan is implemented when the study design phase begins and
ends with the release of the data to the public.
The key elements of the security plan are:
Substituting participant codes for participant identifiers (e.g., using an ID#
versus the name of participant).
Separating participant identifiers from survey data. For example, a list with
participant name, phone number, and case ID must be destroyed after use or
handed over to a supervisor.
Limiting access to identifiable data.
Minimizing hard-copy materials kept.
Storing paper records and materials with identifiers in a secure place in offices,
homes, hotel rooms, cars, airplanes, etc.—any place where the records may be
at a given point in the data collection process.
Shredding “unneeded” survey documents with identifiers ASAP.
Providing security codes (user IDs and passwords) for computerized records.
Training staff to keep laptops secure in participant homes, offices, hotel rooms,
cars, airports, etc.
When necessary, emailing only data “without” identifiers via the Internet. Or
sharing password protected files.
Storing study data on secure network drives.
Restricting access to network directories.
10
Avoiding the production of analysis files and reports with small cells (e.g.,
single, female, Ph.D. statistician in small fishing village in Maine).
Including respondent identification information only as necessary in analysis
files and reports.
Avoiding the production of public-release files with identifying information
and data that can be matched against publicly available databases.
The CHEERS study expects data collectors, to follow during their employment on a
project, all codes from ethics, to technical performance, to work style, and confidentiality.
5)
What to do while conducting interviews.
Make sure that you ask the potential respondent if they want to participate in
the survey or study. Wait for a verbal yes or no.
Give the potential respondent time to make a decision.
If they respond with a verbal “yes” provide the participant with the
informational pamphlet/consent form.
Children under 7 are allowed to participate in this study with the signed
consent of the legal guardian, but no assent is needed from the child. Children
8 to 17 years are allowed to participate in the study with the signed consent of
the legal guardian and a signed assent from the child. Anyone 18 and older
requires only eligibility and a signed consent to participate.
Approach all potential respondents with respect. Do not argue with the
participant. If the potential respondent is rude to you, thank him or her for his
or her time and terminate the interview.
Answer all participants basic questions with knowledge provided to you during
training. However, for extended or hard to answer questions, refer him or her
to the CHEERS supervisor on site.
If an individual appears incompetent to participate, do not ask them to
participate. Thank him or her for his or her time and walk away.
State, “No physical risks are involved in participating in this survey.”
11
You must inform all possible respondents of the incentives: CHEERS t-shirt,
$15 Target gift card and $35 for completing the telephone interviews. Do not
bribe potential respondents. Simply inform them of the incentives and the
benefits of participating in the study, and always thank them for their time,
even if they choose not to participate.
Approach all groups of potential respondents fairly. Do not exclude
respondents based on sex, race, ethnicity, language, physical disability, or age.
-
Exceptions are persons under the age of 18 and persons that appear
incompetent to make the decision to participate and answer questions.
-
We will have bilingual interviewers that speak Spanish. Always attempt
to conduct the interview in English. If the potential participant needs the
interview conducted in Spanish, request one of the bilingual interviewers
to conduct the interview in Spanish. If the respondent understands or
speaks another language besides Spanish and English, we will have to
exclude the respondent from the study. Code the as ineligible.
Do not discuss the respondents’ answers with anyone. In the extreme instance
that a surveyor must discuss a participant’s responses, do so only with
individuals associated with the survey and make certain not to identify the
respondent. These discussions should normally be between the interviewer
and a field supervisor or field manager or data manager on site.
Do not take
ANY
questionnaires home. Edit all questionnaires at the field
location. Questionnaires will always be monitored by supervisory personnel
except during the times when they are secured in a locked receptacle.
QAPP 2
Appendix 1P
CHEERS Survey Training Manual
Water Quality Flier
Water
Qualitv
Information
for the
Public
I
{#'$Y## {'&{f*,##W #,W#M'{#
IRB approval
box
sranrsA
F
p
R
S
VA
L
rxprnrs
JUN 2 i
;n;;c
JU"i\i
Z
0
Z00g
-
-
I
[tTi+i,
l,$'*it'
#?lE
fi
'
rgi'f;A
* u
The Chicaso
River and Calumet
River svstem
Tlrese river systems
are
managed by the Metropolitan
Water Reclamation
District of Greater
Chicago.
Swimming,
jet
skiing
and
water
skiing
not allowed in these
waterways,
but
activities
such
as boatirrg,
fishiug, and
rowiug are allowed. For
more inforrnatiott about the
waterways.
visit:[1p1rryWy.$_!-r._LSi]gaA!:qa\$lC1L4y1qg
For
more infbrrnation
about the Metropolitan
Water
Reclamatiou District,
visit;
It1p...-,1ltUr.
._Ln
s,rd gc:.tlst. i I.
usi
Lakes
and Beaches
For
more intbrnratiorr
about beach
health arrd beaclr
closirrgs
irr
Chicago.
please
visit the
rvebsite
of the Chicago
Park District Beach
Report
http:i,/c!ricasep41:[di.lllig'1.C"e]ff/iftdel.cfnl
rseactiorti
inclefC.ltU/-fqWaglj-Q.-niir-v-ig*-1q-p-Q$jlgn-Q
For
more
information about beach
health,
please
visit to
website
of
the Illinois Department
of
Public
Health Beach
Information
Irttl,rr.uruujdurlo!.
For
rnore irrforr.natiou
about
water quality on
Lake Michigan,
visit
the
website
of the
A I I i a n c e
fo r t
h
e G
reat [,ake
s :
!i!-h;lly'
w
W.glg-?i-tl
iik"e-,:.,Ql.gi.
[atine
fish
causht
in Illinois
rivers and
lakes
General
information,
2007 :
!-rup:ll-r,lw-rr
jslr-dstilir-1s.p-sljaskrqrl$d&$A!vrisvZ"elQ0?.Q2.-0j+:-df
Great
lakes tlsh:
ltllpt'iu:yl
l
The Chicago
River system:
!rtm:/lw--r.y:r.idpli.,ltalqi.-d,U,llstt-\hqallhlfdtitdyr,c-lt!9r1g-111.!1'9r,,rr
QAPP 2
Appendix 2
NEEAR Study Beach Survey
PRIMARY RESPONDENT’S LAST NAME_________________________________________
Study for Beaches Program
2007
Beach Interview
Part A
1/26/2007
Interview status (circle one)
Complete (Part A & B)
Refused
Ineligible
Incomplete
Leaving Late (after 6pm)
Language (circle if in Spanish)
Given in Spanish
Gift received (circle one):
COOLER
TOTE BAG
U.S. Environmental Protection Agency
Centers For Disease Control and Prevention
Research Triangle Park, North Carolina 27711
1600 Clifton Rd
Atlanta, GA 30333
Westat, Inc.
1650 Research Boulevard
Rockville, Maryland 20850-3195
OMB: 2080.0068
Part A data verified (sign) ______________
Expires: 09/30/2008
Part B data verified (sign) ______________
Questionnaire #: ___ ___ ___
Version 1, 1/26/2007
2
BEACH INTERVIEW
Interviewer Name (Part A): ...............................
...................................................
Last
First
Site ID: ........................
Saturday: ______ Sunday: ______ Monday: _______ Friday: ________
Date: ____ / ____ /____
Time: ...........................AM PM (circle one)
X1. Latitude: _________
X2.
Longitude: __________
X3.
Transect Area: ________
Hi, my name is___________________________. I am conducting the
National Beaches Survey
.
Q1.
Have you been interviewed by the National Beaches Survey in the last 28 (4weeks) days?
YES......................................................... 1
Æ
TERMINATE INTERVIEW
NO...........................................................
2
Q1a.
INTERVIEWER BY OBSERVATION: IS THE RESPONDENT 18 OR OLDER?
YES.........................................................
1
NO...........................................................
2
Here is a pamphlet describing the study.
(GIVE RESPONDENT THE CONSENT TRI-FOLD PAMPHLET)
INTERVIEWER:
The federal government is conducting a nationwide research study on the health of swimmers at
marine and Great Lakes beaches. The Environmental Protection Agency (EPA) and the Centers for Disease
Control and Prevention (CDC) are the two agencies that are supporting the study. The study involves a two-part
questionnaire that you complete today and a telephone interview 10-12 days from now. Your participation today
will greatly assist the government in developing better guidelines for safe beach water quality and may improve
beachgoer’s health. Your participation is voluntary and you may stop the interview at any time. There are no risks
involved from participating in this survey. It will take about 10 to 15 minutes of your time for these beach questions
and about the same time for an exit interview at the bath house. At the bathhouse we have a gift for you, when you
leave to go home. After completing the beach and telephone interview, you will also receive a check for the total of
$25. Your responses to the questions will be confidential and your address and other contact information will be
destroyed after you complete the telephone interview and we send you your
Thank You
check.
Q2.
May we continue?
YES....................................................... 1
Æ
START INTERVIEW
NO
2
Æ
REFUSAL, GO TO 5.a.
INTERVIEWER
: Collect GPS coordinates for participants and nonparticipants.
Version 1, 1/26/2007
3
Q2a.
Our survey is primarily for households of one or more persons that live together at the same
address. Do you all live at the same address?
(INTERVIEWER PROBE TO IDENTIFY
HOUSEHOLD. IF MORE THAN ONE HOUSEHOLD WITH A DIFFERENT ADDRESS,
INTERVIEW EACH SEPARATELY.)
YES....................................................... 1
NO......................................................... 2
Q3.
How many members in your household are at the beach today including yourself?
__________ MEMBERS
For the rest of this interview, I will be asking questions mostly about those people in your household who
are here today at the beach.
Q4.
What time did you and your household arrive at the beach today?
(CIRCLE AM OR PM)
___________ AM PM
Q5.
We are interested in asking about the health of your household during the few weeks following your beach
visit. Could you please give me your telephone number so we can get in touch with you in 10-12 days from
now?
YES......................................................... 1
Æ
GO TO Q5B
NO........................................................... 2
Æ
GO TO Q5A
Q5a.
IF “NO,”
Is it for one of the following reasons?
Too busy .................................................
1
No longer interested ...............................
2
Will not be available................................
3
Other reason?.........................................
4
Please specify: .......................................
INTERVIEWER:
Collect GPS Coordinates all persons on beach regardless of participation.
INTERVIEWER:
We will end the interview here since a contact telephone number is required to
complete the telephone interview that I mentioned. Thank you for speaking with us.
Q5b.
10-12 days from now which phone number(s) should we call?
___________________________________________
Q5c.
Is this your home, vacation, or cell phone number?
Home Phone...........................................
1
Vacation Phone.......................................
2
Cell Phone ..............................................
3
Specify other...........................................
4
Version 1, 1/26/2007
4
Q6.
What are the best times to reach you during week days?
(INTERVIEWER: MARK WITH AN “X” ALL DAYS THAT APPLY AND APPROXIMATE TIME SPAN.)
Mon.
Tues.
Wed.
Thurs.
Fri.
Morning
8:00 AM-12:00 PM
(Noon)
Afternoon
12:01 PM-5:00 PM
Evening
5:00 PM-9:00 PM
INTERVIEWER:
We’ll try to reach you during that (those) time(s).
Q6a.
Can I please have your mailing address so that we can send you your $25
Thank You
check?
First Name: ____________________ ....
Last Name ______________________
Address: ......................................................................................................................
City: ..........................................
State: ....................
Zip: ............................
INTERVIEWER:
We will destroy your identifying information after we mail the check.
Q7.
Please tell me the first name of the members of your household at the beach today, their birth dates,
gender, race, ethnicity, and whether they are in diapers.
[INTERVIEWER: IF 8 OR MORE PEOPLE, USE SUPPLEMENTAL QUESTIONNAIRES.]
First Name
Date of Birth
mm/dd/yyyy
Gender
Hispanic
Race*
In Diapers
You
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 2
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 3
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 4
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 5
__ /___ / ____ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 6
___ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 7
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
Person 8
__ /___ / ___ RF DK
M F RF DK
Y N RF DK
Y N RF DK
*Race:
1. White
5. Native Hawaiian or Other Pacific Islander
2. Black or African American
6. Other
3. Asian
7. Refused
4. American Indian or Alaska Native
8. Don’t Know
Version 1, 1/26/2007
5
Q8.
Will (you/all these people at the beach with you today) be living (with you) at the same address(es) during
the next two weeks?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
Q9.
Have any of these household members at the beach today been ill in the past 3 days with . . .
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF
DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. Diarrhea or loose
bowels? ..................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7
8
1 2 7
8
1 2 7
8
1 2 7 8
1 2 7
8
b. Urinary tract
infection
or
1 2 7
8
1 2 7 8
1 2 7 8
1 2 7
8
1 2 7
8
1 2 7
8
1 2 7 8
1 2 7
8
c. Throwing up or
vomiting
? ................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7
8
1 2 7
8
1 2 7
8
1 2 7 8
1 2 7
8
d. Sore throat or
cough?....................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
e. Earache, ear
infection
or runny
1 2 7
8
1 2 7 8
1 2 7 8
1 2 7
8
1 2 7
8
1 2 7
8
1 2 7 8
1 2 7
8
f. Eye infection?............ 1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
g. Rash or itchy skin?.... 1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
h. Sunburn?................... 1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q10.
Are there any household members
not
present at the beach today?
YES......................................................... 1
Æ
GO TO Q10a
NO........................................................... 2
Æ
GO TO Q11
REFUSED............................................... 7
Æ
GO TO Q11
DON’T KNOW......................................... 8
Æ
GO TO Q11
Q10a. Have any household members
not
present at the beach today been ill in the past 3 days
with . . .
Y N RF DK
a. Diarrhea or loose bowels?.....................
1 2 7 8
b. Urinary tract infection or burning
sensation when urinating? ..................
1 2 7 8
c. Throwing up or vomiting? ......................
1 2 7 8
d. Sore throat or cough?............................
1 2 7 8
e. Earache, ear infection or runny ears? ...
1 2 7 8
f.
Eye infection?........................................
1 2 7 8
g. Rash or itchy skin? ................................
1 2 7 8
Version 1, 1/26/2007
6
Q11.
Do you or any household members at the beach today, not including any one that stayed at home, suffer
from any of the following chronic, long-term conditions?
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. Gastrointestinal
problems such as
Crohn’s disease
or irritable bowel
syndrome .......
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
b. Chronic respiratory
diseases such as
asthma or
emphysema
....
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c. Allergies, other
than drug
allergies
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
d. Skin problems
such as psoriasis
or eczema
......
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q12.
How many times do you usually come to this beach each year?
................................................... TIMES
REFUSED...............................................
7
DON’T KNOW.........................................
8
Q13.
How many miles did you travel to the beach today?
__________________________ MILES
REFUSED...............................................
7
DON’T KNOW.........................................
8
Q14.
During the past two weeks, did you (anyone in your household at the beach today) go bathing or swimming
anywhere - at this or some other beach, pool or lake?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
IF NO TO PERSON 1 THROUGH PERSON 8, GO 14c
Version 1, 1/26/2007
7
Q14a. Did you {PERSON} go bathing or swimming anywhere in the past one week (Monday through
Friday) at this or some other beach, pool or lake ?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
Q14b. Did you {PERSON} actually get their head or face wet?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
Q14c. During the past 2 weeks, did you (person) get a sunburn that lasted more than 12 hours?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
ENTER HERE IF NO TO Q14
INTERVIEWER: DID YOU RECORD THE
LONGITUDE AND LATITUDE COORDINATES ON PAGE 1?
THE FOLLOWING IS ENTERED BY THE INTERVIEWER:
X4.
HOW COOPERATIVE WAS THIS HOUSEHOLD?
Very.........................................................
1
Somewhat ...............................................
2
Not at all..................................................
3
X5.
WAS INTERVIEW CONDUCTED IN SPANISH?
YES.........................................................
1
NO...........................................................
2
INTERVIEWER: END OF PART A. PLEASE RETURN QUESTIONNAIRE TO THE BATH HOUSE.
Version 1, 1/26/2007
8
End A
:
Please go to the exit stations when you leave the beach today, before 5:30
PM, to complete Part B of the Beach Interview and to pick-up your beach
gift. Thank you for participating in this portion of the survey.
Version 1, 1/26/2007
9
Interviewer Part A: __________________
Date: ___ / ___ / ____
Questionnaire #: ___ ___ ___
Interviewer Part B
: ____________________
Part B
Beach Survey
Thank you for returning to complete the beach interview. We will have your gift ready for you after we complete the
interview. Some of the questions will be repetitive to ensure accuracy. May we have your name to link it to your
previous answers?
Q15.
Were you the person that we interviewed on the beach or earlier today?
YES.........................................................
1
NO...........................................................
2
Q15a. INTERVIEWER: IF NO, CHECK Q7 TO DETERMINE THE PERSON YOU ARE INTERVIEWING.
Person ___________________________
Q16.
Did you or anyone in your household wade, swim, or play in the water today?
Name Droplist
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
IF NO TO PERSON 1 THROUGH PERSON 8, GO TO Q17
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Q16a.1. Did {PERSON}
immerse their body, not
necessarily {PERSON’s}
head, in water today?.......
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q16a.2. Did {PERSON}
put their face in water or
submerge head in water
today? ..............................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q16a.3. Did {Person} get
water in the mouth today?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
IF NO TO PERSON 1 THROUGH PERSON 8 FOR THE ABOVE QUESTION, Q16a.3, GO TO Q16b.
Version 1, 1/26/2007
10
Q16.a. Did {PERSON} swallow the water?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
[PROGRAMMER NOTE: PUT IN POP UP ASSIST TO SHOW TIME OF QUESTION 4.]
Q16b. Was {PERSON} in the water at the following times today?
READ ONLY FOR TIME PERIODS
THEY WERE AT THE BEACH BASED ON THE ANSWER FROM Q4 ABOVE.
If “Yes,” what part
of the beach did {PERSON} swim in?
INDICATE AREA ON THE MAP
.
[PROGRAMMER NOTE: PREASSIGNED AREA DESIGNATIONS FOR EACH BEACH.]
Person
Times
1
2
3
4
5
6
7
8
Y N
Y N
Y N
Y N
Y N
Y N
Y N
Y N
Before 10:00 am
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
10:00 AM -11:59 AM
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
12:00 PM - 1:59 PM
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
2:00 PM - 3:59 PM
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
4:00 PM - 5:30 PM
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
Area
RF DK
RF DK
RF DK
RF DK
RF DK
RF DK
RF DK
RF DK
INTERVIEWER: MARK AREA WHERE PERSON SWAM MOST OF THE TIME.
Q16c. What total time did {PERSON} stay in the water? We are only interested in time actually in the
water, not the total time at the beach.
(CIRCLE TIME UNITS)
RF
DK
Person 1 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 2 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 3 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 4 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 5 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 6 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 7 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 8 ................ ____ MINUTES
_____.__ HOURS 7
8
Version 1, 1/26/2007
11
Q16d. Did {PERSON} engage in any of the following water-related activities while at the beach today?
(Circle all that apply)
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Swimming
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Swimming laps
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Surfing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Body Surfing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Kite Surfing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Wind Surfing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Sailing (Hobie cat,
Sunfish, etc)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Paddle Boating
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Canoeing or
Kayaking
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Rafting
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Floating on an air
mattress
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Snorkeling
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Scuba Diving
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Jet Skiing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Water Skiing
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Placemarker for sport
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Placemarker for sport
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Did {person} have
contact with water in
a non-circulating pool
or tidal pool?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
INTERVIEWER:
We would like to ask a few questions about things that may help protect swimmers from becoming
ill.
Q17.
What would {PERSON} estimate (your/his/her) total time in direct sunlight was? This does not include
being indoors or under umbrellas, etc.
(CIRCLE TIME UNITS)
Explain to participant that cloudy day is not
considered
RF
DK
Person 1 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 2 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 3 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 4 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 5 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 6 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 7 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 8 ................ ____ MINUTES
_____.__ HOURS 7
8
Version 1, 1/26/2007
12
Q18.
Did {PERSON} engage in any of the following activities while at the beach today?
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a.
collecting sea shells,
rocks, feathers, etc? .....
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
b.
digging in sand or
building sand castles.
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c.
had their body buried
in sand............................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c.1.
Did {person} get
any sand in their
mouth?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
IF YES TO b or c
c.1.a.
After digging in
the sand, or building
sand castles…did
{person} wash their
hands before eating?
(washing of hands may
include the use of
personal water-free
hand sanitizer.)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
d.
playing with algae or
seaweed ....................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
d.1
. Did {person} get
any seaweed in their
mouth?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
IF YES TO d
d.1.a
. After playing with
algae…did {person}
wash their hands before
eating? (washing of
hands may include the
use of personal water-
free hand sanitizer.)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
18.e
Was the sand you dug in or played with dry or wet? (interviewer to read the responses)
1) All dry
2) Mostly dry, some wet
3) Mostly wet, some dry
4) All wet
Version 1, 1/26/2007
13
Q19.
Did {PERSON} cut themselves today or have an open cut when they came to the beach today?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
Q20.
Did {PERSON} wear sunscreen/sunblock today?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
If No, Q23, Otherwise GOTO Q21
Q21.
What was the SPF rating of the sunscreen/sunblock you used most often today?
Enter SPF Level ______________
Q21a. When you used sunscreen/sunblock today, how did you apply it?
Only to certain areas of my body (for example, head and shoulders)
All exposed skin
Q22.
Did you reapply at least once today?
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. Did you
Once?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q23.
Did {PERSON} wear a hat today?
YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
Version 1, 1/26/2007
14
Q23.
If yes, Did the hat have a wide brim or another way to shade face, ears, and back of the neck from the sun?
.............................................................................................................. YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
Q23a.1
Did {PERSON} use protective equipment such as a canopy, umbrella or other type of sunshade today?
................................................................................................
YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 .................................................................................
1
2
7
8
Q23b. Did {PERSON} wear protective clothing, such as a long-sleeved shirt or cover-up?
YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
Version 1, 1/26/2007
15
Q24.
During the summer, if you/(PERSON) go(es) out in the sun repeatedly without sunscreen or protective clothing which
one of these things most usually happens to your/his/her/skin? READ RESPONSES SLOWLY (Choose only one):
First Name
Code
You
Person 2
Person 3
Person 4
Person 5
Person 6
Person 7
Person 8
01 A dark tan
02 Some tanning
03 No tan, maybe some freckles
04 Repeated sunburns
05 OTHER (Specify)
96 NEVER GO OUT IN THE SUN
97 REFUSE
98 DON’T KNOW
Q25.
Did {PERSON} wear insect repellant today?
YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
Q26.
Did you or any member of your household consume food while at the beach today?
YES
NO RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
Version 1, 1/26/2007
16
IF NO, GO to Q27
Was the food . . .
(Circle all that apply)
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. brought from home?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
b. purchased from
vending machines or a
vendor at the beach?
..........
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c. purchased from a
vendor outside the
beach?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q27.
Did you or any member of your household consume any drinks while at the beach today?
YES
NO
RF
DK
Person 1 ........................................................ 1...................2 .................. 7 .................... 8
Person 2 ........................................................ 1...................2 .................. 7 .................... 8
Person 3 ........................................................ 1...................2 .................. 7 .................... 8
Person 4 ........................................................ 1...................2 .................. 7 .................... 8
Person 5 ........................................................ 1...................2 .................. 7 .................... 8
Person 6 ........................................................ 1...................2 .................. 7 .................... 8
Person 7 ........................................................ 1...................2 .................. 7 .................... 8
Person 8 ........................................................ 1...................2 .................. 7 .................... 8
IF NO, GO to Q28
Was the drink . . .
(Circle all that apply)
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. brought from home?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
b. purchased from
vending machines or a
vendor at the beach?
..........
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c. purchased from a
vendor outside the
beach? (Ex. Restaurant,
deli)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Version 1, 1/26/2007
17
Q28.
In the last 48 hours has anyone done the following . . .
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
a. Have you come in
contact with any
animals?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
b. Come into contact
with someone who has
complained of diarrhea,
vomiting, or stomach
illness?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
c. Consumed raw
shell fish?
(Crab, oyster, mussel)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
d. Consumed
rare/raw/undercooked
or meat that is pink in
center?
(Fish, beef, chicken)
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
e. Consumed runny or
raw eggs?
...........................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Thank you for your assistance on this national survey of marine and Great Lake beaches. You can contact us
regarding information about the study at the toll-free number or e-mail address in the pamphlet. We will phone you
in the next 10-12 days with some brief questions about your health. After completing the telephone interview, your
household will receive a $25 check within 30 days of completing the telephone interview.
Gift received (circle one):
COOLER
TOTE BAG
Version 1, 1/26/2007
18
QAPP 2
Appendix 3
NEEAR Telephone Interview
NATIONAL EPIDEMIOLOGICAL AND ENVIRONMENTAL ASSESSMENT OF RECREATIONAL
(NEEAR) WATER STUDY:
BEACHES SURVEY
1/30/2007
INTRO 1
Hello, my name is ____________. May I speak to _______________?
{IF NEEDED: I am calling about a study for the Environmental Protection Agency (EPA). Recently, we
spoke to [BEACH RESPONDENT] at {BEACH}, and we’re calling back to complete the interview.}
1.
AVAILABLE – GO TO INTRO 4
2.
NOT AVAILABLE – GO TO INTRO 2
3.
NEVER HEARD OF PERSON – GO TO INTRO 3
4.
REFUSED – GO TO INTRO 2
INTRO 2
[My name is ____________and] I am calling about a follow-up study for the Environmental Protection
Agency (EPA). Recently, we spoke to (BEACH RESPONDENT) at {BEACH}, and we’re calling to
complete the interview. If s/he isn’t available, I can talk to someone else. Are you a household member
18 years of age or older?
1.
YES -- GO TO AINTRO
2.
NO – May I speak to an adult 18 years of age or older?
3.
REFUSED – COMPLETE NIRF
INTRO 3
Is this number (___) ___ ____ (Verify the number on the call record sheet)?
1.
YES – COMPLETE NIRF
2.
NO -- REDIAL THE NUMBER
INTRO 4
[Hello, my name is _____________, and] I’m calling about a study for the Environmental Protection
Agency (EPA). Recently, we spoke to you at {BEACH} {IF NEEDED: Indiana National Dunes Park
(National Lakeshore)}; and we’re calling back to complete the interview.
1.
CONTINUE
2.
SCHEDULE APPOINTMENT
3.
REFUSED – COMPLETE NIRF
IF ASKED TO CALL BACK OR SCHEDULE APPOINTMENT, SAY: The interview needs to be
completed by [LAST DATE]. When would be the best time to call you before then?
Aintro.
[This is ________________ with the NEEAR Water Study calling.] I’m going to ask some
questions about any swimming or bathing during your initial beach visit or other visits you may have had
in the last 10-12 days and about illnesses that have been experienced in the last week. I will be asking
about the following members of your household …
[READ NAMES OF HH MEMBERS AT THE BEACH.]
{display list of persons showing first name/age/sex.}
[IS RESPONDENT SAME PERSON INTERVIEWED AT THE BEACH?]
YES
1 (
A1
)
NO
2 (
A1a
)
A1. RespName
[May I have just your first name, please?]
_________________
During Beach Visit where you enrolled in this study on {date}
A2
.
(For the persons that were swimmers in the beach interview) Did {PERSON} wear ear plugs while in the
water?
Yes
No
RF
DK
Person 1
1
2
7
8
Person 2
1
2
7
8
Person 3
1
2
7
8
Person 4
1
2
7
8
Person 5
1
2
7
8
Person 6
1
2
7
8
Person 7
1
2
7
8
Person 8
1
2
7
8
A3.
Did {PERSON} wear nose plugs while in the water?
Yes
No RF
DK
Person 1
1
2 7
8
Person 2
1
2 7
8
Person 3
1
2 7
8
Person 4
1
2 7
8
Person 5
1
2 7
8
Person 6
1
2 7
8
Person 7
1
2 7
8
Person 8
1
2 7
8
A4.
Did {PERSON} wear eye goggles or use a face mask while in the water?
Yes
No
RF
DK
Person 1
1
2
7
8
Person 2
1
2
7
8
Person 3
1
2
7
8
Person 4
1
2
7
8
Person 5
1
2
7
8
Person 6
1
2
7
8
Person 7
1
2
7
8
Person 8
1
2
7
8
A5.
During the beach interview, {PERSON} had contact with an animal?
Person 1
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 2
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 3
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 4
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 5
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 6
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 7
Drop down list
Were they unfamiliar to you? Yes No RF DK
Person 8
Drop down list
Were they unfamiliar to you? Yes No RF DK
NEXT QUESTION FOR FEMALE PARTICIPANTS ONLY
A6. Between your beach visit on XXXX date and today were you menstruating (or some other word) or
pregnant?
1
2
3
4
5
6
7
8
Y N
Y N
Y N
Y N
Y N
Y N
Y N
Y N
1 2
1 2
1 2
1 2
1 2
1 2
1 2
1 2
We are now going to switch and ask you questions about activities that have occurred since the
Beach Interview.
B1. AnyoneSwim
................................................................
Have you or any of the people I just mentioned gone bathing or swimming
anywhere since we
talked to [you/ORIGINAL RESPONDENT] at the beach interview on {BEACH INTERVIEW
DATE}? Please include
any bathing or swimming such as at a beach, waterpark, public pool,
private pool, or wading pool.
YES
1 (
B2
)
NO
2 (SymtomIntro)
REFUSED
7 (SymtomIntro)
DON’T KNOW
8 (SymtomIntro)
B2. WhoSwam
Who was it that went bathing or swimming? _______________
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
ASK B3
THROUGH B5 ABOUT EACH PERSON WHO WAS MARKED IN B2. BEGIN WITH THE FIRST
PERSON IN THE LIST WHO WAS MARKED AND CONTINUE WITH ALL OTHER MARKED
PERSONS.
B3a. SameBeach
Did [PERSON] go bathing or swimming at {BEACH} since the beach interview on {BEACH
INTERVIEW DATE}?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B3b. DifferentBeach
Did [PERSON] go bathing or swimming at any other beach?
YES
1 (B3c)
NO
2 (B3d)
REFUSED
7 (B3d)
DON’T KNOW
8 (B3d)
B3c. BeachType
Was this beach at a:
Lake
1
River, or
2
Ocean?
3
OTHER, SPECIFY
6
REFUSED
7
DON’T KNOW
8
B3d. Waterpark
Did [PERSON] go bathing or swimming at a waterpark?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B3e. PublicPool
[Did {PERSON} go bathing or swimming …] at a public pool?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B3f. PrivatePool
[Did {PERSON} go bathing or swimming …] at a private pool?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B3g. WadingPool
[Did {PERSON} go bathing or swimming …] in a wading pool?
[NOTE TO INT: THIS COULD BE A BACKYARD INFLATED POOL.]
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B3h. AnyOtherSwimming
[Did {PERSON} go bathing or swimming …] any other place?
YES
1 (B3i)
NO
2 (B4)
REFUSED
7 (B4)
DON’T KNOW
8 (B4)
CATI EDIT CHECK: IF R ANSWERED YES TO B3a, BUT NO TO B3b THROUGH B3h, ASK, “You’ve
said that (PERSON) went bathing or swimming sometime since (BEACH INTERVIEW DATE). Where
did (you/s/he) go swimming?” CODA B3b-B3h
B3i. OtherSwimLocation
[SPECIFY:] _______________________________
B4. GetFaceWet
Did [you/{PERSON}] actually get [your/{his/her}] face wet while bathing or swimming?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B5. DaysSwam
On which days did [you/{PERSON}] go bathing or swimming? [since {BEACH INTERVIEW
DATE}]
____________________
[CODE ALL THAT APPLY]
INTERVIEWER WILL REFER TO HARD-COPY CALENDAR FOR ALL DATES.
PROG. NOTE: Do not allow a date prior to the BEACH INTERVIEW DATE. Do not allow a date later
than today.
SymptomIntro
I’m going to go through a list of symptoms. Please tell me if anyone has had any of these
symptoms since the interview at {BEACH} on {BEACH INTERVIEW DATE}. Again, the people I
am asking about are…
[
Questions
B6 to B23 are asked about all household members who were at the beach on the
BEACH INTERVIEW DATE.]
B6. AnyStomachAche
Have you or anyone else had a stomachache or abdominal cramping since the interview at
{BEACH} on {BEACH INTERVIEW DATE}?
YES
1 (B6a)
NO
2 (B7)
REFUSED
7 (B7)
DON’T KNOW
8 (B7)
B6a. StomachList
Who? [had a stomachache or abdominal cramps since the interview at {BEACH} on {BEACH
INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons (first name/age/sex).}
REFUSED
7
DON’T KNOW
8
B7. AnyDiarrhea
Has anyone had diarrhea or loose bowels since the interview at {BEACH} on {BEACH INTERVIEW
DATE}? By diarrhea we mean, three or more loose or watery stools in a 24-hour period.
YES
1 (B7a)
NO
2 (B8)
REFUSED
7 (B8)
DON’T KNOW
8 (B8)
B7a. DiarrheaList
Who [had diarrhea or loose bowels since the interview at {BEACH} on {BEACH INTERVIEW
DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons (PERSON).}
REFUSED
7
DON’T KNOW
8
B8. AnyNausea
Has anyone had nausea since the interview at {BEACH} on {BEACH INTERVIEW DATE}?
YES
1 (B8a)
NO
2 (B9)
REFUSED
7 (B9)
DON’T KNOW
8 (B9)
B8a. NauseaList
Who? [had nausea since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons (first name/age/sex).}
REFUSED
7
DON’T KNOW
8
B9. AnyVomiting
Has anyone had throwing up or vomiting? [since the interview at {BEACH} on {BEACH INTERVIEW
DATE}?]
YES
1 (B9a)
NO
2 (B10)
REFUSED
7 (B10)
DON’T KNOW
8 (B10)
B9a. VomitingList
Who had throwing up or vomiting [since the interview at {BEACH} on {BEACH INTERVIEW
DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons (first name/age/sex).}
REFUSED
7
DON’T KNOW
8
B10. AnyUTI
Has anyone had a urinary tract infection or burning sensation when urinating since the interview at
{BEACH} on {BEACH INTERVIEW DATE}?
YES
1 (B10a)
NO
2 (B11)
REFUSED
7 (B11)
DON’T KNOW
8 (B11)
B10a. UTIList
Who? [had a urinary tract infection or burning sensation when urinating since the interview at
{BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons (first name/age/sex).}
REFUSED
7
DON’T KNOW
8
B11. AnyFever
[Has anyone had …] a fever? [since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
YES
1 (B11a)
NO
2 (B12)
REFUSED
7 (B12)
DON’T KNOW
8 (B12)
B11a. FeverList
Who? [had a fever since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B12. AnyHeadache
[Has anyone had …] a headache lasting more than a few hours? [since the interview at {BEACH}
on {BEACH INTERVIEW DATE}?]
YES
1 (B12a)
NO
2 (B13)
REFUSED
7 (B13)
DON’T KNOW
8 (B13)
B12a. HeadacheList
Who? [had a headache since the interview at {BEACH} on {beach interview
date}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B13. AnySoreThroat
[Has anyone had …] a sore throat? [since the interview at {BEACH} on {beach interview date}?]
YES
1 (B13a)
NO
2 (B14)
REFUSED
7 (B14)
DON’T KNOW
8 (B14)
B13a. SoreThroatList
Who? [had a sore throat since the interview at {BEACH} on {beach interview date}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B14. AnyCough
[Has anyone had ] a bad cough? [since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
YES
1 (B14a)
NO
2 (B15)
REFUSED
7 (B15)
DON’T KNOW
8 (B15)
B14a. CoughList
Who? [had a cough since the interview at {BEACH} on {beach interview date}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B15. AnyCold
[Has anyone had …] a cold? [since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
YES
1 (B15a)
NO
2 (B16)
REFUSED
7 (B16)
DON’T KNOW
8 (B16)
B15a. ColdList
Who? [had a cold since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B16. AnyRunnyNose
[Has anyone had…] a runny or stuffy nose? [since the interview at {BEACH} on {BEACH
INTERVIEW DATE}?]
YES
1 (B16a)
NO
2 (B17)
REFUSED
7 (B17)
DON’T KNOW
8 (B17)
B16a. RunnyNoseList
Who? [had a runny nose since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B17. AnyEarache
[Has anyone had …] an earache, ear infection, or runny ears? [since the interview at {BEACH} on
{BEACH INTERVIEW DATE}?]
YES
1 (B17a)
NO
2 (B18)
REFUSED
7 (B18)
DON’T KNOW
8 (B18)
B17a. EaracheList
[Who? [ had an earache since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B18. AnyWateryEyes
[Has anyone had …] watery eyes? [since the interview at {BEACH} on {BEACH INTERVIEW
DATE}?]
YES
1 (B18a)
NO
2 (B19)
REFUSED
7 (B19)
DON’T KNOW
8 (B19)
B18a. WateryEyesList
Who? [ had watery eyes since the interview at {BEACH} on {beach interview
date}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B19. AnyEyeInfection
[Has anyone had …] an eye infection? [since the interview at {BEACH} on {BEACH INTERVIEW
DATE}?]
YES
1 (B19a)
NO
2 (B20)
REFUSED
7 (B20)
DON’T KNOW
8 (B20)
B19a. EyeList
Who? [ had any eye infection since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B20. AnyCuts
(We need to know if the cut became infected after the visit to the beach; When did the cut occur,
it could have occurred after they were at the beach. Check to make sure the information is
conveyed correctly from Beach Interview) [Has anyone had …] an infected cut? [since the
interview at {BEACH} on {BEACH INTERVIEW DATE}?]
YES
1 (B20a)
NO
2 (B21)
REFUSED
7 (B21)
DON’T KNOW
8 (B21)
B20a. CutList
Who? [Had an infected cut since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B21. AnyRash
[Has anyone had …] a rash or itchy skin? [since the interview at {BEACH} on {BEACH
INTERVIEW DATE}?]
YES
1 (B21a)
NO
2 (B22)
REFUSED
7 (B22)
DON’T KNOW
8 (B22)
B21a. RashList
[Who? [Had a rash since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B22. AnySunburn
[Has anyone had …] a sunburn? [since the interview at {BEACH} on {beach interview date}?]
YES
1 (B22a)
NO
2 (B23)
REFUSED
7 (B23)
DON’T KNOW
8 (B23)
B22a. AnySunburnList
Who? [ had sunburn since the interview at {BEACH} on {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B23. ActivitiesIntro
I’d like to ask you about some activities people may have done since the day of the beach interview on
(BEACH INTERVIEW DATE).
B23a. AnyContactAni
[Since the day of the beach interview] Has anyone come into contact with any animals?
YES
1 (B23b)
NO
2 (B24)
REFUSED
7 (B24)
DON’T KNOW
8 (B24)
B23b. AnimalContactList
Who? [ came into contact with animals since {BEACH INTERVIEW DATE}?]
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B23c. ASK FOR EACH PERSON IN A23a:
(Was this animal/Were any of these animals) unfamiliar to
(you/him/her)?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
B23d. What kind of animal or animals were they?
DROP-DOWN MENU WILL ALLOW FOR CHECKING ALL THAT APPLY
1. FISH (AQUARIUM)
2. CATS
3. DOGS
4. POULTRY (CHICKENS, TURKEYS)
5. HORSES
6. COWS
7. SHEEP
8. GOATS
9. AMPHIBIANS (FROGS, SALAMANDERS)
10. REPTILES (SNAKES, TURTLES, LIZARDS)
11. BIRDS (PETS)
12. OTHER, SPECIFY: ________________________
B24a. (Since the day of the beach interview) Has anyone come into contact with someone who
has complained of diarrhea, vomiting, or stomach illness?
YES
1 (B24b)
NO
2 (B25)
REFUSED
7 (B25)
DON’T KNOW
8 (B25)
B24b. PeopleContactList
Who? [had contact with someone complaining of diarrhea, vomiting or stomach illness since
(BEACH INTERVIEW DATE)?
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B25a. (Since the day of the beach interview) Has anyone eaten raw shell fish, such as oysters,
clams, mussels, crabs?
YES
1 (B25b)
NO
2 (B26)
REFUSED
7 (B26)
DON’T KNOW
8 (B26)
B25b. ShellfishList
Who? [has eaten raw shellfish since (BEACH INTERVIEW DATE)?
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B26a.
(Since the day of the beach interview) Has anyone eaten rare or raw meat? This includes pink in
the center/
YES
1 (B26b)
NO
2 (B27)
REFUSED
7 (B27)
DON’T KNOW
8 (B27)
B26b. RawMeatList
Who? [has eaten rare (pink in center) or raw meat since (BEACH INTERVIEW DATE)?
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
B27a. (Since the day of the beach interview) Has anyone eaten runny or raw
eggs?
YES
1 (B27b)
NO
2 (C1INTRO)
REFUSED
7 (C1INTRO)
DON’T KNOW
8 (C1INTRO)
B27b. EggsList
Who? [has eaten runny or raw eggs since (BEACH INTERVIEW DATE)?
[CODE ALL THAT APPLY.]
{response options are the names of all persons}
REFUSED
7
DON’T KNOW
8
IF ANY PERSON WAS MARKED AS HAVING ANY SYMPTOM (B6-B22), ASK ALL PERTINENT
SYMPTOM QUESTIONS FOR THAT PERSON.
BEGIN WITH THE FIRST PERSON IN THE
ENUMERATION WHO WAS MARKED AS HAVING HAD A SYMPTOM, THEN CONTINUE WITH
EACH OTHER PERSON MARKED.
IF NO PERSON WAS MARKED AS HAVING ANY SYMPTOMS, GO TO QUESTION E1.
SECTION C.
C INTRO.
You said that {you/PERSON} experienced some symptoms since {BEACH INTERVIEW DATE}.
Now I would like to ask you about those symptoms.
IF THIS PERSON HAD A STOMACHACHE, GO TO C1 OR ELSE, GO TO C2.
C1. StomachStartDay
On what day did {name/age/sex}’s stomachache or abdominal cramping start?
DATE: ________________________________
REFUSED
7 (C2)
DON’T KNOW
8 (C2)
INTERVIEWER WILL REFER TO HARD-COPY CALENDAR FOR ALL DATES.
PROG. NOTE: Do not allow a date prior to the BEACH INTERVIEW DATE. Do not allow a date later
than today.
C1a. StomachStill
Does {name/age/sex} still have a stomachache or abdominal cramping?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
INTERVIEWER WILL REFER TO HARD-COPY CALENDAR TO NEGOTIATE DATES.
IF THIS PERSON STILL HAS A STOMACHACHE, GO TO C2.
C1b. StomachDays
For how many days did {name/age/sex}’s stomachache or abdominal cramping last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
NOTE: FOR SYMPTOMS LASTING A HALF DAY OR LESS, CODE “00”.
IF THIS PERSON HAD DIARRHEA, GO TO C2. OR ELSE, GO TO C3.
C2. DiarrheaStartDay
On what day did {name/age/sex}’s diarrhea or loose bowels start?
DATE: ________________________________
REFUSED
7 (C3)
DON’T KNOW
8 (C2a)
C2a. DiarrheaStill
Does {name/age/sex} still have diarrhea?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS DIARRHEA, GO TO C2c
C2b. DiarrheaDays
For how many days did {name/age/sex}’s diarrhea last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C2c. DiarrheaNumber
What was the maximum number of bouts or episodes of diarrhea {name/age/sex} experienced in
a 24-hour period?
[NUMBER PER DAY:] |__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD NAUSEA, GO TO C3. OR ELSE, GO TO C4.
C3. NauseaStartDay
On what day did {name/age/sex}’s nausea start?
DATE: ________________________________
REFUSED
7 (C4)
DON’T KNOW
8 (C4)
C3a. NauseaStill
Does {name/age/sex} still have nausea?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS NAUSEA, GO TO C4.
C3b. NauseaDays
For how many days did {name/age/sex}’s nausea last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD VOMITING, GO TO C4. OR ELSE, GO TO C5.
C4. VomitingStartDay
On what day did {name/age/sex}’s throwing up or vomiting start?
DATE: ________________________________
REFUSED
7 (C5)
DON’T KNOW
8 (C5)
C4a. VomitingStill
Is {name/age/sex} still vomiting?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL IS VOMITING, GO TO C4c.
C4b. VomitingDays
For how many days did {name/age/sex}’s vomiting last?
[NUMBER] |__|__|__|
REFUSED
7
DON’T KNOW
8
C4c. VomitingNumber
What was the maximum number of times that {name/age/sex} vomited during a 24-hour period?
[NUMBER] |__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A UTI, GO TO C5. OR ELSE, GO TO C6.
C5. UrinaryTractInfectionStartDay
On what day did {name/age/sex}’s urinary tract infection or burning start?
DATE: ________________________________
REFUSED
7 (C6)
DON’T KNOW
8 (C6)
C5a. UrinaryTractInfectionStill
Does {name/age/sex} still have a urinary tract infection or burning sensation?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A UTI, GO TO C6.
C5b. UrinaryTractInfectionDays
For how many days did {name/age/sex}’s urinary tract infection or burning sensation?
|__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A FEVER, GO TO C6. OR ELSE, GO TO C7.
C6. FeverStartDay
On what day did {name/age/sex}’s fever start?
DATE: ________________________________
REFUSED
7 (C7)
DON’T KNOW
8 (C7)
C6a. FeverStill
Does {name/age/sex} still have a fever?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A FEVER, GO TO C6c.
C6b. FeverDays
For how many days did {name/age/sex}’s fever last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C6c. FeverTempTaken
Was {name/age/sex}’s temperature taken using a thermometer?
YES
1 (C6d)
NO
2 (C7)
REFUSED
7 (C7)
DON’T KNOW
8 (C7)
C6d. FeverTemp
What is the highest temperature that {name/age/sex} has had since {BEACH
INTERVIEW DATE}?
[TEMPERATURE:] __
___
Range: 98.6 to 106.9
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A HEADACHE, GO TO C7. OR ELSE, GO TO C8.
C7. HeadacheStartDay
On what day did {name/age/sex}’s headache start?
DATE: ________________________________
REFUSED
7 (C8)
DON’T KNOW
8 (C8)
C7a. HeadacheStill
Does {name/age/sex} still have a headache?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A HEADACHE, GO TO C8.
C7b. HeadacheDays
For how many days did {name/age/sex}’s headache last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A SORE THROAT, GO TO C8. OR ELSE, GO TO C9.
C8. SoreThroatStartDay
On what day did {name/age/sex}’s sore throat start?
DATE: ________________________________
REFUSED
7 (C9)
DON’T KNOW
8 (C9)
C8a. SoreThroatStill
Does {name/age/sex} still have a sore throat?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A SORE THROAT, GO TO C8c.
C8b. SoreThroatDays
For how many days did {name/age/sex}’s sore throat last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C8c. SoreThroatAllergy
Was this sore throat related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A BAD COUGH, GO TO C9. OR ELSE, GO TO C10.
C9. CoughStartDay
On what day did {name/age/sex}’s bad cough start?
DATE: ________________________________
REFUSED
7 (C10)
DON’T KNOW
8 (C10)
C9a. CoughStill
Does {name/age/sex} still have a bad cough?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A BAD COUGH, GO TO C9c.
C9b. CoughDays
For how many days did {name/age/sex}’s cough last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C9c. CoughAllergy
Was this bad cough related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A COLD, GO TO C10. OR ELSE, GO TO C11.
C10. ColdStartDay
On what day did {name/age/sex}’s cold start?
DATE: ________________________________
REFUSED
7 (C11)
DON’T KNOW
8 (C11)
C10a. ColdStill
Does {name/age/sex} still have a cold?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A COLD STILL, GO TO C10c.
C10b. ColdDays
For how many days did {name/age/sex}’s cold last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C10c. ColdAllergy
Was this cold related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A RUNNY OR STUFFY NOSE, GO TO C11, OR ELSE, GO TO C12.
C11. RunnyNoseStartDay
On what day did {name/age/sex}’s runny or stuffy nose start?
DATE: ________________________________
REFUSED
7 (C12)
DON’T KNOW
8 (C12)
C11a. RunnyNoseStill
Does {name/age/sex} still have a runny or stuffy nose?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS A RUNNY NOSE, GO TO C11c.
C11b. RunnyNoseDays
For how many days did {name/age/sex}’s runny or stuff nose last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C11c. RunnyNoseAllergy
Was this runny or stuffy nose related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD A EARACHE, GO TO C12. OR ELSE, GO TO C13.
C12. EaracheStartDay
On what day did {name/age/sex}’s earache, ear infection or runny ears start?
DATE: ________________________________
REFUSED
7 (C13)
DON’T KNOW
8 (C13)
C12a. EaracheStill
Does {name/age/sex} still have an earache, ear infection or runny ears?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS AN EARACHE, GO TO C12c.
C12b. EaracheDays
For how many days did {name/age/sex}’s earache, ear infection or runny ears last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C12c. EaracheAllergy
Was this earache, ear infection or runny ears related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD WATERY EYES, GO TO C13. OR ELSE, GO TO C14.
C13. WateryEyesStartDay
On what day did {name/age/sex}’s watery eyes start?
DATE: ________________________________
REFUSED
7 (C14)
DON’T KNOW
8 (C14)
C13a. WaterEyesStill
Does {name/age/sex} still have watery eyes?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS WATERY EYES, GO TO C13c.
C13b. WateryEyesDays
For how many days did {name/age/sex}’s watery eyes last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C13c. WateryEyesAllergy
Were the watery eyes related to allergies?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD AN EYE INFECTION, GO TO C14. OR ELSE, GO TO C15.
C14. EyeInfectionStartDay
On what day did {name/age/sex}’s eye infection start?
DATE: ________________________________
REFUSED
7 (C15)
DON’T KNOW
8 (C15)
C14a. EyeInfectionStill
Does {name/age/sex} still have the eye infection?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS AN EYE INFECTION, GO TO C15.
C14b. EyeInfectionDays
For how many days did {name/age/sex}’s eye infection last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
IF THIS PERSON HAD AN INFECTED CUT, GO TO C15. OR ELSE, GO TO C16.
(Make sure information about the cut date is being relayed)
C15. CutStartDay (Maybe CutInfectDay)
On what day did {name/age/sex}’s cut first get infected?
DATE: ________________________________
REFUSED
7 (C16)
DON’T KNOW
8 (C16)
C15a. CutStill
Does {name/age/sex} still have an infected cut?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
IF THIS PERSON STILL HAS AN INFECTED CUT, GO TO C15c.
C15b. CutDays
For how many days did {name/age/sex}’s infected cut last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C15c. Where were [you/PERSON#n] cut?
[Mark all that apply.]
Location
Check if positive
1. ankle
2. arms
3. armpits
4. back
4a. Was that the upper or lower back?
5. breast
6. buttocks
7. chest
8. ears
9. face
10. feet
10a. Was it on the soles or the top of the feet/foot?
11. genitalia
12. groin
13. hands
13a. Was it on the back of the hand, the palm, or the fingers?
14. legs
15. mouth
16. neck
17. scalp
18. stomach
19. throat
20. other
specify:
21. refused
IF THIS PERSON HAD RASH, GO TO C16. OR ELSE, GO TO C17.
C16. RashStartDay
On what day did {name/age/sex}’s rash, itchy skin, or skin infection start?
DATE: ________________________________
REFUSED
7 (C17)
DON’T KNOW
8 (C17)
C16a. RashStill
Does {name/age/sex} still have the rash, itchy skin, or skin infection?
YES
1
NO
2 (C17)
REFUSED
7 (C17)
DON’T KNOW
8 (C17)
IF THIS PERSON STILL HAS SKIN PROBLEM, GO TO C16c.
C16b. RashDays
For how many days did {name/age/sex}’s rash, itchy skin, or skin infection last?
|__|__|__|
REFUSED
7
DON’T KNOW
8
C16c. Where were/was your/{name/age/sex}’s rash?
[Mark all that apply.]
Location
Check if positive
1. ankle
2. arms
3. armpits
4. back
4a. Was that the upper or lower back?
5. breast
6. buttocks
7. chest
8. ears
9. face
10. feet
10a. Was that on the sole or the top of the foot?
11.
genitalia
12.
groin
13. hands
13a. Was that on the back of the hand, the fingers or the palm?
14. legs
15. mouth
16. neck
17. scalp
18. stomach
19. throat
20. other
specify:
21. refused
22. don’t know
IF THIS PERSON HAD SUNBURN, GO TO C17. OR ELSE, GO TO QUESTION D1.
(When did they get sunburned. Was the information relayed)
C17. Sunburn
On which parts of the body was {name/age/sex} sunburned?
[Mark all that apply.]
Location
Check if positive
1. Face or head
2. Neck or
shoulders
3. Back
5. Chest or
abdomen
6. Arms or hands
7. Legs or feet
8. Other
Specify:
9. Refuse
11. Don’t know
ASK D1 ONLY ONCE FOR EACH PERSON REPORTING SYMPTOMS (C1-C172). START WITH
PERSON #1.
D1.
When (your/PERSON’s) (illness/condition) began, (were you/was s/he)
working for pay either inside or outside the home? Please include jobs for which (you
were/s/he was) self-employed.
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
[PROG NOTE: Remaining Section D questions will be asked for all household members (1-12)
reporting symptoms of the following syndromes:
Gastrointestinal/Diarrhea (symptoms – stomach ache, diarrhea, nausea, vomiting)
Eye Infection (symptoms – watery eyes, eye infection)
Upper Respiratory (symptoms – sore throat, cough, runny nose, cold)
Ear Infection (symptom – earache)
Urinary Tract Infection (symptom – urinary tract infection)
Skin (symptom – cuts, rash, sunburn)
Data will not be collected on headache and fever if they occur in isolation and are not linked to
specific syndromes.]
(Do this for each symptom for each person)
DINTRO
You said [you/PERSON] had [LIST OF SYMPTOMS MAKING UP SYNDROME].
[READ FOR FIRST SYNDROME FOR FIRST PERSON: We would now like to discuss how
this (illness/condition) affected (your/his/her) daily activities
.]
DOES D1=01 (Working for pay/business)
Yesgo to D2
No go to D4
D2.
During (your/his/her) illness, did (you/s/he) miss any time from work, for example because
(you/s/he) called in sick or took time off to see a doctor?
YES
1 (D3)
NO
2 (D4)
REFUSED
7 (D4)
DON’T KNOW
8 (D4)
D3.
How many days? |__|__|__| days (IF IN HOURS, i.e. <1 DAY, THEN CODE AS ZERO)
REFUSED
7
DON’T KNOW
8
D4.
Did this illness prevent (you/him/her) from performing daily activities such as school, recreation, or
vacation activities, or work around the home?
YES
1
NO
2 (D6)
REFUSED
7 (D6)
DON’T KNOW
8 (D6)
D5.
How many days?
|__|__|__|
days (IF IN HOURS, i.e. <1 DAY, THEN CODE AS ZERO)
REFUSED
7
DON’T KNOW
8
D6
. Did (your/his/her) illness cause other household members to lose time at work?
YES
1
NO
2 (D8a)
REFUSED
7 (D8a)
DON’T KNOW
8 (D8a)
D7.
IF Yes: How many days?
|__|__|__| days (IF IN HOURS, i.e. <1 DAY, THEN CODE AS ZERO)
REFUSED
7
DON’T KNOW
8
Next, I am going to ask you some questions about the treatment and diagnosis of (your/his/her) illness.
You said that (you/PERSON) suffered from (SYMPTOMS MAKING UP EACH ILLNESS).
D8a.
Did (you/s/he) consult a healthcare provider over the phone about this (illness/condition)?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
D8b.
Did (you/s/he)
visit
a health care provider?
YES
1
NO
2 (D8e)
REFUSED
7 (D8e)
DON’T KNOW
8 (D8e)
D8c
. How many times?
_______ #TIMES
REFUSED
7
DON’T KNOW
8
D8d
. What illness did the health care provider say (you/s/he) had?
_______________
REFUSED
7
DON’T KNOW
8
D8e.
Did (you/s/he)
visit
an emergency room?
YES
1
NO
2 (D9a)
REFUSED
7 (D9a)
DON’T KNOW
8 (D9a)
D8f.
How many times?
_______ #TIMES
REFUSED
7
DON’T KNOW
8
D8g.
Were you admitted to the hospital?
YES
1
NO
2 (D9a)
REFUSED
7 (D9a)
DON’T KNOW
8 (D9a)
D8h.
How many days (were you/was s/he) hospitalized?
_______ # DAYS
REFUSED
7
DON’T KNOW
8
D8i.
(Were you /Was s/he) given intravenous fluids?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
D9a.
Did (you/s/he) receive a prescription for an antibiotic or other drug for this (illness/condition)?
YES
1
NO
2 (D10a)
REFUSED
7 (D10a)
DON’T KNOW
8 (D10a)
D9b.
About how much of your own or your household’s money was spent altogether for these
prescription medicines?
Amount $ ______________________
Amount to nearest dollar
D10a
. Did (you/s/he) use any over-the-counter medications, including things like special drinks, only
because of this (illness/condition)?
YES
1
NO
2 (E1)
REFUSED
7 (E1)
DON’T KNOW
8 (E1)
D10b
.
About how much of your own or your household’s money was spent altogether for over-
the-counter medicines?
Amount $ ________________________
Amount to nearest dollar
EXIT STATEMENT
IF THERE IS ANOTHER PERSON IN THE ENUMERATION WHO WAS MARKED AS HAVING ANY
SYNDROME, GO BACK TO D1 FOR THAT PERSON AND ASK APPROPRIATE QUESTIONS FROM
D1
THROUGH
D10b
OR ELSE, IF THERE IS NO OTHER PERSON IN THE ENUMERATION WHO NEEDS TO BE ASKED ABOUT,
GO TO
E1
.
E1.
Before today, were you aware that people could become ill by swimming at the beach?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
E2.
After today, will you change the way you use the water at the beach? Or Will you change your recreational
use of water/activities at the beach?
YES
1
NO
2
REFUSED
7
DON’T KNOW
8
(We might want to move this to the front. Might make more sense. Moving it may bias)
Q1.
Did you or anyone in your household wade, swim, or play in the water on ___/___/___?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
IF NO TO PERSON 1 THROUGH PERSON 8, GO TO Q20
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Q1a.1
. Did {PERSON}
immerse their body, not
necessarily {PERSON’s}
head, in water today? ..........
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
Q1a.2.
Did {PERSON} put
their face in water or
submerge head in water
today?..................................
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
CONTINUE
Person
1
2
3
4
5
6
7
8
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Y N RF DK
Q1a.3.
Did {Person} get
water in the mouth today?
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
1 2 7 8
IF NO TO THE ABOVE QUESTION, GO TO QUESTION Q2..
Q1a.4
.Did {PERSON} gag or cough after getting water in their mouth?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
IF NO TO PERSON 1 THROUGH PERSON 8, GO TO Q16d
Q1a.5. Did {PERSON} swallow the water?
YES
NO
RF
DK
Person 1.................................................. 1 .................2 ................ 7.................. 8
Person 2.................................................. 1 .................2 ................ 7.................. 8
Person 3.................................................. 1 .................2 ................ 7.................. 8
Person 4.................................................. 1 .................2 ................ 7.................. 8
Person 5.................................................. 1 .................2 ................ 7.................. 8
Person 6.................................................. 1 .................2 ................ 7.................. 8
Person 7.................................................. 1 .................2 ................ 7.................. 8
Person 8.................................................. 1 .................2 ................ 7.................. 8
Q2.
What total time did {PERSON} stay in the water? We are only interested in time actually in the water, not
the total time at the beach.
(CIRCLE TIME UNITS)
RF
DK
Person 1 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 2 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 3 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 4 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 5 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 6 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 7 ................ ____ MINUTES
_____.__ HOURS 7
8
Person 8 ................ ____ MINUTES
_____.__ HOURS 7
8
This completes our telephone interview and your participation. I’d like to verify your address so we can mail a
check for $25 to {BEACH RESPONDENT}. (VERIFY ADDRESS ON CALL SHEET.) You will receive your
check in 30 days and thank you for your participation in this study. Thank you for taking the time to talk with me.
Goodbye.
IF RESP DOESN’T HAVE BROCHURE:
You can call 1-888-422-3072, from 8 am to 4:30 pm, Monday through Friday, Eastern time; mention you
are calling about the NEEAR study.
Website: NEEAR Water Study
You can also e-mail
neear_water_study@epa.gov
.
37
QAPP 2
Appendix 4A
Eligibility Screener
University of lllinois, Chicago
CHEERS Study
Eligibility
Screener & Refusal Tally sheet 2008
Hello. My name is
IYOUR
NAM$
and I an'r
part
of the
CI{EERS
field team. I'm
going
to ask
you
a few
questions
to
see
if
you
are eligible to be
parl
of our research study.
l.
What
outdoor activity will
you
be doing today:
Are
you
also
going
to Sll/IM,
BOOGIE
BOARD, WATER/JET
SKI
or
GO
TUBING.
If
"YES",
terminste recruiting. NO PRIMARY
CONTACT
ACTIVITY.
2. I{ave
you (or
your
child) enrolled in the
CIJEERS study in the
past
2l days?
Yes
(terminate
recruiting)
No
3. Have
you (or
you
child)
participated
in any water recreational
activity
(such
as
swimming. skiing, kayaking, fishing,
boating etc) at a lake, river
or beach in
the
past
48
hours? SWIMMING IN A POOL
IS OK.
Yes
(terminate
recruiting)
No
DOG WALKERS-
If they
waded
in the water with their dogs, they are ineligible.
4.
ls
it
OK for us to contact
you
by
phone
3 times over the next three weeks and ask
you
questions
about
your
health?
Yes
No
(terminate
recruiting)
5.
WATER-RECREATORS-
Will
you
be back by
(tell
them
what time we
plan
on
wrapping up
for
the
day) today?
If
"No--
Let them know
it is important
that we complete
this survey before
we
leave
this
location
today. If
participant
cannot
return to complete
the
survey-
they are
"NOT
ELIGIBLE".
If
"NOT
ELIGIBLE'1
I'm sorry, but
you
will not be
eligible for
enrolling in the study
now.
Please
look
for
the CHEERS
team in the future
and
you
may be
able to enroll then.
If ELIGIBLE:
Proceed
to consent
process.
Note:
Participants
are eligible
even
if they live
outside the
Chicago aren
or Illinois.
sTART$A
F
tr'
X{
# WA
l-
ExplnEs
,jtJN
2
i
?0aj8
JLJI\
2
0
2tj0g
uiirVEr{$iiY
ri iLi,l$Lrl$
At
uf{luAG0
liiSTlTtlTi0i\iAL
ltEV
IHVf
SOARD
CHEERS
Eligibility Screener
&
Refusal Tally Sheet,
Version
2,41312008
QAPP 2
Appendix 4B
Refusal Tally Sheet
1)
t
Refusal
Tallv
Sheet
Date
Researcher
Location
LJ
*Please
circle or more
of the following
options:
No time
:NT
Confidentiality concerns:
Concems
Language barrier:
Lang
Ineligible
Water
Activity:1t626
No
Reason:NR
Recent Water
ContacF RWC
No ParenF NP
Return Late:
RL
Currently Enrolled= CE
Lives
out
of
US: Non-US
No Phone: NPh
CHEERS
Eligibility
Screener & Refusal
Tally
Sheet,
Version
2, March 2008
Time
No:
Adults
No:
chitd
Reason
for
Ineligibility
*
Reason
for
refugal*
M
F
M
F
Lang
NP
CE NPh
IWA
RWC
RL
Non-US
NT
NR
Concerns
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concerns
Lang
NP
CE
NPh
IWA
RWC
RL
Non-US
NT NR
Concerns
Lang
NP
CE NPh
IWA
RWC
RL
Non-US
NT NR
Concerns
Lang
NP
CE
NPh
IWA
RWC
RL
Non,US
NT NR
Concerns
Lang NP
CE NPh
IWA
RWC
RL
Non-US
NT NR
Concerns
Lang
NP
CE NPh
IWA
RWC
RL
Non-US
NT NR
Concerns
Lang
NP
CE
NPh
IWA
RWC
RL Non-US
NT
NR
Concerns
Lang
NP
CE
NPh
IWA
RWC
RL Non-US
NT
NR
Concerns
Lang
NP
CE NPh
IWA RWC
RL Non-US
NT
NR
Concerns
Lang
NP
CE NPh
IWA
RWC RL
Non-US
NT NR
Concems
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT NR
Concems
Lang
NP
CE NPh
IWA
RWC
RL
Non-US
NT
NR
Concerns
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concems
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concems
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concems
Lang NP
CE
NPh
IWA RWC
RL
Non-US
NT
NR
Concems
Lang
NP
CE
NPh IWA
RWC
RL
Non-US
NT
NR
Concems
Lang NP
CE NPh
IWA
RWC
RL Non-US
NT NR
Concems
Lang NP
CE
NPh
IWA
RWC RL
Non-US
NT
NR
Concerns
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT NR
Concerns
Lang NP
CE NPh
IWA
RWC RL
Non-US
NT NR
Concems
Lang NP
CE NPh
IWA
RWC RL
Non-US
NT
NR
Concems
Lang
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concems
Lans
NP
CE NPh
IWA
RWC
RL Non-US
NT NR
Concerns
Lans
NP
CE NPh
IWA RWC
RL
Non-US
NT
NR Concems
Lans
NP
CE NPh
IWA
RWC
RL Non-US
NT
NR
Concems
Lans
NP CE NPh
IWA
RWC
RL
Non-US
NT NR
Concerns
Lang
NP
CE NPh IWA
RWC
RL
Non-US
NT NR
Concems
Lang NP
CE NPh
IWA
RWC
RL Non-US
NT NR
Concerns
Lang
NP
CE NPh
IWA RWC
RL Non-US
NT
NR Concerns
Lang NP
CE NPh IWA
RWC
RL Non-US
NT NR
Concerns
Lanq
NP CE NPh IWA
RWC
RL Non-US
NT
NR Concerns
CHEERS QAPP 2
APPENDIX 6: FIELD SURVEY B
CHEERS Part B
PB_S2loc
Location:
1 = River
2 = Lake
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF RIVER:
PB_S2
Enter River Location:
1 = Skokie Rowing Center
2 = Clark Park
3 = River/Honan Park
4 = North Avenue
5 = Alsip
6 = Worth
7 = Little Calumet River
8 = 28 Street
9 = OTHER (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF LAKE:
PB_S3
Enter General Area Waterways Location:
1 = Leone Beach
2 = Montrose Harbor
3 = Wilson Beach
4 = Belmont Harbor
5 = Diversey Harbor
6 = Jackson Park Harbor
7 = Skokie Lagoons
8 = Des Plaines River
9 = Kankakee River
10 = Fox River
11 = OTHER (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Intro
"Thank you for returning to complete the survey. We will have your t-shirt
and gift-card ready for you after we complete the survey. May I have your name
so that we can link it to your previous answers?
--------------------------------------------------------------------------------
PB_Q1First
"What is your first name?"
--------------------------------------------------------------------------------
PB_Q1Last
"What is your last name?"
--------------------------------------------------------------------------------
PB_Q9
"Did you engage in any water recreational activities at the <location> today?":
1 = Yes
2 = No
Æ
SKIP TO Q12 SERIES
DON’T KNOW
Æ
SKIP TO Q12 SERIES
REFUSAL
Æ
SKIP TO Q12 SERIES
--------------------------------------------------------------------------------
IF YES TO PB_Q9:
PB_Q9a
"What water recreational activities did you engage in while at the <location> today?”
Check All That Apply. If 'NONE' be sure no others are checked.
1 = Boating
2 = Canoeing
3 = Kayaking
4 = Rowing
5 = Rafting
6 = Fishing on a boat
7 = Fishing at the pier, shore or dock
8 = None of the above
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q9b
"Did you (read activities from list) also while at the <location> today?
Check All That Apply.
1 = Jet ski?
2 = Water ski?
3 = Go tubing?
4 = Boogie board?
5 = Use a waverunner?
6 = Swim?
7 = Go sailing?
8 = None of the above?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF PB_Q9b RESPONSE IS “SWIM”:
PB_Q9c
"You said you swam today. Was it accidental or intentional?":
1 = Accidental
2 = Intentional
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF PB_Q9b RESPONSE IS “INTENTIONAL”
INEL
"Thank you for participating in the CHEERS study.
We will not be calling you for the follow-up telephone survey.”
(INTERVIEWER): Be sure they receive their t-shirt and $15 gift card.
--------------------------------------------------------------------------------
ASK Q10 SERIES FOR EACH ACTIVITY:
PB_Q10SAIL.i_H
"For how long did you <FILL ACTIVITY>?
Enter in hours and minutes.
Enter a number between 0 and 18 for hours.
Enter a number between 0 and 59 for minutes
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.ii
"Where did you launch with your <FILL ACTIVITY>?"
1 = Shore
2 = Pier or dock
3 = Boat launch
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.iii
"Where did you exit with your <FILL ACTIVITY>?"
1 = Shore
2 = Pier or dock
3 = Boat launch
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.iv
"Did you travel upstream or downstream?"
1 = Upstream
2 = Downstream
3 = Both
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.v
"Did any part of your body get wet at all today?"
1 = Yes
2 = No
Æ
SKIP TO PB_Q10SAIL.vii
DON’T KNOW
Æ
SKIP TO PB_Q10SAIL.vii
REFUSAL
Æ
SKIP TO PB_Q10SAIL.vii
--------------------------------------------------------------------------------
IF YES TO PB_10SAIL.v:
PB_Q10SAIL.vi
"How wet did you get? Would you say..."
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched, or,
4 = Submerged?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.vi_feet
"How wet did your feet or legs get? Would you say..."
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched,
4 = Submerged, or,
5 = Not wet at all?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.vi_hands
"How wet did your hands or arms get? Would you say...":
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched,
4 = Submerged, or,
5 = Not wet at all?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.vi_torso
"How wet did your torso (that is your stomach or back) get? Would you say...":
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched,
4 = Submerged, or,
5 = Not wet at all?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.vii
"Did your face or head get wet?":
1 = Yes
2 = No
Æ
SKIP TO PB_Q10SAIL.xvii
DON’T KNOW
Æ
SKIP TO PB_Q10SAIL.xvii
REFUSAL
Æ
SKIP TO PB_Q10SAIL.xvii
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.vii:
PB_Q10SAIL.viii
"How wet did your face or head get? Would you say..."
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched, or,
4 = Submerged?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.ix
"Did you get any water in your mouth today?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.xv
"Did you swallow any water while <FILL ACTIVITY> today?"
1 = Yes
2 = No
Æ
SKIP TO
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xv:
PB_Q10SAIL.xvi
"Would you say it was...
(PROBE): Your best estimate is fine."
1 = A drop or two,
2 = A teaspoon, or,
3 = One or more mouthfuls?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.xvii
"Did you get wet while launching the <FILL ACTIVITY>?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.xviii
"Did your <FILL ACTIVITY> flip over or capsize today?"
1 = Yes
2 = No
Æ
SKIP TO PB_Q10SAIL.xxiii_A
DON’T KNOW
Æ
SKIP TO PB_Q10SAIL.xxiii_A
REFUSAL
Æ
SKIP TO PB_Q10SAIL.xxiii_A
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xviii:
PB_Q10SAIL.xix
"How many times?"
1 = One
2 = Two
3 = More than twice
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xviii:
PB_Q10SAIL.xix_H
"How long did you stay in the water after capsizing?”
Enter in hours and minutes.
Enter a number between 0 and 18 for hours.
Enter a number between 0 and 59 for minutes.
--------------------------------------------------------------------------------
PB_Q10SAIL.xxiii_A
"Did you wade into the water or stand in the water while <FILL ACTIVITY> today?"
1 = Yes
2 = No
Æ
SKIP TO PB_Q10SAIL.Rubeyes
DON’T KNOW
Æ
SKIP TO PB_Q10SAIL.Rubeyes
REFUSAL
Æ
SKIP TO PB_Q10SAIL.Rubeyes
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xxiii_A:
PB_Q10SAIL.xxiii_B
"Did you wear waders or hip boots?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xxiii_A:
PB_Q10SAIL.xxiii_CH
"How long did you stay in the water?”
Enter in hours and minutes.
Enter a number between 0 and 18 for hours.
Enter a number between 0 and 59 for minutes.
--------------------------------------------------------------------------------
PB_Q10SAIL.Rubeyes
"Did you rub your eyes while <FILL ACTIVITY> today?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q10SAIL.xxiv
"You said you were boating. Was it on a power boat?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q10SAIL.xxiv:
PB_Q10SAIL.xxv
"How long is your boat?”
Enter a number in feet from 1 to 100.
(PROBE): Approximately how many feet?"
--------------------------------------------------------------------------------
ASK ONLY FOR BOAT ACTIVITIES:
PB_Q10SAIL.xxvi
"How many people, not including yourself, were on the boat with you today?”
Enter a number from 0 to 97.
--------------------------------------------------------------------------------
ASK IF RESPONSE TO PB_Q10SAIL.xxvi IS MORE THAN “1”:
PB_Q10SAIL.xxvii
"How many of these people, not including yourself, are enrolled in the CHEERS study?”
Enter a number from 0 to 97.
--------------------------------------------------------------------------------
ASK FOR EACH PERSON:
PB_Q10SAIL.Roster.Person[01].Q1cName
"What is the full name of the (first/next) person who was on the boat with you,
who is also enrolled in the CHEERS study?":
--------------------------------------------------------------------------------
ASK FOR EACH PERSON:
PB_Q10SAIL.Roster.Person[01].Q1cGender
"What is their gender?"
1 = Male
2 = Female
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR FISHING ACTIVITIES:
PB_Q10SAIL.xxviii
"How many fish did you catch while <FILL ACTIVITY> today?"
--------------------------------------------------------------------------------
ASK ONLY FOR FISHING ACTIVITIES:
PB_Q10SAIL.xxvix
"What kind of bait did you use <FILL ACTIVITY> today? Did you use...
Check All That Apply.
1 = Live bait such as worms or minnows?,
2 = Artificial lures?,
3 = Natural lures such as corn, fish eggs or meat?,
4 = Some other type of bait? (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
ASK ONLY FOR FISHING ACTIVITIES:
PB_Q10SAIL.xxx
"Do you plan on eating any of the fish you caught today?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q11.a
"Did you eat during or after your activities at the <LOCATION>?
(NOTE): Only record any eating before they left the location.
1 = Yes
2 = No
Æ
SKIP TO PB_Q11.d
DON’T KNOW
Æ
SKIP TO PB_Q11.d
REFUSAL
Æ
SKIP TO PB_Q11.d
--------------------------------------------------------------------------------
IF YES TO PB_Q11a:
PB_Q11.b
"Did you clean your hands before eating?"
1 = Yes
2 = No
Æ
SKIP TO PB_Q11.d
DON’T KNOW
Æ
SKIP TO PB_Q11.d
REFUSAL
Æ
SKIP TO PB_Q11.d
--------------------------------------------------------------------------------
IF YES TO PB_Q11b:
PB_Q11.c
"Did you use...
Read All Choices. Check All That Apply.
(NOTE): <LOCATION> water doesn't count.
1 = Soap,
2 = A hand sanitizer,
3 = Hand wipes, or,
4 = Just rinse your hands?
5 = Other (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q11.d
"Did you drink anything during or after your activities at the <LOCATION> today?
(NOTE): Only record any drinking before they left the location.
1 = Yes
2 = No
Æ
SKIP TO CE_1Intro
DON’T KNOW
Æ
SKIP TO CE_1Intro
REFUSAL
Æ
SKIP TO CE_1Intro
--------------------------------------------------------------------------------
IF YES TO PB_Q11d:
PB_Q11.e
"Did you clean your hands before drinking?"
1 = Yes
2 = Sometimes
3 = No
Æ
SKIP TO PB_Q11g
DON’T KNOW
Æ
SKIP TO PB_Q11g
REFUSAL
Æ
SKIP TO PB_Q11g
--------------------------------------------------------------------------------
IF YES OR SOMETIMES TO PB_Q11e:
PB_Q11.f
"Did you use...
Read All Choices. Check All That Apply.
(NOTE): <LOCATION> water doesn't count."
1 = Soap,
2 = A hand sanitizer,
3 = Hand wipes, or,
4 = Just rinse your hands?
5 = Other (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q11d:
PB_Q11.g
"How many ounces did you drink at the <LOCATION> today?
(PROBE): Your best estimate is fine.
Use Visual Aids As Appropriate:
Can = 12 ounces
Small bottle = 8 ounces
Regular bottle = 16 ounces
Or Check their water bottle.
Enter a number from 1 to 500.
--------------------------------------------------------------------------------
ASK THE Q12 SERIES AND Q13 SERIES FOR RESPONDENTS WHO DID NOT DO ANY
WATER REC ACTIVITIES TODAY:
PB_Q12[
"What activity did you participate in today? Did you...
Read All Categories. Check All That Apply.":
1 = Roller blade?,
2 = Run?,
3 = Walk?,
4 = Cycle?,
5 = Golf?,
6 = Play baseball?,
7 = Play softball?,
8 = Play soccer?,
9 = Play tennis?,
10 = Walk your dog?,
11 = Do any other activity? (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q13
"Did you at any point in time today come in contact with the <LOCATION> water?"
1 = Yes
2 = No
Æ
SKIP TO CE_1Intro
DON’T KNOW
Æ
SKIP TO CE_1Intro
REFUSAL
Æ
SKIP TO CE_1Intro
--------------------------------------------------------------------------------
PB_Q13a
"Did any part of your body get wet at all today?"
1 = Yes
2 = No
Æ
SKIP TO CE_1Intro
DON’T KNOW
Æ
SKIP TO CE_1Intro
REFUSAL
Æ
SKIP TO CE_1Intro
--------------------------------------------------------------------------------
PB_Q13b
"Did your face or head get wet?"
1 = Yes
2 = No
Æ
SKIP TO CE_1Intro
DON’T KNOW
Æ
SKIP TO CE_1Intro
REFUSAL
Æ
SKIP TO CE_1Intro
--------------------------------------------------------------------------------
IF YES TO PB_Q13b:
PB_Q13c
"How wet did your face or head get?"
1 = Sprinkle or few drops,
2 = Splashed,
3 = Drenched, or,
4 = Submerged?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q13d
"Did you get any <LOCATION> water in your mouth today?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q13e
"Did you swallow any <LOCATION> water today?"
1 = Yes
2 = No
Æ
SKIP TO CE_1Intro
DON’T KNOW
Æ
SKIP TO CE_1Intro
REFUSAL
Æ
SKIP TO CE_1Intro
--------------------------------------------------------------------------------
IF YES TO Q13e:
PB_Q13f
"Would you say it was...
(IF NECESSARY): Your best estimate is fine."
1 = A drop or two,
2 = A teaspoon, or,
3 = One or more mouthfuls?
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1Intro
"Now I am going to ask you a few questions about your current health status.
--------------------------------------------------------------------------------
CE_1a1
"Do you have any ongoing stomach or intestinal problems,
otherwise known as gastrointestinal problems?"
1 = Yes
2 = No
Æ
SKIP TO CE_1b
DON’T KNOW
Æ
SKIP TO CE_1b
REFUSAL
Æ
SKIP TO CE_1b
--------------------------------------------------------------------------------
IF YES TO CE_1a1:
CE_1a2
"Which of the following do you have? Do you have...
Check All That Apply.
1 = Crohn's disease?,
2 = Inflammatory bowel disease?,
3 = Irritable bowel syndrome?,
4 = Ulcers?,
5 = Gastritis?,
6 = Acid Reflux?,
7 = Lactose intolerance?,
8 = OTHER (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1b
"Do you have any ongoing respiratory problems such as asthma,
bronchitis or emphysema?":
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1c
"Do you have diabetes?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1d
"In the past 7 days, have you taken oral antibiotics?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1e
"Do you have any condition that makes you more likely to get infections?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_1f
"In the past 48 hours, have you taken any antacids such as Tums, Rolaids and Mylanta?
(NOTE): Also includes Pepsid AC, Zantac, Prilosec."
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_2
"On average, how many bowel movements do you have in a day?
(PROBE): By bowel movements we mean pooping.
0) Less than one
1) One
2) Two
3) Three or more
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_2a
"Do you wear contact lenses?"
1 = Yes
2 = No
3 = Sometimes
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3a
"Now I am going to ask you a few questions about how you are feeling today.
Are you currently experiencing any of the following symptoms?
Abdominal cramps or stomach ache?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3b
"Diarrhea or loose bowels?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3c
"Nausea?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3d
"Vomiting?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3e
"Fever?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3f
"Headache?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3g
"Sore throat?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3h
"A bad or persistent cough?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3i
"A cold, or a runny or stuffy nose?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3j
"Ear ache or ear infection?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3k
"Eye irritation?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3l
"Drainage or crusting in your eyes?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3cut
"Any areas on your skin where there are cuts?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3bug
"Any areas on your skin where there are bug bites?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3m
"Any areas on your skin that are sore or draining?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_3n
"Rash or itchy skin?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
CE_cut[
"Where are the cuts located? Check All That Apply."
1 = RIGHT SIDE, HEAD/FACE,
2 = RIGHT SIDE, NECK,
3 = RIGHT SIDE, UPPER ARM,
4 = RIGHT SIDE, ELBOW,
5 = RIGHT SIDE, FORE ARM,
6 = RIGHT SIDE, HAND,
7 = RIGHT SIDE, FINGERS,
8 = RIGHT SIDE, THIGH,
9 = RIGHT SIDE, KNEE,
10 = RIGHT SIDE, LOWER LEG,
11 = RIGHT SIDE, ANKLE/FOOT,
12 = RIGHT SIDE, TOES,
13 = RIGHT SIDE, ABDOMEN,
14 = RIGHT SIDE, CHEST,
15 = RIGHT SIDE, BACK,
16 = RIGHT SIDE, (SPECIFY),
17 = LEFT SIDE, HEAD/FACE,
18 = LEFT SIDE, NECK,
19 = LEFT SIDE, UPPER ARM,
20 = LEFT SIDE, ELBOW,
21 = LEFT SIDE, FORE ARM,
22 = LEFT SIDE, HAND,
23 = LEFT SIDE, FINGERS,
24 = LEFT SIDE, THIGH,
25 = RIGHT SIDE, KNEE,
26 = LEFT SIDE, LOWER LEG,
27 = LEFT SIDE, ANKLE/FOOT,
28 = LEFT SIDE, TOES,
29 = LEFT SIDE, ABDOMEN,
30 = LEFT SIDE, CHEST,
31 = LEFT SIDE, BACK,
32 = LEFT SIDE, (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF CUT ON RIGHT SIDE AT OTHER LOCATION:
CE_cutRO_text
"Where else on your right side did you cut yourself?"
--------------------------------------------------------------------------------
IF CUT ON LEFT SIDE AT OTHER LOCATION:
CE_cutLO_text
"Where else on your left side did you cut yourself?"
--------------------------------------------------------------------------------
CE_bug[
"Where are the bug bites or cuts located? Check All That Apply."
1 = RIGHT SIDE, HEAD/FACE,
2 = RIGHT SIDE, NECK,
3 = RIGHT SIDE, UPPER ARM,
4 = RIGHT SIDE, ELBOW,
5 = RIGHT SIDE, FORE ARM,
6 = RIGHT SIDE, HAND,
7 = RIGHT SIDE, FINGERS,
8 = RIGHT SIDE, THIGH,
9 = RIGHT SIDE, KNEE,
10 = RIGHT SIDE, LOWER LEG,
11 = RIGHT SIDE, ANKLE/FOOT,
12 = RIGHT SIDE, TOES,
13 = RIGHT SIDE, ABDOMEN,
14 = RIGHT SIDE, CHEST,
15 = RIGHT SIDE, BACK,
16 = RIGHT SIDE, (SPECIFY),
17 = LEFT SIDE, HEAD/FACE,
18 = LEFT SIDE, NECK,
19 = LEFT SIDE, UPPER ARM,
20 = LEFT SIDE, ELBOW,
21 = LEFT SIDE, FORE ARM,
22 = LEFT SIDE, HAND,
23 = LEFT SIDE, FINGERS,
24 = LEFT SIDE, THIGH,
25 = RIGHT SIDE, KNEE,
26 = LEFT SIDE, LOWER LEG,
27 = LEFT SIDE, ANKLE/FOOT,
28 = LEFT SIDE, TOES,
29 = LEFT SIDE, ABDOMEN,
30 = LEFT SIDE, CHEST,
31 = LEFT SIDE, BACK,
32 = LEFT SIDE, (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF BUG BITES ON RIGHT SIDE ON OTHER LOCATION:
CE_bugRO_text
"Where else on your right side did you have bug bites?"
--------------------------------------------------------------------------------
IF BUG BITES ON LEFT SIDE ON OTHER LOCATION:
CE_bugLO_text
"Where else on your left side did you have bug bites?"
--------------------------------------------------------------------------------
CE_sore[
"Where are the sores or draining skin located? Check All That Apply."
1 = RIGHT SIDE, HEAD/FACE,
2 = RIGHT SIDE, NECK,
3 = RIGHT SIDE, UPPER ARM,
4 = RIGHT SIDE, ELBOW,
5 = RIGHT SIDE, FORE ARM,
6 = RIGHT SIDE, HAND,
7 = RIGHT SIDE, FINGERS,
8 = RIGHT SIDE, THIGH,
9 = RIGHT SIDE, KNEE,
10 = RIGHT SIDE, LOWER LEG,
11 = RIGHT SIDE, ANKLE/FOOT,
12 = RIGHT SIDE, TOES,
13 = RIGHT SIDE, ABDOMEN,
14 = RIGHT SIDE, CHEST,
15 = RIGHT SIDE, BACK,
16 = RIGHT SIDE, OTHER (SPECIFY),
17 = LEFT SIDE, HEAD/FACE,
18 = LEFT SIDE, NECK,
19 = LEFT SIDE, UPPER ARM,
20 = LEFT SIDE, ELBOW,
21 = LEFT SIDE, FORE ARM,
22 = LEFT SIDE, HAND,
23 = LEFT SIDE, FINGERS,
24 = LEFT SIDE, THIGH,
25 = LEFT SIDE, KNEE,
26 = LEFT SIDE, LOWER LEG,
27 = LEFT SIDE, ANKLE/FOOT,
28 = LEFT SIDE, TOES,
29 = LEFT SIDE, ABDOMEN,
30 = LEFT SIDE, CHEST,
31 = LEFT SIDE, BACK,
32 = LEFT SIDE, OTHER (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF SORES / DRAINING SIDE ON RIGHT SIDE ON OTHER LOCATION:
CE_soreRO_text
"List other sites on right side where R has sores or draining skin."
--------------------------------------------------------------------------------
IF SORES / DRAINING SIDE ON LEFT SIDE ON OTHER LOCATION:
CE_soreLO_text
"List other sites on left side where R has sores or draining skin."
--------------------------------------------------------------------------------
CE_rash[
"Where is the rash located? Check All That Apply."
1 = RIGHT SIDE, HEAD/FACE,
2 = RIGHT SIDE, NECK,
3 = RIGHT SIDE, UPPER ARM,
4 = RIGHT SIDE, ELBOW,
5 = RIGHT SIDE, FORE ARM,
6 = RIGHT SIDE, HAND,
7 = RIGHT SIDE, FINGERS,
8 = RIGHT SIDE, THIGH,
9 = RIGHT SIDE, KNEE,
10 = RIGHT SIDE, LOWER LEG,
11 = RIGHT SIDE, ANKLE/FOOT,
12 = RIGHT SIDE, TOES,
13 = RIGHT SIDE, ABDOMEN,
14 = RIGHT SIDE, CHEST,
15 = RIGHT SIDE, BACK,
16 = RIGHT SIDE, (SPECIFY),
17 = LEFT SIDE, HEAD/FACE,
18 = LEFT SIDE, NECK,
19 = LEFT SIDE, UPPER ARM,
20 = LEFT SIDE, ELBOW,
21 = LEFT SIDE, FORE ARM,
22 = LEFT SIDE, HAND,
23 = LEFT SIDE, FINGERS,
24 = LEFT SIDE, THIGH,
25 = RIGHT SIDE, KNEE,
26 = LEFT SIDE, LOWER LEG,
27 = LEFT SIDE, ANKLE/FOOT,
28 = LEFT SIDE, TOES,
29 = LEFT SIDE, ABDOMEN,
30 = LEFT SIDE, CHEST,
31 = LEFT SIDE, BACK,
32 = LEFT SIDE, (SPECIFY),
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF RASH ON RIGHT SIDE ON OTHER LOCATION:
CE_rashRO_text
"Where else on your right side do you have a rash?"
--------------------------------------------------------------------------------
IF RASH ON LEFT SIDE ON OTHER LOCATION:
CE_rashLO_text
"Where else on your left side do you have a rash?"
--------------------------------------------------------------------------------
PB_Q15
"Are you currently sunburned?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q2a
"In the past 72 hours have you come in contact with someone who had
vomiting or diarrhea?"
1 = Yes
2 = No
Æ
SKIP TO PB_Q2b
DON’T KNOW No
Æ
SKIP TO PB_Q2b
REFUSAL No
Æ
SKIP TO PB_Q2b
--------------------------------------------------------------------------------
IF YES TO PB_Q2a:
PB_Q2a1
"Were any of those contacts with people who live with you, such as family members or
roommates?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q2b
"In the past 72 hours have you come in contact with someone who had
a cold, cough or sore throat?"
1 = Yes
2 = No No
Æ
SKIP TO PB_Q2c
DON’T KNOW No
Æ
SKIP TO PB_Q2c
REFUSAL No
Æ
SKIP TO PB_Q2c
--------------------------------------------------------------------------------
IF YES TO PB_Q2b:
PB_Q2b1
"Were any of those contacts with people who live with you, such as family members or
roommates?":
(
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q2c
"In the past 72 hours have you come in contact with someone who had
an eye infection?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3a
"In the last 48 hours have you touched a cat or dog?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3b
"In the last 48 hours have you touched any animals other than cats or dogs,
including farm animals or animals at a petting zoo?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3c
"In the last 48 hours have you
Eaten any sushi or raw shell fish such as crab, oyster, or mussels?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3d
"In the last 48 hours have you eaten any rare, raw, or undercooked meat?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3e
"In the last 48 hours have you eaten any runny or raw eggs?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3f
"In the last 48 hours have you eaten a prepackaged sandwich?"
NOTE: Please do not include any home-made sandwiches or hot sandwiches.
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3g
"In the last 48 hours have you eaten any fresh fruit, vegetables, or salad greens?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q3h
"In the last 48 hours have you eaten a hamburger?"
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q16
"When was the last time you were involved in any water activity before today?"
1 = Past 3 days,
2 = 4-7 days,
3 = More than 7 days ago but less than 30 days (one month),
4 = 30 days(one month) or more ago
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
PB_Q17
"Approximately how many miles did you travel today to get here today?
Enter a number between 0 and 500.
Enter 0 for less than a mile.
--------------------------------------------------------------------------------
PB_Q18a
"(INTERVIEWER): Do not ask children this question.
Did you use any tobacco while at the <LOCATION> today?"
1 = Yes
2 = No
Æ
SKIP TO Q19River
DON’T KNOW
Æ
SKIP TO Q19River
REFUSAL
Æ
SKIP TO Q19River
--------------------------------------------------------------------------------
IF YES TO PB_Q18a:
PB_Q18b
"Was that cigarettes, cigars, a pipe, or chewing tobacco?"
1 = Cigarettes,
2 = Cigars,
3 = Pipe,
4 = Chewing tobacco,
5 = Other (SPECIFY)
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
IF YES TO PB_Q18a:
PB_Q18c
"How much did you use?
(PROBE): How many cigarettes, cigars, pipes did you smoke or pinches of tobacco did you
chew?
Enter a number from 1 to 100.
--------------------------------------------------------------------------------
PB_Q19River
"In the past 12 months, how many times have you used the Chicago River for any
water
recreational activities?”
Enter a number from 0 to 365.
(PROBE): Your best estimate is fine."
--------------------------------------------------------------------------------
PB_Q19Lake
"In the past 12 months, how many times have you used Lake Michigan for any
water recreational
activities?”
Enter a number from 0 to 365.
(PROBE): Your best estimate is fine."
--------------------------------------------------------------------------------
PB_Q19Lagoon
IF PBS2loc = SKOKIE LAGOON, DES PLAINES RIVER, KANKAKEE RIVER, FOX RIVER OR
OTHER GENERAL AREA WATERWAY:
"In the past 12 months, how many times have you used the (SPECIFY GENERAL AREA
WATERWAY LOCATION) for any
water recreational activities?”
Enter a number from 0 to 365.
(PROBE): Your best estimate is fine."
--------------------------------------------------------------------------------
PB_Q19
"On a scale from 0 to 10 where 0 is not at all risky and 10 is very risky, can you tell me how
much of a health risk you think it is to do water sports on the Chicago River?
(PROBE): We are not asking about safety,
only health."
--------------------------------------------------------------------------------
PB_Q8Addr
"May I please have your mailing address so that we can send you
your $35 'Thank You' check once you have completed the final telephone interview? You should
receive your check in about 6-8 weeks.
Enter Street Address. Verify Spelling."
--------------------------------------------------------------------------------
PB_Q8City
Enter City. Verify Spelling."
--------------------------------------------------------------------------------
PB_Q8State
Enter State. Use Standard 2-Letter Abbreviation."
--------------------------------------------------------------------------------
PB_Q8Zip
Enter 5-Digit Zip Code."
--------------------------------------------------------------------------------
PB_Thanks
"Thank you for your assistance on this survey. We will call you 2, 5 and 21 days
from today. If you have any questions or concerns you can reach us at the numbers
provided in the consent document.
--------------------------------------------------------------------------------
IF RESPONDENT DECLINED TO GIVE ADDRESS:
PB_InelA
"I'm sorry. In order to be eligible to participate in the rest of the
survey we would need your mailing address to mail your 'Thank you' check.
Thank you for your time."
--------------------------------------------------------------------------------
PB_Phone
(INTERVIEWER): Was the interview completed in person or over the phone?
1 = In person
2 = Over the phone
--------------------------------------------------------------------------------
PB_Proxy
Was this interview completed by a proxy respondent?
1 = Yes
2 = No
DON’T KNOW
REFUSAL
--------------------------------------------------------------------------------
QAPP 2
Appendix 5
Field Survey A
UNIVERSITY OF ILLINOIS, CHICAGO
CHEERS FIELD SURVEY
PART A
Interviewer Name (Part A): ...............................
...................................................
Last
First
CAWs: Location…
…………….Drop down menu: Skokie Rowing Center, Clark Park, North Avenue, Alsip, Worth,
Little Calumet River, 28 street, other
If other: Free text
General Area Waterways: Location
…………..Drop down menu: Leone Beach, Montrose Harbor, Wilson Beach,
Belmont Harbor, Diversey Harbor, Jackson Park Harbor, Skokie Lagoons, Des Plaines River, Kankakee River, Fox
River, other
If other: Free text
ENTER 6-digit Case Identification Number: _____________
Date: ____ / ____ /____
Start Time OF Field Survey A:
AM / PM
(computer to populate this field)
(computer to populate this field)
Hi, my name is___________________________. In order for us to find out more about people like you who
participate in outdoor activities, I will ask you a few questions about yourself.
Q1. Please tell me your first name, last name and birth month/year. (INTERVIEWER: CODE GENDER.)
First Name
Last Name
Date of Birth
mm/yyyy
Gender
__ / ___ RF DK
M F RF DK
Note: PROGRAM decides if this is proxy interview, based on child’s age. If child is below 7 years of age
interviewer is prompted to request parent to answer the
questions.
Q1a. Do you consider yourself to be … SELECT ALL THAT APPLY.
1. White,
5. Native Hawaiian or Other Pacific Islander, or
2. Black or African American,
6. Asian?
3. Hispanic,
7. Other: Specify ________________________
8. Refused
4. American Indian or Alaska Native, 9. Don’t Know
Q1b. Are you here
today
with any people who live with you, such as family members or roommates? Y N RF DK,
or If No (Skip to Q2)
Q1c. How many people who you live with are here today? Enter Number. (Please do not include yourself)
Q1d. How many of those people are enrolled or enrolling in the CHEERS study today? Enter Number. (Please do
not include yourself)
Field Survey A, Version 10, January 2008
2
Q2.
Some people swim, canoe, kayak, fish, SCUBA dive, or participate in water recreational activities at
places such as beaches, water parks, public pools, private pools, or wading pools. During the past
7 days, have
you participated in any water recreational activities anywhere?
YES
1 Go To Q3
NO
2 Go To Q5
REFUSED
7 Go To Q5
DON’T KNOW
8 Go To Q5
Q3. Where did you engage in these water recreational activity/ activities? Check all that apply.
PROBE: If BEACH,
ask – was that an ocean, lake or river?
Location
Lake
Y N RF DK
If YES , Were any of these activities at Lake
Michigan
River
Y N RF DK
If YES , Were any of these activities at
Chicago River or Calumet-Sag channel
Lagoon
Y N RF DK
If YES, Were any of these activities at
Skokie Lagoon
Water park
Y N RF DK
Public Pool
Y N RF DK
Private pool
Y N RF DK
Wading pool
Y N RF DK
Ocean
Y N RF DK
Other
Specify:
Q4. During any of these water recreational activities………
a. Did any part of your body get wet at all?
If NO, GoTo Q5
b. If YES, did your face/head get wet?
Y N RF DK
Y N RF DK
c. Did you get any water in your mouth?
If NO, GoTo Q5
d. If YES, did you swallow any water?
Y N RF DK
Y N RF DK
Field Survey A, Version 10, January 2008
3
Q5. As mentioned earlier we are interested in asking about your health in the next 3 weeks
. May I please have
the best phone number where we can reach you at over the next
3 weeks?
YES.........................................................
1
?
GO TO Q6a
NO...........................................................
2
?
terminate survey
(IF NECESSARY): As mentioned earlier we will be calling 2,5, and 21 days from today to ask you a few
questions about your health.
INTERVIEWER:
We will end the interview here since a contact telephone number is required to complete the
telephone interview that I mentioned. Thank you for speaking with us.
Q6a.
Which
is the best
phone number(s)
to reach you at during this time
? INTERVIEWER: COLLECT AS
MANY
PHONE NUMBERS AND EXTENTIONS AS
POSSIBLE.
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00 noon
Afternoon
12:01 PM-5:00 PM
Evening
5:01 PM- 9:00 PM
other
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00 noon
Afternoon
12:01 PM-5:00 PM
Evening
5:01 PM- 9:00 PM
other
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00 noon
Afternoon
12:01 PM-5:00 PM
Evening
5:01 PM- 9:00 PM
other
INTERVIEWER: Thank you
. We’ll try to reach you during that (those) time(s).
End A
:
IF non-water recreator proceed directly to Survey B.
IF water recreator ask: Will you be returning by (mention time) to complete Survey B?
IF “YES”- remind them to return to the CHEERS tent by (mention time) to complete Survey B and collect
their gift card and t-shirt.
Interviewer make sure the data manager has the list of participants who will return to complete survey B.
End Time for Field Survey A________AM /PM
(computer to populate this field)
Field Survey A, Version 10, January 2008
4
Field Survey A, Version 10, January 2008
5
QAPP 2
Appendix 7
Telephone Follow-up Interview
Follow up Telephone Interview, Version 7, January 2008
UNIVERSITY OF IILINOIS, CHICAGO
CHEERS
FOLLOW-UP TELEPHONE SURVEY
ENTER 6-digit Case Identification Number:_____________________
A1. Hello, my name is ____________. May I speak to (RESPONDENT NAME: _______________)?
1.
AVAILABLE – GO TO A4
2.
NOT AVAILABLE – GO TO A2
3.
NEVER HEARD OF PERSON – GO TO A3
4.
REFUSED – Too busy ................................................
No longer interested ...............................
Will not be available................................
Other reason?.........................................
Please specify: .......................................
A2. Recently, we spoke to (RESPONDENT) at [fill River/Lake location], and we’re calling to complete the interview.
If s/he isn’t available- Could you please tell us what is the best time to reach him/her?
DATE:
TIME:
A3. Is this number (___) ___ ____ ? (VERIFY THE NUMBER ON THE CALL RECORD SHEET)
1.
YES – COMPLETE NIRF
2.
NO -- REDIAL THE NUMBER
A4. I’m calling about a follow-up health survey for the CHEERS study. Recently, we spoke to you at [fill River/Lake
location]{IF NEEDED: Specify the location and date}; and we’re calling back to complete the interview.
1.
YES- CONTINUE Goto Section B
2.
NO- SCHEDULE APPOINTMENT
3.
NO- REFUSED - Too busy
No longer interested ...........................
Will not be available............................
Other reason?.....................................
Please specify: ...................................
IF ASKED TO CALL BACK OR SCHEDULE APPOINTMENT, SAY: The interview needs to be completed by
[LAST DATE]. When would be the best time to call you before then?
DATE:
TIME:
2
Follow up Telephone Interview, Version 7, January 2008
Symptom Intro
B.
Now I am going to ask you a few questions about how you have been feeling since we last spoke.
Since we last spoke on (fill Date of survey or last telephone interview) [fill number of days] days
ago.
Note: Go through each symptom. If YES to any symptom, then Go to symptom detail questions. If NO to
any symptom, Go to next symptom on the list. If NO to all symptoms, then skip to Section D.
Symptom
Y N RF DK
Symptom Detail
B1. Abdominal cramps or stomach ache
Y N RF DK
If Yes, Complete B1a- B1d.
B2. Diarrhea or loose bowels
Y N RF DK
If Yes, Complete B2a- B2d
B3. Nausea
Y N RF DK
If Yes, Complete B3a- B3d
B4. Vomiting
Y N RF DK
If Yes, Complete B4a- B4d
B5. Fever
Y N RF DK
If Yes, Complete B5a- B5e
B6. Headache
Y N RF DK
If Yes, Complete B6a- B6c
B7. Sore throat
Y N RF DK
If Yes, Complete B7a-B7d
B8. Cough
Y N RF DK
If Yes, Complete B8a-Be
B9. A Cold or a runny or stuffy nose
Y N RF DK
If Yes, Complete B9a-B9d
B10. Earache or ear infection
Y N RF DK
If Yes, Complete B10a-B10d
B11. Eye irritation
Y N RF DK
If Yes, Complete B11a-B11d
B12. Drainage or crusting in eyes
Y N RF DK
If Yes, Complete B12a-B12d
B13. Any areas on your skin that are sore or
draining
Y N RF DK
If Yes, Complete B13a-B13h
B14. Rash or Itchy skin
Y N RF DK
If Yes, Complete B14a-B14f
B. Symptom Detail
INTERVIEWER WILL REFER TO HARD-COPY CALENDAR TO NEGOTIATE DATES.
B1a. On what date did the abdominal cramping or stomach ache start?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B1b.
Do you still have the abdominal cramping or stomach ache?
YES
NO Go to B1c.
REFUSED Go to B1c.
DON’T KNOW Go to B1c.
B1c.
On what date did the abdominal cramps or stomach ache end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B1d. Was this menstrual cramps? Ask only for female participants 12 years or older.
3
Follow up Telephone Interview, Version 7, January 2008
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B2a. On what date did the diarrhea or loose bowels start?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B2b. Do you still have diarrhea or loose bowels?
YES
NO Go to B2c.
REFUSED Go to B2c.
DON’T KNOW Go to B2c.
B2c. On what date did the diarrhea or loose bowels end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B2d. What is the maximum number of times you had diarrhea in a 24-hour period?
[NUMBER PER DAY:] |__|__|__|
REFUSED
97
DON’T KNOW
98
B3a. On what date did the nausea start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B3b.
Do you still have nausea?
YES
NO Go to B3c.
REFUSED Go to B3c.
DON’T KNOW Go to B3c.
B3c. On what date did the nausea end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
4
Follow up Telephone Interview, Version 7, January 2008
B3d. Are you pregnant? Ask only adult females.
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B4a. On what date did you start vomiting?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B4b. Do you still have the vomiting?
YES
NO Go to B4c.
REFUSED Go to B4c.
DON’T KNOW Go to B4c.
B4c. On what date did the vomiting end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B4d. Are you pregnant? Ask only adult females.
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B5a. On what date did the fever start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B5b. Do you still have fever?
YES
NO Go to B5c.
REFUSED Go to B5c.
DON’T KNOW Go to B5c.
B5c. On what date did the fever end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
5
Follow up Telephone Interview, Version 7, January 2008
B5d. Was your temperature taken using a thermometer?
YES
1
NO
2 Go To B6a
REFUSED
97 Go To B6a
DON’T KNOW
98 Go To B6a
B5e. What is the highest temperature that you had?
[TEMPERATURE:] _____
Range: 98.6 to 106.9
REFUSED
97
DON’T KNOW
98
B6a. On what date did the headache start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B6b. Do you still have a headache?
YES
NO Go to B6.
REFUSED Go to B6c.
DON’T KNOW Go to B6c.
B6c. On what date did the headache end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B7a. On what date did the sore throat start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B7b. Do you still have a sore throat?
YES
NO Go to B7c.
REFUSED Go to B7c.
DON’T KNOW Go to B7c.
6
Follow up Telephone Interview, Version 7, January 2008
B7c. On what date did the sore throat end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B7d. Do you think this sore throat was related to allergies?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B8a. On what date did the cough start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B8b. Do you still have a cough?
YES
NO Go to B8c.
REFUSED Go to B8c.
DON’T KNOW Go to B8c.
B8c. Did you cough up phlegm?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B8d. On what date did the cough end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B8e. Do you think this cough was related to allergies?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B9a. On what date did your cold or runny or stuffy nose start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
7
Follow up Telephone Interview, Version 7, January 2008
B9b. Do you still have a cold or runny or stuffy nose?
YES
NO Go to B9c.
REFUSED Go to B9c.
DON’T KNOW Go to B9c.
B9c.
On what date did the cold or runny or stuffy nose end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B9d. Do you think was this cold or runny or stuffy nose was related to allergies?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
B10a. On what date did the earache/ear infection start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B10b. Do you still have an earache?
YES
NO Go to B10c
REFUSED Go to B10c
DON’T KNOW Go to B10c
B10c. On what date did the earache end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B10d. Was this earache or ear infection in your……..
Left ear,
Right ear
Both ears
REFUSED
97
DON’T KNOW
98
B11a. On what date did the eye irritation start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
8
Follow up Telephone Interview, Version 7, January 2008
B11b. Do still have eye irritation?
YES
NO Go to B11c
REFUSED Go to B11c
DON’T KNOW Go to B11c
B11c. On what date did the eye irritation end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B11d. Was the irritation in your…
Left eye,
Right eye
Both eyes
REFUSED
97
DON’T KNOW
98
B12a. On what day did the eye drainage or crusting start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B12b. Do you still have eye drainage or crusting?
YES
NO Go to B12c
REFUSED Go to B12c
DON’T KNOW Go to B12c
B12c.
On what date did the eye drainage or crusting end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B12d. Was this drainage or crusting in your…..
Left eye,
Right eye,
Both eyes
REFUSED
97
DON’T KNOW
98
9
Follow up Telephone Interview, Version 7, January 2008
B13a. Where
is the soreness or drainage located?
Right side
Left side
Head/ Face
Y N RF DK
Head/ Face
Y N RF DK
Neck
Y N RF DK
Neck
Y N RF DK
Upper Arm
Y N RF DK
Upper Arm
Y N RF DK
Fore Arm
Y N RF DK
Fore Arm
Y N RF DK
Elbow
Y N RF DK
Elbow
Y N RF DK
Hand
Y N RF DK
Hand
Y N RF DK
Fingers
Y N RF DK
Fingers
Y N RF DK
Thigh
Y N RF DK
Thigh
Y N RF DK
Lower leg
Y N RF DK
Lower leg
Y N RF DK
Ankle/ Foot
Y N RF DK
Ankle/ Foot
Y N RF DK
Abdomen
Y N RF DK
Abdomen
Y N RF DK
Chest
Y N RF DK
Chest
Y N RF DK
Back
Y N RF DK
Back
Y N RF DK
Knee
Y N RF DK
Knee
Y N RF DK
Toes
Y N RF DK
Toes
Y N RF DK
Other: Specify
Y N RF DK
Other: Specify
Y N RF DK
B13d.
On what day did you first notice soreness or drainage?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
B13e. Do you still have the soreness or drainage?
YES
NO Go to B13f
REFUSED Go to B13f
DON’T KNOW Go to B13f
B13f. On what date did the soreness or drainage end?
DATE: ______ / _____ /
200_
REFUSED
97
DON’T KNOW
98
B13g. Do you think this soreness or drainage was related to insect bites?
Y
N
RF
DK
B13h. Do you think this soreness or drainage was related to allergies?
Y
N
RF
DK
B14a. On what day did the rash or itchy skin start?
DATE: ______ / _____ /
2007
REFUSED
97
DON’T KNOW
98
10
Follow up Telephone Interview, Version 7, January 2008
B14b. Do you still have the rash or itchy skin?
YES
NO Go to B14c
REFUSED Go to B14c
DON’T KNOW Go to B14c
B14c. On what date did the rash or itchy skin end?
DATE: ______ / _____ /
200_
REFUSED
97
B14d. Where is the rash located?
Right side
Left side
Head/ Face
Y N RF DK
Head/ Face
Y N RF DK
Neck
Y N RF DK
Neck
Y N RF DK
Upper Arm
Y N RF DK
Upper Arm
Y N RF DK
Fore Arm
Y N RF DK
Fore Arm
Y N RF DK
Elbow
Y N RF DK
Elbow
Y N RF DK
Hand
Y N RF DK
Hand
Y N RF DK
Fingers
Y N RF DK
Fingers
Y N RF DK
Thigh
Y N RF DK
Thigh
Y N RF DK
Lower leg
Y N RF DK
Lower leg
Y N RF DK
Ankle/ Foot
Y N RF DK
Ankle/ Foot
Y N RF DK
Abdomen
Y N RF DK
Abdomen
Y N RF DK
Chest
Y N RF DK
Chest
Y N RF DK
Back
Y N RF DK
Back
Y N RF DK
Knee
Y N RF DK
Knee
Y N RF DK
Toes
Y N RF DK
Toes
Y N RF DK
Other: Specify
Y N RF DK
Other: Specify
Y N RF DK
B14e. Do you think this rash or itchy skin was related to insect bites?
Y
N
RF
DK
B14f. Do you think this rash or itchy skin was related to allergies?
Y
N
RF
DK
NOTE: IF ANY OF THE SYMPTOMS (B1-B14) WERE REPORTED IN THE FIELD SURVEY B OR PREVIOUS
PHONE SURVEY, THEN ASK QB15 –
B15. Have you had a 48 hour symptom free period since you reported the (symptom) during the field
survey B or previous telephone interview on (enter date)?
Y
N
11
Follow up Telephone Interview, Version 7, January 2008
RF
DK
C. You said you had [LIST OF SYMPTOMS FROM B1-B14]. We would now like to discuss how this illness or
these symptoms affected your daily activities
.
C1. Did these symptoms prevent you from performing daily activities such as school, work or
recreation?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
C2 For how long were you prevented from performing daily activities?
|__|__|__|
days (IF IN HOURS, i.e. <1 DAY, THEN CODE AS ZERO)
REFUSED
97
DON’T KNOW
98
C3.
Did you consult a healthcare provider over the phone or in person about any of these symptoms?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
If YES to C3 then ask C4-C8
C4
What illness did the health care provider say (you) had, if any?
_______________
NONE
REFUSED
97
DON’T KNOW
98
C5
Did you visit an emergency room for this illness?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
C6
Were you admitted in the hospital for this illness?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
12
Follow up Telephone Interview, Version 7, January 2008
C7
Did you receive a prescription for an antibiotic or other drug to treat this illness?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
C8
Did you use any over-the-counter medications, including things like special drinks, for these
symptoms?
YES
1
NO
2
REFUSED
97
DON’T KNOW
98
D. Now I am going to ask you a few questions about your recent water recreational activities .
D1. Since we last spoke on (Fill date of last interview) x days ago, have
you participated in any water
recreational activities
anywhere?
YES
1 D2
NO
2 Go to E1
REFUSED
7 Go to E1
DON’T KNOW
8 Go to E1
D2.
Since we last spoke on (Fill date of last interview) x days ago, on how many days have
you participated
in these water recreational activities ?
Enter number: _____________
D3. Record date of water recreational activities since last interview. Enter dates: _________________---
D4. Where did you engage in these water recreational activity/activities? Check all that apply. If Beach,
ask – Was it ocean, lake or river?
Location
Lake
Y N RF DK
If YES , ask if Lake Michigan
River
Y N RF DK
If YES , ask if Chicago River or Calumet-Sag
channel
Lagoon
Y N RF DK
If YES, ask if Skokie Lagoon
Beach
Y N RF DK
Water park
Y N RF DK
Public Pool
Y N RF DK
Private pool
Y N RF DK
Wading pool
Y N RF DK
Ocean
Y N RF DK
Other
Specify:______________
13
Follow up Telephone Interview, Version 7, January 2008
D5.
During any of these water recreational activities………
a. Did any part of your body get wet at all?
b. If YES, Did your face or head get wet?
Y N RF DK
Y N RF DK
c. Did you get any water in your mouth?
If NO, Go to D6
d. If YES, did you swallow any water?
Y N RF DK
Y N RF DK
Y N RF DK
E. I’d like to ask you about some other exposures you may have had since we last spoke [fill date of survey
or last telephone interview].
E1. Since we last spoke on [fill date of survey or last telephone interview]……………..
a. Have you touched any cat or dog?
Y N RF DK
b. Have you touched any other animals, other than cats or
dogs, including farm animals or animals at a petting zoo?
Y N RF DK
d. Have you eaten any sushi or raw shell fish (such as crab,
oyster, or mussels)?
Y N RF DK
d. Have you eaten any rare/raw/undercooked meat?
Y N RF DK
e. Have you eaten any runny or raw eggs?
Y N RF DK
f. Have you eaten a pre-packaged sandwich?
Y N RF DK
h. Have you eaten any fresh fruit, vegetables, or salad
greens?
Y N RF DK
i. Have you eaten a hamburger?
Y N RF DK
14
Follow up Telephone Interview, Version 7, January 2008
E2.
Since we last spoke [fill date of survey or last telephone interview], have you come into contact with someone
who had ……………
a. Vomiting or diarrhea?
Y N RF DK
IF YES Goto E3
b. A cold or sore throat or cough?
Y N RF DK
IF YES Goto E3
c. An eye infection?
Y N RF DK
IF YES Goto E3
E3. Were any of those contacts with people who live with you, such as family members or roommates?
Yes GOTO E3a
No GOTO F
E3a. How many of those people were enrolled in this CHEERS study?
ENTER NUMBER:____________________
E3b. And what are their names?
First and Last Name
Gender
I
RF DK
M F RF DK
RF DK
M F RF DK
RF DK
M F RF DK
RF DK
M F RF DK
15
Follow up Telephone Interview, Version 7, January 2008
F. INTERVIEWER: Reconfirm phone numbers and call back times.
F1. Which is the best phone number(s) to reach you at during this time? INTERVIEWER: COLLECT AS
MANY PHONE NUMBERS AND EXTENTIONS AS POSSIBLE.
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00
noon
Afternoon
12:01 PM-5:00 PM
other
Evening
5:01 PM- 9:00 PM
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00
noon
Afternoon
12:01 PM-5:00 PM
other
Evening
5:01 PM- 9:00 PM
Home phone
Vacation phone
cell phone
Morning
8:00 AM-12:00
noon
Afternoon
12:01 PM-5:00 PM
Evening
5:01 PM- 9:00 PM
other
EXIT STATEMENT
Note: If this is the Day 2 or Day 5 interview then ask for a date and time to schedule next telephone interview. Make sure to
inform the participant that interview must be completed within a certain period.
Note: Schedule appointment for home
visit by the nurse if any symptoms need clinical evaluation.
INTERVIEWER: Thank you for your assistance on this survey. We will call you again in about (x) days to
complete the next telephone interview. After completing the final telephone interview, you will receive a $35
check within 30 days.
At the end of day 21 telephone interview. This completes our telephone interview and your participation. I’d like to verify
your address so we can mail a check for
$35
to you. (VERIFY ADDRESS ON CALL SHEET.) You will receive your check
in 30 days. Thank you for your participation in this study. Goodbye.
F2. INTERVIEWER: Was this interview with a proxy respondent?
YES
NO
F3. INTERVIEWER: Is there anything you want to add about respondent comprehension, any issues with the
answers to specific items, or anything else that affects data quality?
YES Specify:_____________________________
NO
INTERVIEWER NAME: FIRST______________LAST________________________
16
Follow up Telephone Interview, Version 7, January 2008
PROGRAM: Home visit triggers.
If YES to 1 or more of the following symptoms –
B1. Abdominal cramps or stomach ache
Stool sample (ONLY IF THIS IS
NOT
MENSTURAL
CRAMPS)
GOTO G1
B2. Diarrhea or loose bowels
Stool sample Goto G1
B4. Vomiting
Stool sample Goto G1
B3. Nausea
Stool sample. Only if present
along with symptoms B1, B2 or
B4. OR with FEVER. Goto G1
B12. Drainage from eyes or crusting in
eyes. Please ask if there is any discharge
from the eyes? If YES, only then get a
sample. If it is only a sore area we do not
want a sample.
Eye discharge sample. Goto G2
Obtain sample only if there is any
eye discharge.
B13. Any areas on your skin that are sore
or sore or draining
Skin discharge sample. Goto G2
Decision tree for home visits and stool collection: This is followed when the survey triggers the home visit
or stool sample collection.
G1. INTERVIEWER: We will need a stool sample from you.
1.
Have you already collected a stool sample? Y N (Goto G9 )
2.
If YES, When did you collect it? (How many hours back?)
3.
Have you called the courier service to schedule a pick-up? Y N (Goto G5)
4.
If YES- Thank participant. Continue to finish survey.
5.
If participant has collected the stool sample in the past 6 hours, but not called the courier
service then- Will you be at home in the next 2 hours? Y N (Goto G8 )
6.
If YES, may I send the courier service home to pick-up the sample? Y N (Goto G8)
7.
If YES, confirm address and contact courier service to pick-up sample in the next 2 hours.
Thank participant and continue to finish the survey.
8.
If NO, Ok- will you call the courier service to set up a convenient time to pick-up the sample.
(The interviewer will have all details of the courier service and how samples need to be collected and
stored- if needed by the participant)
9.
Do you have a stool kit available? Y N (Goto G13)
10.
If YES, Do you understand how to use the kit? Y N (Goto G12)
11.
If YES, please follow the instructions on the stool kit and collect the sample. Kindly contact
the courier service using the number on the kit for a pick-up. (The interviewer will have all details of
the courier service and how samples need to be collected and stored- if needed by the participant)
12.
If NO, The interviewer will have all details of the courier service and how samples need to be
collected and stored- if needed by the participant.
17
Follow up Telephone Interview, Version 7, January 2008
13.
OK- We will send you a stool kit. After that please follow the instructions on the stool kit and
collect the sample. Kindly contact the courier service using the number on the kit for a pick-up.
END: Is there any other question or concern regarding the stool collection that I can answer? If YES,
Specify:________________________________
G2. INTERVIEWER: A CHEERS study clinician will call you to schedule a home visit. The clinician may
examine your eyes and take an eye swab, or swab your skin (depending on the symptom). Interviewer has
to confirm the address and pass on details to the CHEERS nurse.
If a participant calls in with symptoms in between their day 2, 5 and 21 survey then we follow the same
decision tree. However, in addition we collect other case details too:
1. Case ID
2. Participant First and Last Name
3. Symptom experienced
4. Start date
5. Does symptom still persist? If NO- then end date.
6. Go through G1 or G2 based on home visit or stool collection trigger.
18
QAPP 2
Appendix 8: CHEERS Pilot Study Protocol
& IRB Approval Letter
QAPP 2
Appendix 8A
CHEERS Pilot Study Protocol and IRB Approval Letter
Pilot Consent Document
Flesch-Kincaid Grade level 8.9
Leave box empty - For office use only
University of Illinois at Chicago
CONSENT FOR PARTICIPATION IN RESEARCH
“
PILOT STUDY FOR EVALUATING THE CHICAGO WATER RECREATION STUDY
FIELD AND TELEPHONE QUESTIONNAIRES”
(To be administered in English)
Why am I being asked?
The University of Illinois at Chicago (UIC) School of Public Health is planning a research study
on the health of people in the Chicago area who take part in outdoor recreation. You are being
asked to be in this pilot study because you are active in outdoor recreation in or around Lake
Michigan and the Chicago River. We ask that you read this form and ask any questions you may
have before agreeing to be in the research.
Your taking part in this research is voluntary. Your decision whether or not to be in the study
will not affect your current or future relationship with the University of Illinois at Chicago
(UIC). If you decide to be part of the research, you may withdraw at any time without affecting
that relationship.
Why is this research being done?
We want to know if the questionnaires developed for a research study are user friendly. Results
could be used for improving the quality of the questionnaires to be used in a larger study.
What is the purpose of this research?
The main purpose of this pilot study is to evaluate and refine the field and telephone
questionnaires developed for a larger study on outdoor recreators in the Chicago area.
What procedures are involved?
If you agree to be in this pilot study, we would ask you to do the following things:
¾
We will first ask you to read and sign this consent form.
2 of 5
Chicago Water Recreation Study Pilot Consent, June 2007
¾
Then we will ask you a few questions, about your current health and your recreational
activity today. This will take about 8-12 minutes.
¾
We then will ask for your feedback about the questionnaire. This will take about 10 to 15
minutes.
¾
We will ask you for your address and phone number, as we will try to contact you.
¾
We will call you 1 or 2 days from today to ask you about how you feel. This will take
about 10 minutes.
¾
We then will ask your feedback on the telephone questionnaire that will take about 10-15
minutes.
What are the potential risks and discomforts?
The research has no major physical risks or discomforts other than the time involved. You may
feel uncomfortable while answering certain questions during the survey/interview. You have the
right to refuse to answer any questions at any time. We will do the telephone follow-up at a time
that you tell us is convenient for you.
You may be concerned about how we will store/protect your data. We will protect your
confidentiality and to keep your answers private.
When the results of the research are
published or discussed in conferences, no information will be included that would reveal
your identity.
Are there benefits to taking part in the research?
There are no direct benefits to the participant from being in this pilot study.
The results from this pilot study may help future research subjects better understand the survey
questions being asked
.
Will I be told about new information that may affect my decision to participate?
During the course of the pilot study, you will be informed of any significant new findings (either
good or bad), such as changes in the risks or benefits resulting from participation in the research
or new alternatives to participation that might cause you to change your mind about continuing
in the study. If new information is provided to you, your consent to continue participating in this
study will be re-obtained.
What about privacy and confidentiality?
The only people who will know that you are a research subject are members of the research
team. No information about you, or provided by you during the research will be disclosed to
others without your written permission, except:
- If necessary to protect your rights or welfare (for example, if you are injured and need
emergency care or when the UIC Institutional Review Board monitors the research or
consent process); or
3 of 5
Chicago Water Recreation Study Pilot Consent, June 2007
- If required by law.
We respect your privacy. All computer files with identifiers will be password-protected. Any
hard copies of the forms/files will be stored in locked cabinets and will be destroyed as soon as
data collection is complete. When the results of the research are published or discussed in
conferences, no information will be included that would reveal your identity.
What if I am injured as a result of my participation?
No injury is expected to occur as a result of your participation in this research. In the event
Injury related to this research, treatment would be available through the UIC Medical Center.
However, you or your third party payer, if any, will be responsible for payment of this treatment.
If you feel you have been injured, you may contact Jacqueline Wuellner at 312-996-3395.
What are the costs for participating in this research?
There are no costs to you for participating in this research.
Will I be reimbursed for any of my expenses or paid for my participation in this research?
You will receive a total of up to $ 25 in gift certificates for participating in this research
according to the following schedule:
If you decide to participate, we will give you a $15 gift card after you have answered the
questions today.
After completing the survey and final telephone interview: a $10 check will be sent to you in the
mail.
Can I withdraw or be removed from the study?
Your participation in this research is VOLUNTARY. If you choose not to participate, that will
not affect your relationship with UIC or your right to health care or other services to which you
are otherwise entitled. If you decide to participate, you are free to withdraw your consent and
discontinue participation at any time without affecting your future care at UIC.
Who should I contact if I have questions?
The research contact for this study is Dr. Sam Dorevitch. You may ask any questions you have
now. If you have questions later, you may contact the Dr.Dorevitch at: 312-355-3629 or 312-
413-1739.
What are my rights as a research subject?
If you feel you have not been treated according to the descriptions in this form, or you have any
questions about your rights as a research subject, you may call the Office for the Protection of
4 of 5
Chicago Water Recreation Study Pilot Consent, June 2007
Research Subjects (OPRS) at 312-996-1711 (local) or 1-866-789-6215 (toll-free) or e-mail
OPRS at uicirb@uic.edu
.
Remember:
Your participation in this research is voluntary. Your decision whether or not to participate will
not affect your current or future relations with the University. If you decide to participate, you
are free to withdraw at any time without affecting that relationship. You will be given a copy of
this form for your information and to keep for your records.
Signature of Subject or Legally Authorized Representative
I have read (or someone has read to me) the above information. I have been given an opportunity
to ask questions and my questions have been answered to my satisfaction. I agree to participate
in this research. I have been given a copy of this form.
Signature
Date
Printed Name
Signature of Researcher
Date (must be same as subject’s)
Printed name of Researcher
Signature of Witness (if appropriate)
Date (must be same as subject’s)
Printed name of Witness (if appropriate)
5 of 5
Chicago Water Recreation Study Pilot Consent, June 2007
QAPP 2
Appendix 8B
CHEERS Pilot Study Protocol and IRB Approval Letter
Field Pilot Survey Evaluation Form
PILOT FIELD SURVEY EVALUATION FORM
Interviewer Name_______________________________
Participant Name _______________________________
Date of interview _______________________________
Time of interview_______________________________
1.
Were there any questions that may be confusing for other people who will take this
survey?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
2.
How difficult was it to answer all of the questions?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
3.
Is there anything that you think would be helpful to add to or take out of the
questionnaire?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Field questionnaire evaluation, June 2007
2
4.
Which questions were confusing or difficult to answer?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
5.
What do you think about the length of the questionnaire?
Notes:
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
6.
Did any of the questions make you feel uncomfortable? If so, which ones?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
7.
How would you feel if a nurse or doctor would examine your eyes, skin and ears
after you took the survey?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Field questionnaire evaluation, June 2007
3
8. What do you think would be good ways to recruit people who (mention activity of
participant) for this study?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
9.
We want to know how wet people get when they (mention the participant’s water-
recreational activity). What would be good questions to ask people in order to find
that out?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
10. Do you have any other suggestions for us when we do a research study about water
contact, outdoor recreation, and health?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Field questionnaire evaluation, June 2007
4
QAPP 2
Appendix 8C
CHEERS Pilot Study Protocol and IRB Approval Letter
Pilot Study Telephone Evaluation Form
PILOT TELEPHONE QUESTIONNAIRE EVALUATION FORM
Interviewer Name_______________________________
Participant Name _______________________________
Date of call ___________________________________
Time of call ___________________________________
1.
Were there any questions that you think may be confusing for other people who
will take this survey?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
2.
How difficult was it to answer the questions?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
3.
Is there anything that you think would be helpful to add or take out of the
questionnaire?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Telephone questionnaire evaluation form, June 2007
2
4.
Which questions were confusing or difficult to answer?
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
5.
What do you think about the amount it took to finish the interview ?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
6.
Did any of the questions about your health make you uncomfortable?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
7.
Would you have any concerns if a nurse or physician came to your home to
collect a stool sample and or examine your eyes, skin or ears if you got sick after
your activity at the lake or river?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Telephone questionnaire evaluation form, June 2007
3
8.
How would you feel about answering this telephone interview three times over a
three week period?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
9.
On the whole, would you be willing or interested in participating in the CHEERS
study?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
10. If so, how much money do you think we should pay people for their time in
answering the questions at the lake or river, and by phone?
Y N RF DK
Notes:__________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Telephone questionnaire evaluation form, June 2007
4
QAPP 2
Appendix 8D
CHEERS Pilot Study Protocol and IRB Approval Letter
Pilot Study IRB Approval Letter
Page 2 of 3
Approval Notice
Initial Review – Expedited Review
June 22, 2007
Samuel Dorevitch, MD, MPH
Environmental and Occupational Health
2121 W. Taylor St.
442 S.P.H.W., M/C 923
Chicago, IL 60612-0690
Phone: (312) 355-3629 / Fax: (312) 996-0064
RE:
Protocol # 2007-0420
“Pilot Study for Evaluating the Chicago Water Recreation Study Field and
Telephone Questionnaires”
Dear Dr. Dorevitch:
Members of Institutional Review Board (IRB) #3 reviewed and approved your research protocol
under expedited review procedures [45 CFR 46.110(b)(1) and 21 CFR 56.110(b)(1)] on June 19,
2007. You may now begin your research
Please note,
Jacqueline Wuellner’s
research training expired on 12/31/2006 and she may not
participate in the conduct of the research until an amendment (adding her key research
personnel) has been submitted and approved confirming that her continuing education
requirement has been completed. You may refer her to the OPRS website at
http://tigger.uic.edu/depts/ovcr/research/protocolreview/irb/education/continuing.shtml
where continuing education offerings are available."
Your research meets the requirement(s) for the following category - Expedited Review Approval
Category 45 CFR 46.110(b)(1) and /or 21 CFR 56.110(b)(1):
(7)
Research on individual or group characteristics or behavior (including but not limited to
research on perception, cognition, motivation, identity, language, communication, cultural beliefs
or practices and social behavior) or research employing survey, interview, oral history, focus
group, program evaluation, human factors evaluation, or quality assurance methodologies.
Please note the following information about your approved research protocol:
Protocol Approval Period:
June 19, 2007 - June 17, 2008
Approved Subject Enrollment #:
50
Page 3 of 3
Additional Determinations for Research Involving Minors:
These determinations have not
been made for this study since it has not been approved for enrollment of minors.
Performance Sites:
UIC
Sponsor:
Metropolitan Water Reclamation District of Greater
Chicago
Research Protocol(s):
a) Epidemiologic Study of Recreational Use of the Chicago Area Waterways; March 28,
2007
Informed Consent(s):
a) Chicago Water Recreation Study Pilot Consent, June_2007
Please note the Review History of this submission
:
Receipt Date
Submission Type Review Process
Review Date
Review Action
06/13/2007
Initial Review
Expedited
06/19/2007
Approved
Please remember to:
Æ
Use only the IRB-approved and stamped consent document(s) enclosed with this letter
when enrolling new subjects.
Æ
Use your
research protocol number
(2007-0420) on any documents or correspondence with
the IRB concerning your research protocol.
Æ
Review and comply with all requirements of the,
"
UIC Investigator Responsibilities, Protection of Human Research Subjects
"
Please note that the UIC IRB has the right to ask further questions, seek additional
information, or monitor the conduct of your research and the consent process.
We wish you the best as you conduct your research. If you have any questions or need further
help, please contact the OPRS office at (312) 996-1711 or me at (312) 355-2939. Please send any
correspondence about this protocol to OPRS at 203 AOB, M/C 672.
Sincerely,
Jewell Hamilton, MSW
IRB Coordinator, IRB # 3
Office for the Protection of Research Subjects
Enclosure(s):
1. UIC Investigator Responsibilities, Protection of Human Research Subjects
2. Informed Consent Document(s):
a) Chicago Water Recreation Study Pilot Consent, June_2007
cc:
Rosemary Sokas, MD, MOH, Environmental and Occupational Health, M/C 922
QAPP 2
Appendix 8E
CHEERS Pilot Study Protocol and IRB Approval Letter
Pilot Study Protocol
CHEERS Pilot Study Protocol 2007
The procedure for the pilot field testing is outlined below:
a. Participants will be enrolled at pre-determined sites along the Chicago River,
and Lake Michigan. Three groups of participants will be enrolled: 1)
recreators with water exposure at the Chicago Area Waterways (CAWs), 2)
recreators with water exposure at Lake Michigan and other general use
waterways, and 3) recreators without water exposure. We are interested in
administering the questionnaires to specific user groups such as (but not
limited to) kayakers, boaters, fishers, bikers and golfers or baseball players.
b. Approximately 50 participants will be recruited for the pilot study over a 1
week period.
c. CHEERS study personnel will approach individuals and explain the purpose
of the pilot study. Only English speaking adults (18 years or older) will be
eligible to participate in the pilot study. Interested individuals will be given a
consent form to read, and ask any questions necessary for clarification.
d. Individuals will be told that in order to participate in this pilot study they will
be required to:
•
Sign an informed consent for the pilot study (Appendix A)
•
Provide an address (so their stipend can be mailed to them) and
telephone number (for administering the telephone survey).
•
Complete an 8-12 minute questionnaire about their health, outdoor
activities and other exposures, and provide feedback on the
questionnaire. The whole process should take no more than 30
minutes.
•
Complete a 10-minute telephone questionnaire (after 1 or 2 days)
about their health after the outdoor activity, and give us some feedback
about the telephone questionnaire. The whole process should take no
more than 30 minutes.
•
Participants will be given a $15 gift card for completing the field
questionnaire and will be sent a $10 check in the mail after completing
the telephone questionnaires.
e. Following the consent process the Field Clinical Evaluation Form (described
in QAPP# 3), without the clinical assessment, Field Survey A (Appendix 5 of
QAPP 2) and Field Survey B (Appendix 6 of QAPP 2) will be administered.
The questionnaires will be administered by one CHEERS staff member, while
a second staff member will record the process by making notes on the paper
copy of the questionnaire. Questions that are followed by a long pause before
the respondent provides an answer (potentially indicating difficulties in
comprehension) will be noted. The observer will also note problems with
questions including but not limited to wording difficulties, confusing syntax,
question order, and ease of cognition. The observer will note any problems
with the administration of the questionnaire, as well as any clarifications
request by the participant. The administrators will also make notes of any
problems after the questionnaire has been completed. The total duration of
administering each survey will be recorded. The field pilot study will be
conducted using paper forms or a portable computer.
f. After the field questionnaires are administered, the participant will be asked to
evaluate any problems with the questionnaire using the field pilot survey
evaluation form (Appendix B). Specific questions will be asked to direct the
discussion. These questions, listed below, are not strict guidelines and should
facilitate discussion of the questionnaire. Researchers will be instructed to
allow subjects to converse freely about the questionnaire while covering
topics of difficulty, appropriateness, and areas of potential improvement of the
questionnaire.
g. At the end of the discussion participants will be given a $ 15 gift card for a
coffee shop or supermarket. The administrator will also make a note of the
best time to call in the next two days for telephone follow-up.
h. A CHEERS staff member will call the participant within 2 days of their field
interview. The day and time will be based on the convenience and availability
of the participant. The CHEERS member will administer a 10 minute
Telephone Follow-up Interview (Appendix 7 of QAAP 2). After completing
the interview participants will be asked to evaluate any problems with the
questionnaire. Specific questions, outlined in the field telephone interview
evaluation form (Appendix C) will be asked to prompt conversation. These
questions are not strict guidelines and are designed to promote discussion of
the questionnaire. Researchers will be instructed to allow subjects to converse
freely about the questionnaire while covering topics of difficulty,
appropriateness, and areas of potential improvement of the questionnaire.
i. At the end of the telephone interview participants will be thanked for their
time and told that a $10 check will be mailed to them within the next 2 weeks.
j. All data collected in the field and telephone interviews will be stored in a
locked file cabinet.
k. Evaluation of pilot data: The SPM will develop a list questions that were
associated with long pauses prior to response, clarifications requested by
participants, and responses to the evaluation questions that followed the
administration of the questionnaires. The SPM will also meet with CHEERS
personnel who conducted the pilot interviews within two days of the each
interview to discuss their impressions. Following the last pilot interview, the
SPM will present a summary of findings to the SRL project manager, staff
who participated in the pilot, and other CHEERS key personnel.
Opportunities to improve the clarity of the questionnaires, and the
incorporation of new questionnaires based on respondent suggestions will be
discussed.
l. Time-table for pilot study: The pilot study will be conducted by the SPM and
other CHEERS staff trained in the use of the BLAISE/CAPI software. The
evaluation meeting will take place in the third week of July, and an
amendment to the CHEERS study IRB protocol will be submitted listing these
modifications.
These changes will also be submitted to SRL for
programming. These questionnaires will only be used in the field study
following IRB approval.
m. Locations: The pilots study will be conducted at various Lake Michigan and
Chicago River locations. These sites and dates for conducting the pilot survey
have been selected to target specific groups of recreators: for example, CAWs
boaters at the Worth launch, paddlers at Clark Park, lake fishers and boaters at
Jackson Park Harbor and Montrose Harbor, and lake kayakers at Wilson
Beach. CAWs fishers are expected to be recruited at the River Park/Honan
Park site. Unexposed recreators engaging a variety of activities are found all
of the above locations.
n. Human research subjects protections: All members of CHEERS pilot study
team will be IRB trained/certified. The protocol will not be implemented until
IRB approval is obtained (Appendix D). All hard copies of forms with
identifiers will be stored in locked cabinets. All personal identifiers will be
removed from the dataset and destroyed following the completion of the pilot
study. Participants will not be identified individually in any of the discussions
or reports of this pilot study.
o. Staff: The field coordinator will have overall responsibility for identifying
sites and times to start the pilot study. The SPM will be responsible for
training staff to administer the questionnaire using the CAPI system, to
perform the pilot study evaluation, and to record findings on the pilot study
field data collection sheet. Staff members will work in groups of two or more.
p. The telephone pilot survey will be conduct at UIC using a computer
programmed in BLAISE for CATI, by landline telephone.
QAPP 2
Appendix 9
CAWs Use Survey Data Sheet
CAWs Use Survey Data Sheet
Page__of________
1. Location: _____________________________
2. Date:
______________
(MM / DD / YY)
3. CHEERS Staff Names:____________________
_______________________________________
3. Start Time of observation: ________________
(AM / PM)
4. Stop Time of observation: ________________
(AM / PM)
Tally of beginning of activity (launch, entry). If present at time of start of observation, then circle the tally mark.
Boating
Canoeing
Diving/
jumping
Fishing
stationary
Jet
skiing
Rowing
Sailing
Swimming
Tubing
Wading
Water
skiing
Other
(indicate)
__:00
__:10
__:20
__:30
__:40
__:50
Weather Conditions:
Cloud Cover
100%
50%
10%
0%
Precipitation Present
Heavy
Light
Mist
Clear
Precipitation 24 Hour
Heavy
Light
Mist
Clear
Precipitation 24 Hour
Heavy
Light
Mist
QAPP 2
Appendix 10
IRB Exemption for Use Survey
2007-0386
Page 2 of 4
June 8, 2007
Exemption Granted
June 8, 2007
Samuel Dorevitch, MD, MPH
Environmental and Occupational Health
2121 W. Taylor St.
442 S.P.H.W., M/C 923
Chicago, IL 60612-0690
Phone: (312) 355-3629 / Fax: (312) 996-0064
RE:
Research Protocol # 2007-0386
“Recreational Use Profile of the Chicago Area Waterways”
Dear Dr. Dorevitch:
Your Claim of Exemption was reviewed on June 7, 2007 and it was determined that your
research protocol meets the criteria for exemption as defined in the U. S. Department of Health
and Human Services Regulations for the Protection of Human Subjects [(45 CFR 46.101(b)].
You may now begin your research
The specific exemption category under 45 CFR 46.101(b) is:
(2) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures,
interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a
manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any
disclosure of the human subjects' responses outside the research could reasonably place the subjects at risk of
criminal or civil liability or be damaging to the subjects' financial standing, employability, or reputation.
You are reminded that investigators whose research involving human subjects is determined to
be exempt from the federal regulations for the protection of human subjects still have
responsibilities for the ethical conduct of the research under state law and UIC policy. Please be
aware of the following UIC policies and responsibilities for investigators:
1. Amendments You are responsible for reporting any amendments to your research protocol
that may affect the determination of the exemption and may result in your research no
longer being eligible for the exemption that has been granted.
2. Record Keeping You are responsible for maintaining a copy all research related records in
a secure location in the event future verification is necessary, at a minimum these
documents include: the research protocol, the claim of exemption application, all
questionnaires, survey instruments, interview questions and/or data collection instruments
2007-0386
Page 3 of 4
June 8, 2007
associated with this research protocol, recruiting or advertising materials, any consent
forms or information sheets given to subjects, or any other pertinent documents.
3.
Final Report When you have completed work on your research protocol, you should
submit a final report to the Office for Protection of Research Subjects (OPRS).
4.
Information for Human Subjects UIC Policy requires investigators to provide information
about the research protocol to subjects and to obtain their permission prior to their
participating in the research. The information about the research protocol should be
presented to subjects in writing or orally from a written script. When appropriate, the
following information must be provided to all research subjects participating in exempt
studies:
a. The researchers affiliation; UIC, JBVMAC or other institutions,
b. The purpose of the research,
c. The extent of the subject’s involvement and an explanation of the procedures to be
followed,
d. Whether the information being collected will be used for any purposes other than the
proposed research,
e. A description of the procedures to protect the privacy of subjects and the
confidentiality of the research information and data,
f. Description of any reasonable foreseeable risks,
g. Description of anticipated benefit,
h. A statement that participation is voluntary and subjects can refuse to participate or can
stop at any time,
i. A statement that the researcher is available to answer any questions that the subject
may have and which includes the name and phone number of the investigator(s).
j. A statement that the UIC IRB/OPRS or JBVMAC Patient Advocate Office is available
if there are questions about subject’s rights, which includes the appropriate phone
numbers.
Please be sure to:
ÆUse
your research protocol number (listed above) on any documents or correspondence with
the IRB concerning your research protocol.
We wish you the best as you conduct your research. If you have any questions or need further
help, please contact me at (312) 355-2908 or the OPRS office at (312) 996-1711. Please send any
correspondence about this protocol to OPRS at 203 AOB, M/C 672.
Sincerely,
Charles W. Hoehne
Assistant Director, IRB # 2
Office for the Protection of Research Subjects
Enclosure(s): None
cc:
Rosemary Sokas, MD, MOH, Environmental and Occupational Health, M/C 922
2007-0386
Page 4 of 4
June 8, 2007
QAPP 2
Appendix 11
IRB Approval for Survey Methods Protocol
Page 2 of 4
Approval Notice
Initial Review – Expedited Review
June 26, 2007
Samuel Dorevitch, MD, MPH
Environmental and Occupational Health
2121 W. Taylor St.
442 S.P.H.W., M/C 923
Chicago, IL 60612-0690
Phone: (312) 355-3629 / Fax: (312) 996-0064
RE:
Protocol # 2007-0436
“Epidemiologic Study of Recreational Use of the Chicago Area Waterways”
Dear Dr. Dorevitch:
Members of Institutional Review Board (IRB) #3 reviewed and approved your research protocol
under expedited review procedures [45 CFR 46.110(b)(1) and 21 CFR 56.110(b)(1)] on June 22,
2007. You may now begin your research.
Your research meets the requirement(s) for the following categories - Expedited Review Approval
Category 45 CFR 46.110(b)(1) and /or 21 CFR 56.110(b)(1):
(3)
Prospective collection of biological specimens for research purposes by noninvasive means.
(7)
Research on individual or group characteristics or behavior (including but not limited to
research on perception, cognition, motivation, identity, language, communication, cultural beliefs
or practices and social behavior) or research employing survey, interview, oral history, focus
group, program evaluation, human factors evaluation, or quality assurance methodologies.
Please note the following information about your approved research protocol:
Protocol Approval Period:
June 22, 2007 - June 20, 2008
Approved Subject Enrollment #:
9330
Additional Determinations for Research Involving Minors:
These determinations have not
been made for this study since it has not been approved for enrollment of minors.
Performance Sites:
UIC
Sponsor:
Metropolitan Water Reclamation District of Greater
Chicago
Research Protocol(s):
a) Epidemiologic Study of Recreational Use of the Chicago Area Waterways; March 28,
2007
Page 3 of 4
Recruitment Material(s):
a) "Be part of a research study about water quality and your health!" Flyer; CHEERS
ver.1_June 18, 2007
Informed Consent(s):
a) CHEERS (Parental Permission), Ver. 1, June 18, 2007
b) CHEERS (Consent), Version. 1, June 18, 2007
Assent(s):
a) CHEERS_assent_ver.1 June 18, 2007
Please note the Review History of this submission
:
Receipt Date
Submission Type Review Process
Review Date
Review Action
06/18/2007
Initial Review
Expedited
06/22/2007
Approved
Please remember to:
Æ
Use only the IRB-approved and stamped consent document(s) enclosed with this letter
when enrolling new subjects.
Æ
Use your
research protocol number
(2007-0436) on any documents or correspondence with
the IRB concerning your research protocol.
Æ
Review and comply with all requirements of the,
"
UIC Investigator Responsibilities, Protection of Human Research Subjects
"
Please note that the UIC IRB has the right to ask further questions, seek additional
information, or monitor the conduct of your research and the consent process.
We wish you the best as you conduct your research. If you have any questions or need further
help, please contact the OPRS office at (312) 996-1711 or me at (312) 355-2939. Please send any
correspondence about this protocol to OPRS at 203 AOB, M/C 672.
Sincerely,
Jewell Hamilton, MSW
IRB Coordinator, IRB # 3
Office for the Protection of Research Subjects
Enclosure(s):
1. UIC Investigator Responsibilities, Protection of Human Research Subjects
2. Informed Consent Document(s):
a) CHEERS (Parental Permission), Ver. 1, June 18, 2007
b) CHEERS (Consent), Version. 1, June 18, 2007
3. Assent Document(s):
a) CHEERS_assent_ver.1 June 18, 2007
4. Recruiting Material(s):
a) "Be part of a research study about water quality and your health!" Flyer;
CHEERS ver.1_June 18, 2007
Page 4 of 4
cc:
Rosemary Sokas, MD, MOH, Environmental and Occupational Health, M/C 922
QAPP 2
Appendix 12
Thermal Wristband Barcoding Technology
1
Zebra Wrist Band Solutions
Web Link :http://healthcare.infologixsys.com/products/Products/Barcode/Patient-
Identification-Wristbands/Zebra-Wrist-Band-Solutions/default.asp
A premium direct thermal wristband that provides automated ID verification for
hospitals and other facilities seeking to boost efficiency and accuracy. Wrist band labels
are also available with UV varnish, which provides excellent chemical resistance for
printed information. Thermal wristbands are ideal for any environment where the
accuracy of information is critical to providers.
Zebra wristbands and thermal printers are designed to perform together as a
complete, optimal bar code wristband print solution -- ensuring the highest quality results
for uncompromised patient safety. Easy to operate and load, Zebra's all-in-one solution
makes it extremely simple for anyone to quickly create legible, accurate, reliable bar
coded wristbands. As a result, CHEERS team members can quickly print Case ID
wristbands for each subject with minimal instruction.
2
Zebra Z-Band Direct Wristbands
•
Direct thermal printing
•
Clinically proven antimicrobial coating
•
Pressure-sensitive adhesive tab
•
Six color options
•
Three sizes: adult, pediatric and infant
Z-Band QuickClip Wristbands
•
Direct thermal printing
•
Clinically proven antimicrobial coating
•
Secure, clip closure
•
Antimicrobial coating
•
Six color options
•
Two sizes: Adult (1 3/16" x 11") and infant (1" x 7")
The Z-Band Zebra direct thermal wristbands feature an inorganic ionic silver coating that
kills microorganisms that come into contact with it. As the silver ions are taken into the
microorganisms, they react and bond to the cellular enzyme. This inhibits the
microorganism enzyme activity and multiplication, which eliminates them.
Zebra’s antimicrobial coating has been clinically proven to prevent the growth and
survival of the following microorganisms on patient id wristbands:
•
S. aureus
•
P. aeruginosa
•
E. coli
Z-Band Zebra Direct Thermal Wristbands
•
Priners are easy to use
: within seconds you can print and secure the patient
identification wristband.
•
Highly durable
: they’re specially coated to resist water, soaps, chemicals and
other elements so the printed bar codes remain crisp and scannable for up to 14
days. Zebra patient wristbands are scannable after repeated exposure to water and
common solvents, including isopropyl alcohol, ethyl rubbing alcohol, Betadine,
and Purell.
•
Zebra patient wristbands offer superior tensile strength.
•
Zebra patient wristbands are unlikely to cause skin irritation.
3
Benefits with Zebra Patient Identification
Designed to work together to create a complete bar coded wristband solution, Zebra's
direct thermal printers and Z-Band direct thermal wristbands with antimicrobial coating:
•
Produce bar codes that remain legible and scannable for the duration of a subjects
participation in the field surveys.
•
Significantly reduce errors by automating access to accurate participant
information.
•
Satisfy participant safety and privacy standards set by IRB.
Zebra Barcode Printer - Model S4M
•
Affordable choice for high-volume wristband printing
•
Full 8" wristband roll capacity
•
Wide range of connectivity options
Zebra Barcode Thermal Printer - Model H 2824-Z
•
Economical and compact
•
Print on a variety of patient id wristband types and sizes
4
QAPP 2
Appendix 13
Survey Methods Field Report
CHEERS
Field Progress Report/Unusual Occurrence Report for Data Managers
Location:
Date:
Time:
Staff Members:
Shift 1:
Shift 2:
Data:
Consent
Forms
Field Survey A
Field Survey B
1. Equipment and Supplies Issues: (battery/scanner issues, start up etc)
2. Data Transfer Issues:
Case ID range given out today-
Laptop numbers used in the field today-
Data downloaded from all laptops: YES
NO
3. Interviewer Issues: (absenteeism, non-compliance etc)
_________’W’ did not show up on time.
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
__________________________________________________
Was time sheet completed? YES
NO
Was field supervisor informed about any interviewer issues? YES NO
4. Participant Issues: (Lost wrist bands, incomplete surveys etc)
John Doe lost his wrist band. We looked up his consent form and completed survey B.
Case ID 200902 and 200967 did not return to complete survey B.
5. Site Factors: (weather, set up site etc)
QAPP 2
Appendix 14: Field Maps of Recruitment Areas
QAPP 2
Appendix 14A
Maps of Recruitment Areas
Alsip Boat Launch
Alsip Boat Launch – CAWS
Tent & table set-up
(tack symbol)
:
1. Near Boat Launch and parking lot
**CAWS Use Survey Required**
Mobile recruiting: See circled area for recruiting area – use mobile unit as necessary.
Site Permission: Cook County Right of Entry Agreement (in emergency about site
permission, contact Vito Benignole at 708-906-1561 (cell))
Teams/Clubs: None
General Target Participants:
•
Boaters
•
Fishermen
•
Bikers, walkers, runners and other non-water recreators
QAPP 2
Appendix 14B
Maps of Recruitment Areas
Belmont Harbor
Belmont Harbor – GUW
Tent & table set-up
(tack symbol)
:
1. 41/N. Lake Shore to parking lot on North side of harbor
2. Belmont Ave. to parking lot on South side of harbor
Mobile recruiting: See circled areas. Mobile recruiting necessary from each tent location.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
General Target Participants:
•
Boaters (not sailors)
•
Fishermen
•
Bikers, walkers and runners and other non-water recreators
QAPP 2
Appendix 14C
Maps of Recruitment Areas
Burnham Harbor
Burnham Harbor - GUW
Tent & table set-up
(tack symbol)
:
1. Near parking lot and paths
Mobile recruiting: See circled areas. Area on other side of harbor also possibility if there
are enough staff and someone is willing to walk all the way over there.
NOTE: Data manager – get 1 extra mobile recruiting folder from supply manager.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: None
General Target Participants:
•
Fishermen and boaters
•
Bikers, runners and other non-water recreators
QAPP 2
Appendix 14D
Maps of Recruitment Areas
Busse Lake Boating Center
Busse Lake Boating Center - GUW
Tent & table set-up
(tack symbol)
:
1. Near boat rental facility
Mobile recruiting: See circled areas.
NOTE: Data manager – get 1 extra mobile
recruiting folders from supply manager.
Site Permission
: Cook County Right of Entry Agreement (in emergency about site
permission, contact Vito Benignole at 708-906-1561 (cell))
General Target Participants:
•
Boaters, paddlers and rowers
•
Fishermen
•
Bikers, walkers and runners and other non-water recreators
QAPP 2
Appendix 14E
Maps of Recruitment Areas
Chicago Dragon Boat Race
Chicago Dragon Boat Race – CAWS
(
Ping Tom Park, 300 W. 19
th
St., Sat. 7/26)
Tent & table set-up
tack symbol)
:
1. On grass near main pavilion – location is flexible as long as it is in a visible place.
**CAWS Use Survey Required**
Supply Manager:
No vehicles are allowed on the park site between 7:00am - 4:00pm. All unloading of
equipment by car must be done strictly before 7:00am
on the day of the races. There
is only one entrance into the park and this pathway is relatively narrow. To navigate
vehicles whilst there is on foot traffic has been seen in the past to be incredibly dangerous
and hazardous. As a direct result of this, the Chicago Police is strongly enforcing this
rule this year. In addition, on the day of the event the park is still open to the general
public therefore increasing the amount of pedestrians in the park on this day. However,
should you wish to unload your equipment on foot and utilize hand trolleys, you are
welcome to do so. The event is scheduled to finish at approximately 4:00pm and again,
no vehicles are allowed before this time.
Parking:
In terms of parking facilities in Chinatown, there is street and metered parking as well as
2 Chinatown Parking Lots, both located on Wentworth Avenue (between 19th St. and
Cermak Road). Parking between 3-12 hours costs $16, whilst 12-24 hours costs $25. I
would strongly recommend you arrive early as possible as previous years have proven
that parking fills up by 9:00am. Parking in general on most days here in Chinatown is
limited, and it will be even more so on the day of the event. You have been warned!
Mobile recruiting: None. No extra mobile recruiting folders necessary.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office), and Chicago Dragon
Boat Race/ Chinatown Chamber of Commerce.
Teams/Clubs: None
General Target Participants:
•
Paddlers –
Dragon boat racers
•
Bikers, walkers, runners and other non-water recreators
QAPP 2
Appendix 14F
Maps of Recruitment Areas
Chicago Sprints
Chicago Sprints @ Lincoln Park Boat Club - GUW
Tent & table set-up: Near registration/ check-in tent (see LPBC staff for directions).
Time: 7:30am – 7pm
Parking: Supply manager –drop off supplies and park on street, either Canon or
Stockton. If you don't mind paying the zoo fees, just park in the zoo lot by the boathouse.
NOTE: let coaches and others who check in know about the study, they'll be the ones who
decide whether and when to send their athletes to you.
Mobile Recruiting: May be useful if team members do not want to come to table.
Site Permission: LPBC – Steve Neumann
Teams/Clubs: Lincoln Park Boat Club
Target Participants:
•
Rowers involved with the Chicago Sprints tournament –
BE CAREFUL with
ELIGIBILTY!
•
Non water
recreators
in vicinity
QAPP 2
Appendix 14G
Maps of Recruitment Areas
Clark Park
Clark Park – CAWS (
3400 N. Rockwell St., Chicago IL 60618)
Tent & table set-up
tack symbol)
:
1. Near Chicago River Canoe & Kayak rental facility
**CAWS Use Survey Required**
Mobile recruiting: See circled areas. No extra mobile recruiting folders necessary.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: None
General Target Participants:
•
Paddlers
•
Fishermen
•
Bikers, walkers, runners and other non-water recreators
QAPP 2
Appendix 14H
Maps of Recruitment Areas
Crystal Lake
Crystal Lake - GUW
Recruiting sites: Boat launch
Site Permission: Crystal Lake Rowing Club, contact Arne Arnesen, (815) 356-9215.
Teams/Clubs: Crystal Lake Rowing Club – only participants recruiting.
Recruiting Method: Tent and table set-up. No mobile recruiting necessary.
QAPP 2
Appendix 14I
Maps of Recruitment Areas
Diversey Harbor
Diversey Harbor - GUW
Tent & table set-up
(tack symbol)
:
1. Northern boat launch
Mobile recruiting: See circled areas. No extra mobile folders needed.
Site Permission
: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs
: none
General Target Participants:
•
Boaters (no sailboats)
•
fishermen
•
Bikers, walkers and runners and other non-water recreators
QAPP 2
Appendix 14J
Maps of Recruitment Areas
Lake Arlington
Lake Arlington – Sea Kayak Extravaganza - GUW
Tent & table set-up
(tack symbol)
:
1. In between both boat docks
Mobile recruiting: See circled area.
Site Permission: Lake County Forest Preserve Right of Entry Agreement.
Clubs/Teams: The Northwest Passage – Contact: Nancy Vedder.
General Target Participants:
•
Paddlers, rowers and boaters
•
Fishermen
•
Bikers, walkers and runners and other non-water recreators
QAPP 2
Appendix 14K
Maps of Recruitment Areas
Leone Beach
Leone Beach - GUW
Tent & table set-up
(tack symbol)
:
1. Next to Kayak Chicago rental facility at beach
Mobile recruiting: At DM’s discretion.
Site Permission
: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs:
None
General Target Participants:
•
Kayakers from
Kayak Chicago
rental facility
•
Fishermen
•
Non-water recreators at park south of rental facility – baseball diamonds and
basketball court
QAPP 2
Appendix 14L
Maps of Recruitment Areas
Lincoln Park Boat Club
Lincoln Park Boat Club - GUW
Tent & table set-up (
tack symbol)
:
1. Near Lincoln Park Boat Club boathouse - lagoon is between Lake Shore Drive and
Cannon Dr.
Mobile Recruiting: See circled areas. No extra mobile recruiting folders necessary.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: Lincoln Park Boat Club – events, classes, recreators. Contact: Steve
Neumann.
General Target Participants:
•
Paddlers and rowers at the boat club –
make sure to go through eligibility
screening!!
•
Bikers, walkers, runners and other non-water recreators
QAPP 2
Appendix 14M
Maps of Recruitment Areas
Mayor Daley’s Fishing Festival
Mayor Daley’s River Fishing Festival – CAWS
Tent & Table set-up
(tack symbol)
:
1. N. Columbus & East Wacker.
Directions for SM: Go Roosevelt to Columbus drive (just east of state and west of LSD). Go north on
Columbus. You'll drive underground at Randolph (near Frank Ghery Band Shell), keep going north (you're
now on lower columbus). Look out for 'CENTRAL AUTO POUND' signs, they will help to guide you. Pass
Lake. Take a left at 'South Water'... this is where it gets tricky. You want to turn onto the part of the street
that looks like a ramp heading downward (on the map it is called the 'service level' or something). Drive
down the ramp. Stetson should be the next street. Take a right. You're now on the 'service level' it is one
level below both lower wacker and lower columbus. Stetson deadends at the Wacker Service level. Take a
right. Go straight past the Columbus service level street and head straight for the autopound at the very end
of the street (if you look to your left, you'll see a gap in the concrete/fence that opens to the bikepath and a
grassy knoll w/ bike rental place -- that's your goal). The street (Wacker Service level) does a U-turn @
autopound, follow the turn. Pull up in front (or near) the gap in the gate/concrete. Look for your team...
unload... have a great CHEERS day.
**CAWS Use Survey Required**
Site Permission: Chicago Park District Right of Entry Agreement. In emergency concerning park officers,
contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: None
General Target Participants:
•
Fishermen
•
Non-water okay but not priority
Mobile recruiting ESSENTIAL! DM – get 2 extra mobile folders from SM
QAPP 2
Appendix 14N
Maps of Recruitment Areas
Montrose Beach
Montrose Beach - GUW
Tent & table set-up
(tack symbol)
:
1. Next to Kayak Chicago rental facility at beach
2. Near harbor – ONLY TABLES & CHAIRS IF AVAILABLE
Mobile recruiting: See circled areas.
NOTE: Data manager – get 2 extra mobile
recruiting folders from supply manager.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: None
General Target Participants:
•
Kayakers from
Kayak Chicago
rental facility
•
Fishermen and boaters at north and south sides of harbor
•
Bikers, runners and other non-water recreators
QAPP 2
Appendix 14O
Maps of Recruitment Areas
North Ave. at Kingsbury
North Ave. @ Kingsbury – CAWS
Tent & table set-up
(tack symbol)
:
1. On pavement/sidewalk down the hill past the parking lot by Old Navy.
**CAWS Use Survey Required**
Mobile recruiting: No mobile recruiting necessary. See circled area for general recruiting.
Site Permission: Private property, we’re there at the invitation of the rowing club.
Contact: Mike Wallin – head coach.
Teams/Clubs:
•
Lincoln Park Juniors (LPJ)
General Target Participants:
•
Rowers on LPJ team
•
Non-water recreators (sometimes rowers go to a gym to work out during practice
– they are still eligible as unexposed)
QAPP 2
Appendix 14P
Maps of Recruitment Areas
North Ave. at Magnolia
North Ave. @ Magnolia – CAWS
Tent & table set-up
(tack symbol)
:
1. On pavement/sidewalk outside of kayak/rowing facility (unless otherwise given
permission to come inside gates).
**CAWS Use Survey Required**
Mobile recruiting: Mobile recruiting not necessary. See circled area for general
recruiting.
Site Permission: Metropolitan Water Reclamation District
Teams/Clubs:
•
Kayak Chicago – rental facility & tours (contact: Dave Olsen)
•
University of Chicago
•
Ignatius Chicago Crew
•
Lincoln Park Boat Club men’s and women’s rowing teams (contact: Steve
Neumann)
General Target Participants:
•
Rowers and paddlers
•
Non-water recreators
QAPP 2
Appendix 14Q
Maps of Recruitment Areas
Skokie Lagoons
Skokie Lagoons - GUW
Tent & table set-up (
tack symbol)
:
1. Tower Rd. Boat launch
Mobile recruiting: See circled areas. No extra mobile recruiting folders necessary.
Site Permission: Cook County Right of Entry Agreement (in emergency about site
permission, contact Vito Benignole at 708-906-1561 (cell))
Teams/Clubs: Chicago Kayak Club, Chicago River Canoe & Kayak, and The Northwest
Passage.
NOTE: Classes given by Chicago Kayak and The Northwest Passage teach
rolling/rescuing; therefore, those people are not eligible for the study.
General Target Participants:
•
Kayakers and canoers put in at the boat launch, along with some boaters who are
usually fishing off their boats.
•
Fishermen need to be searched for – they are not usually near the boat launch.
•
Bikers – more of a priority here than at other sites, but all non-water recreators
eligible
QAPP 2
Appendix 14R
Maps of Recruitment Areas
Skokie Rowing Center
Skokie Rowing Center – CAWS
3220 Oakton St., Skokie IL 60076 (aka Dammrich Rowing Center)
Tent & table set-up
(tack symbol)
:
1. Next to pavilion where Chicago R. Canoe & Kayak sets up.
DO NOT
set up under the pavilion.
**CAWS Use Survey Required**
Mobile recruiting: See circled area. No extra folders needed, but recruit non-water recreators in rest of
the park north of tent.
Site Permission: Village of Skokie
Teams/Clubs:
•
Chicago River Canoe & Kayak has a rental facility (open 10am – 6pm) and hosts classes Sat. &
Sun. 10am and 1pm. Contact: Ryan Chew.
•
Northwestern University rowing club
•
North Park University Vikings (women’s crew),
note: cannot accept incentives
•
North Park University men’s club
•
New Trier High School Rowing
•
Loyola Academy – students
DO NOT
have permission to participate in study
•
Woodlands Academy – we do not have any agreement with them
General Target Participants:
•
Paddlers and rowers
•
Fishermen (occasionally)
•
Bikers, walkers and runners are okay but not priority.
QAPP 2
Appendix 14S
Maps of Recruitment Areas
Solidarity Drive
Solidarity Drive - GUW
Tent & table set-up
(tack symbol)
:
1. On E. Solidarity Dr. in between Aquarium and Planetarium
OR
2. In front of Aquarium
Mobile recruiting: See circled areas. There is a lot of ground to cover so do what you can
with the staffing available.
NOTE: Data manager – get 2 extra mobile recruiting folders from supply manager.
Site Permission: Chicago Park District Right of Entry Agreement. In emergency
concerning park officers, contact Brian Loll, 773-761-8674 (office).
Teams/Clubs: None
General Target Participants:
•
Fishermen and boaters
•
Bikers, runners and other non-water recreators
QAPP 2
Appendix 14T
Maps of Recruitment Areas
Tampier Lake
Tampier Lake Boating Center - GUW
Tent & table set-up
(tack symbol)
:
1. Next to boat launch
Mobile recruiting: See circled areas. No extra mobile recruiting folders needed.
Site Permission: Cook County Right of Entry Agreement (in emergency about site
permission, contact Vito Benignole at 708-906-1561 (cell))
Teams/Clubs: None
General Target Participants:
•
Kayakers and canoers
•
Fishermen and boaters
•
Non-water recreators
QAPP 2
Appendix 14U
Maps of Recruitment Areas
Worth Boat Launch
Worth Boat Launch – CAWS
Table & tent set-up
(tack symbol)
:
1. Near Boat Launch
**CAWS Use Survey Required**
Mobile recruiting: See circled areas.
NOTE: Data manager – get 1 extra mobile
recruiting folder from supply manager.
Site Permission: Cook County Right of Entry Agreement (in emergency about site
permission, contact Vito Benignole at 708-906-1561 (cell))
Teams/Clubs: None
General Target Participants:
•
Boaters
•
Fishermen by aeration SEPA site
(star marker)
•
Bikers, walkers, runners and other non-water recreators
QAPP 2
Appendix 15:
Location Specific Flier for Lake
IRB approval
box
$TAR?$A
F F ffi
S VA
L
sxprnes
JUN
?
'1
?ilijfi
JUN
2
0
Z00g
UitllV#i$;Ti
$F
tLr-rr{{Jlti
fft
CH|CAG0
lfil$Tt'ru
ri$$,rAL
i{EVt
Ew
g0AR0
?#nrt{*^ft
{ft{{&##,
-$&#ffi:{#'
PLEASE
NOTE:
THE
CHEERS
STUDY
IS
IN
PROGRESS
TODAY
AT
THE LAKE
As
part
of our
efforts
to improve
public
health,
the
lJniversity
of
Illinois
at Chicago
(UIC)
School
of
pubiic
Health
is conducting
a
three-year
study
called
"CHEERS,"
'lhe
Chicago,
Health.
Environmental
Exposure
and
Recreation
Stucly.
In this
study,
researchers
will evaluate
the
health
of
people
who exercise
o.,i.Jc,orr.
some
who have
water
contact,
and some
who
do
not.
This
research
will take
place
on
different
clays,
at dif-ferept
locations
on or
near
Lake
Michigan,
the Chicago
and
Calumet
River
systems'
and
other
rivers
apd
lagoons
in the
Chicagg
area.
If
you
participate
in
two short
survey
interviews
today,
you
will
receive
a
$15
gift card
and
a
T-shirt
today.
After
completing
three
10
minute
telephone
follow-
up
intervier",
on.i
the
next
three
weekso
a
check
for
$35
check
wilt
be
mailed
to
you
after
your last
home
telephone
interview.
If
you
are
selected
for
a
follow-up
home
visit
by
research
nurses'
you
will
receive
a
check
for
an
additional
$75.
purpose
of
the Survey.
Toclay's
survey
is
part
of a
research
project that
will
help
to better
understand
the
ielationship
between
outdoor
recreation,
water
quality, and,
people's
health'
When
the
research
project
is completed,
the
results
will
help
develop
better
guidelines
for recreational
water
quality.
your
Cooperation
is
Extremely
Valuable
and
Will
be
Greatly
Appreciated.
of
course,
your
participation
in
today's
survey
is
voluntary
-
whether
you
are
interviewed
today
is entirely
up
to
you' All
ihe
int'brmation
that
you
provide
will
be
kept
confidential.
what
to
Expect
Today.
All
interviewers
are
wearing
CHEERS
t-shirts
and
name
badges.
If
you
are
eligible.
statf
will
interview
your for
about
3
minutes.
The
second
part
of
the
interview
will be
conducted
afti,
yo.r finish
your outdooi
activity
today-
This
will
take
about
8-
10
rninutes'
When
you
go home.
We
will
contact
you
three
times
over
the
next
three
weeks.
to ask
you
about
your
health.
on
completion
of
the
last
telephone
interview,
we
will
send
you
a
$35
check.
If
you
are
selected
fbr
a
home
visit.
we may
request
a
stool
sample,
or
research
staff
may
visit
you at
home'
If
you were
to
get sick.
those
results
couid
irelp
you
and
your doctor
by
identifying
germs that
may
have
made
you
ill'
you
would
receive
an
additional
check
for
$75
for
the
home
visit'
Local
Support
for
GHEERS:
Several
cities,
including
chicago,
Evanston,
Skokie,
worth.
and
Alsip
are
helpir-rg
ulC
with this
project.
For
any
questions
call
the
CHEERS
project coordinator
at
(312)
99G2094'
wE
THANK
yoil
rN
ADVANCE
FoR
YouR
TIME
AND
cooPERATIoN!!
#tr'ffi
CHEERS
flyer,
Lake,
Versioti
1,112312008
QAPP 2
Appendix 16: Adult Consent Form
The purpose of asking you questions is to learn more about any illness you may experience and
how you were exposed. It will take 8-10 minutes or less to complete. Answering the
questionnaire is voluntary. You may choose not to answer any question for any reason
In addition, some participants will be selected for a home visit by study staff. If you are selected,
this could involve collecting a sample (such as a stool sample or a “pink eye” sample) so the
laboratory can analyze any infection you may have. If a stool sample is required, we’ll need to
collect 3 samples over a one week period. The purpose of getting stool samples is to test for
bacteria, viruses, and parasites. This testing involves no risk to you. We will store your specimen
indefinitely and potentially test the samples for other microbes, or for chemicals related to health
or the environment, in the future.
What are the potential risks and discomforts?
The research has no major physical risks or discomforts other than the time involved and the
potential loss of privacy during the follow-up home visit. If you undergo the study nurse’s
examination of your eyes, ears or skin, you may feel a little uncomfortable, while you are being
checked. You may feel a little uncomfortable while answering certain questions during the
survey/interview. You have the right to refuse to answer any questions at any time. If you are
selected for a home visit, we will call to set up an appointment based on your convenience before
we make the visit.
You may be concerned about how we will store/protect your data. We will do our best to protect
your confidentiality and to keep the results of any test (in case we ask you for stool or eye/skin
discharge samples) private.
When the results of the research are published or discussed in
conferences, no information will be included that would reveal your identity.
Are there benefits to taking part in the research?
There are no direct benefits to you from being in this research. If a stool or other sample is
collected, you will be notified of the results, along with advice to share those results with your
physician. If you do not have a physician we will give you a directory of low cost clinics in the
Chicago area. We will also provide you with information sheets about this condition.
The results from this study may or may not benefit people who particiapte in outdoor activities
along Lake Michigan and Chicago River. Our findings could help establish a set of better water
quality standards.
Will I be told about new information that may affect my decision to participate?
During the course of the study, you will be informed of any significant new findings (either good
or bad), such as changes in the risks or benefits resulting from participation in the research or
new alternatives to participation, that might cause you to change your mind about continuing in
the study. If new information is provided to you, your consent to continue participating in this
study will be re-obtained.
2 of 5
CHEERS Version 5, July, 2008
What about privacy and confidentiality?
The only people who will know that you are a research subject are members of the research
team. No information about you, or provided by you during the research will be disclosed to
others without your written permission, except:
- if necessary to protect your rights or welfare (for example, if you are injured and need
emergency care or when the UIC Institutional Review Board monitors the research or
consent process); or
- if required by law. This could happen if we collect a stool sample from you and we find
that you have an infection caused by certain germs (like Salmonella and several others).
We will be required by law to notify the Illinois Department of Public Health, which
helps track cases of infections. The Illinois Department of Public Health will not share
your personal information.
We respect your privacy. All computer files with identifiers will be password-protected. Any
hard copies of the forms/files will be stored in locked cabinets and will be destroyed as soon as
data collection is complete. When the results of the research are published or discussed in
conferences, no information will be included that would reveal your identity.
What if I am injured as a result of my participation?
No injury is expected to occur as a result of your participation in this research. In the event of
injury related to this research, treatment will be available through the UIC Medical Center.
However, you or your third party payer, if any, will be responsible for payment of this treatment.
If you feel you have been injured, you may contact Sara Wuellner at 312-996-2094.
What are the costs for participating in this research?
There are no costs to you for participating in this research.
Will I be reimbursed for any of my expenses or paid for my participation in this research?
You could receive a total of up to $125 in cash and gift cards for participating in this research
according to the following schedule:
If you decide to participate, we will give you a T-shirt plus a $15 gift card after you have
answered the questions before you go home today.
Six to eight weeks after completing the three follow-up telephone interviews: $35 (Remember
the final follow-up phone call will be 3 weeks from today)
If you are selected for a home visit, after providing an eye or skin drainage swab, or 3 separate
stool samples: $75
Money will be paid in the form of a check made out to you and mailed to your home address.
Alternately, if you are enrolling in the study as a member of a rowing, boating, cycling, running,
or other sports team/club, you have the option to donate part or all of your compensation to the
team.
3 of 5
CHEERS Version 5, July, 2008
Can I withdraw or be removed from the study?
Your participation in this research is VOLUNTARY. If you choose not to participate, that will
not affect your relationship with UIC or your right to health care or other services to which you
are otherwise entitled. If you decide to participate, you are free to withdraw your consent and
discontinue participation at any time without affecting your future care at UIC.
Who should I contact if I have questions?
The researcher conducting this study is Dr. Sam Dorevitch. You may ask any questions you
have now. If you have questions later, you may contact the researchers at: 312-996-2094.
What are my rights as a research subject?
If you feel you have not been treated according to the descriptions in this form, or you have any
questions about your rights as a research subject, you may call the Office for the Protection of
Research Subjects (OPRS) at 312-996-1711 (local) or 1-866-789-6215 (toll-free) or e-mail
OPRS at
uicirb@uic.edu
.
Remember:
Your participation in this research is voluntary. Your decision whether or not to participate will
not affect your current or future relations with the University. If you decide to participate, you
are free to withdraw at any time without affecting that relationship.
You will be given a copy of this form for your information and to keep for your records.
4 of 5
CHEERS Version 5, July, 2008
Signature of Subject or Legally Authorized Representative
I have read (or someone has read to me) the above information. I have been given an opportunity
to ask questions and my questions have been answered to my satisfaction. I agree to participate
in this research. I have been given a copy of this form.
Signature
Date
Printed Name
Signature of Researcher
Date (must be same as subject’s)
Printed name of Researcher
Signature of Witness (if appropriate)
Date (must be same as subject’s)
Printed name of Witness (if appropriate)
OPTIONAL
I don’t want to receive any money for this research. Please donate my incentives to:
My team/club. The name of the team/club is___________________________________
Friends of the Chicago River
The CHEERS research study
Signature__________________________________
Date__________________
5 of 5
CHEERS Version 5, July, 2008
QAPP 2
Appendix 17: Parental Consent Form
recreation on the day you child enrolls in the study and briefly about how the child is feeling and
their recent activities. Continuing in the study will involve you or your child answering a series
of telephone questions 2, 5 and 21 days from the day your child enrolls in the study.
The purpose of asking the questions is to learn more about any illness your child may experience
and how they were exposed. It will take 8-10 minutes to complete. Answering the questionnaire
is voluntary. You and your child may choose not to answer any question for any reason.
In addition, some participants will be selected for a home visit by study staff. If your child is
selected, this could involve collecting a sample (such as a stool sample or a “pink eye” sample)
so the laboratory can analyze any infection they may have. If a stool sample is required, we’ll
need to collect 3 samples over a one week period. The purpose of getting stool samples is to test
for bacteria, viruses and parasites. This testing involves no risk to your child. We will store
your child’s specimen indefinitely and potentially test the samples for other microbes, or for
chemicals related to health or the environment, in the future.
What are the potential risks and discomforts?
The research has no major physical risks or discomforts other than the time involved and the
potential loss of privacy during the follow-up home visit (if your child falls ill). If your child
undergoes the study nurse’s examination of his or her eyes, ears or skin, he or she may feel a
little uncomfortable while being checked. Your child may feel uncomfortable while answering
certain questions during the survey/interview. You and your child have the right to refuse to
answer any question at any time. We will call for follow-up phone interviews at a time
convenient for you and your child.
You may be concerned about how we will store/protect your child’s data. We will do our best to
protect your child’s confidentiality and to keep the results of any test (in case we ask your child
for stool or eye/skin discharge samples) private.
When the results of the research are
published or discussed in conferences, no information will be included that would reveal
your child’s identity.
Are there benefits to taking part in the research?
There are no direct benefits to your child from being in this research. If a stool or other sample is
collected, you will be notified of the results, along with advice to share those results with your
physician. If you do not have a physician will be given a directory of low cost clinics. You will
also be given a printed fact sheet about the condition.
The results of this study may or may not benefit people who particiapte in out door activities
along Lake Michigan and Chicago rivers and lakes. Our findings could help create a set of better
water quality standards.
.
Will I be told about new information that may affect my/ my child’s decision to
participate?
2 of 5
CHEERS Parental Consent, Version 5, July 9, 2008
During the course of the study, you and your child will be informed of any significant new
findings (either good or bad), such as changes in the risks or benefits resulting from participation
in the research or new alternatives to participation, that might cause you to change your mind
about continuing in the study. If new information is provided to you, you and your child will be
asked to consent again to continue participating in this study.
What about privacy and confidentiality?
The only people who will know that your child is a research subject are members of the research
team. No information about your child, or provided by you during the research will be disclosed
to others without written permission from you, except:
- if necessary to protect his/her rights or welfare (for example, if your child is injured and
need emergency care or when the UIC Institutional Review Board monitors the research
or consent process); or
- if required by law. This could happen if we collect a stool sample from your child and we
find that he or she has an infection caused by certain germs (like Salmonella and several
others). We will be required by law to notify the Illinois Department of Public Health,
which helps track cases of infections. The Illinois Department of Public Health will not
share your child’s personal information.
We respect your child’s privacy. All computer files with identifiers will be password-
protected. Any hard copies of the files will be stored in locked cabinets and will be destroyed as
soon as data collection is complete. When the results of the research are published or discussed
in conferences, no information will be included that would reveal you and your child’s identity.
What if my child is injured as a result of my participation?
No injury is expected to occur as a result of your participation in this research. In the event of
injury related to this research, treatment will be available through the UIC Medical Center.
However, you or your third party payer, if any, will be responsible for payment of this treatment.
If you feel your child has been injured, you may contact Sara Wuellner at 312-996-2094.
What are the costs for participating in this research?
There are no costs to you/your child for participating in this research.
Will my child be reimbursed for any of my expenses or paid for my participation in this
research?
Your child could receive a total of up to $125 in cash and gift cards for participating in this
research according to the following schedule:
If your child decides to participate, they will be given a T-shirt plus a $15 gift card after they
have answered the questions before they go home on the day they enroll in the study.
Six to eight weeks after completing the three follow-up telephone interviews: $35 (Remember
the final follow-up phone call will be 3 weeks from the day they enroll in the study)
3 of 5
CHEERS Parental Consent, Version 5, July 9, 2008
If your child is selected for a home visit, after providing an eye or skin drainage swab or 3
separate stool samples: $ 75
Money will be paid in the form of a check made out to your child and mailed to your home
address. Alternately, your child has the option to donate their compensation to the rowing team
to which they belong, if they so desire.
Can my child withdraw or be removed from the study?
Your child’s participation in this research is VOLUNTARY. If you/your child choose not to
participate, that will not affect your relationship with UIC or your right to health care or other
services to which you are otherwise entitled. If you decide to allow your child to participate, you
are free to withdraw your consent and discontinue participation at any time without affecting
your future care at UIC.
Advance consent
Children (and adults) can enroll in this research as often as every 21 days. Each time they would
receive the same set of financial incentives (gift cards and checks). This research study will seek
to recruit members of rowing teams and other sports team/clubs several times during the 2008
season. If your child is on a team/club, you can check a box on the signature page of this form,
that would allow your child to enroll and re-enroll into the study when the CHEERS research
staff are recruiting members of the team, even if you aren’t present. You can withdraw your
permission at any point by contacting the CHEERS team at (312)996-2094 or (312)355-3629.
Who should I contact if I have questions?
The researchers conducting this study are Dr. Sam Dorevitch and Dr. Preethi Pratap. You may
ask any questions you have now. If you have questions later, you may contact the researchers at:
312-996-2094.
What are my child’s rights as a research subject?
If you feel your child has not been treated according to the descriptions in this form, or you have
any questions about your child’s rights as a research subject, you may call the Office for the
Protection of Research Subjects (OPRS) at 312-996-1711 (local) or 1-866-789-6215 (toll-free) or
e-mail OPRS at uicirb@uic.edu
.
Remember:
Your child’s participation in this research is voluntary. Your decision whether or not to allow
your child to participate will not affect your current or future relations with the University. If you
agree to participation, you and your child are free to withdraw at any time without affecting that
relationship.
You will be given a copy of this form for your information and to keep for your records.
4 of 5
CHEERS Parental Consent, Version 5, July 9, 2008
Signature of Subject or Legally Authorized Representative
I have read (or someone has read to me) the above information. I have been given an opportunity
to ask questions and my questions have been answered to my satisfaction. I agree to allow my
child to participate in this research. I have been given a copy of this form.
OPTION 1
My child may enroll in this research whenever they are eligible.
My child may only enroll within 21 days of the date I sign this form.
My child may only enroll on the date I sign this form.
OPTION 2
I don’t want my child to receive any money for this research. Please donate his/her incentive
gift card and check(s) to:
His/her team/club. The name of the team/club is_______________________________
Friends of the Chicago River
The CHEERS research study
_________________________
Child’s Printed Name
Signature of parent or guardian
Date
Printed name of parent or guardian
Signature of Researcher
Date
Printed name of Researcher
5 of 5
CHEERS Parental Consent, Version 5, July 9, 2008
QAPP 2
Appendix 18: Assent Form
l,eave box cnrpty
-
[]or
ollicc
usc
only
$TAnTsApPR0VALET
,iul'{
?1;il,1$
JUi\i
z0
-.-if,Sii&1,$[it'S$l!#'*3T#$
4.
5.
)
6.
8.
University
of
Illinois at Chicago
ASSENT
TO
PARTICIPATE
IN RESBARCH
..CHEERS:
'I'he
Chicago
Health.
Environmental
Exposures
and Recreation
Study
l.
My name
is
We are
asking
you to take
part
in
a research
study because
we are trying
to learn
more
about the
health of
people
(like you)
who
play
and exercise
outdoors
in and around
Chicago
area
rivers
and lakes.
lf
you
agree
to
be
in this study,
we will ask
you
a f'ew
questions
today
befbre
and afier
your
activity.
We
will also
call
you
in a
f'ew days to ask
you
a
few
questions about how
you
are
f'eeling.
If
you get
sick
we may come
to
your
home
to see
you
and
collect a
sample
if necessary.
This is safe
and
does
not
put
you
in any
danger.
You
will
learn
more about
how
safe
it is
for
you
and
your
family
to exercise
outdoors.
If
you get
sick.
a nurse
may come
to
your
home
fbr a
quick
check-up.
Your
family
will
be
paid
becar"rse
yoll
are
in the
research
proiect.
Please
talk this
over
with
your parents
before
you
decide
whether or
not to.ioin
the
study.
We
will also
ask
your
parents to
give
their
permission
fbr
you
to take
part
in this study'
But
even
if
your parents say
"yes"
you
can still
decide
not
to do
this.
If
you don't
want
to be
in
this study,
you don't
have
to.
Remember,
being
in this study
is
up to
you and
no one
will
be
upset
if
you
don't
want to or
even
if
you
change
your
mind
later
and
want
to
stop.
you
can
ask
any
questions that
you have
about
the study.
If
you
have
a
question
later
that
you didn't
think
of
now,
you
can
call
Sara
Wuellner
at 312-996-2094
or
ask
me
next
time.
g.
Signing
your name
at
the
bottom
means
that
you
agree
to
be
in this
study.
You
and
your
pur.ntr
will be
given a
copy
of
this
form
after
you
have
signed
it'
Name
of
Subiect
Signatr-rre
CHEERS
Asseent.
Version
2,
January
l6'
2008
lof
I
Age
Date
Grade
in School
QAPP 2
Appendix 19: Study Questionnaire Variables for Data Analysis
Appendix: Questionnaire Variable for Data Analysis
1. Health End-points: Acute Gastrointestinal Illness
Health-end points
AGI – Acute GI illness
1. Single symptom definition: the presence of any one of the following symptoms, as determined by telephone follow-up on day 2, 5
or 21 + No symptoms at baseline.
i. Abdominal cramps or stomach ache
ii. Diarrhea
1. Any
2.
≥2
bouts/24 hour
3.
≥3
bouts/24 hour
iii. Nausea
iv. Vomiting
2. Syndrome definitions :
i. Any two or more of the following symptoms + No symptoms at baseline
Vomiting
Diarrhea
Nausea
Stomach ache/abdominal cramps
ii. Highly credible GI illness (HCGI): in accordance with the definition posited by Cabelli et al (1982) + No symptoms at baseline
i.
vomiting, or
ii.
diarrhea associated with fever, or
iii. stomach ache with fever, or
iv. nausea with fever, or
v.
diarrhea with impact on daily activities
iii. EPA study GI illness definitions + No symptoms at baseline (Wade et al, 2006)
i.
≥3
loose stools/24 hours, or
ii.
vomiting+ stomachache, or
iii. nausea + stomachache, or
iv. nausea with impact on activity, or
v.
stomachache with impact on activity
2. Health End-point: Ear and Eye Illness
Health endpoints
Non-GI Illness Ear
1. Single symptom definition: the presence of any one of the following symptoms, as determined by telephone follow-up on day 2, 5 or 21
+ No symptoms at baseline
i. Earache or ear infection
2. Clinically proven: the presence of any one of the following symptoms, as determined by clinical evaluation during home visits (could be
left or right ear for each symptom)
i. Earache or pain with traction
ii.
Discharge
iii. Canal edema
iv. Canal erythema
v. Diagnosed with ear infection
vi. Antibiotic treatment
Non-GI Illness Eyes
1. Single symptom definition: the presence of any one of the following symptoms, as determined by telephone follow-up on day 2, 5 or 21
+ No symptoms at baseline
i. Eye irritation
ii. Drainage or crusty eyes
3. Health End-point: Dermal
Non-GI Illness Dermal
1. Single symptom definition: the presence of any one of the following symptoms, as determined by telephone follow-up on day 2, 5 or 21 +
No symptoms at baseline
i. Any area on the skin that are red, or sore, or draining
ii. Rash or Itchy skin
2. Clinically proven: the presence of any one of the following symptoms, as determined by clinical evaluation during home visits (could be
left or right ear for each symptom)
i. Warmth around the wound
ii. Tenderness
iii. Erythema
iv. Discharge
v. Antibiotic treatment (including type of antibiotic)
4. Health End-point: Upper Respiratory
Health endpoints
Non-GI Illness Upper Respiratory Illness
1. Single symptom definition: the presence of any one of the following symptoms, as determined by telephone follow-up on day 2, 5
or 21 + No symptoms at baseline
i. Sore throat
ii. Cough
iii. Cold/ Runny or stuffy nose
2. Syndrome definitions:
i. Any two or more of the following symptoms + No symptoms at baseline
Headache
Sore throat
Cough
Cold or runny or stuffy nose
Fever
Note: Only fever or headache with out any other symptoms is not considered an upper respiratory illness
ii. Respiratory illness in accordance with the definition posited by Colford et al (2007)
Cough with phlegm
5. Potential Confounder and/or Effect Modifiers: Demographics and others
Demographics and other
1.
Age
2.
Gender
3.
Race
4.
Telephone number
5. Address
5.
Distance traveled to the lake/river for outdoor activity
6. Concern about doing water sports in the Chicago River
6.Effect Modifiers and/or confounders: Pre-exposure Non-water-related GI factors
Effect Modifiers
A. Pre-exposure and health
1. Non-water related GI illness exposures
1.
Animal contact
a.
dog/cat
b.
other animal (petting zoo/farm)
2.
Food exposure
a.
Raw shell fish
b.
Raw meats
c.
raw eggs
d. Store bought cold sandwich
e. Store bought cold salad
d. Fresh produce
e.
Hamburger
3.
Exposure to someone ill
a.
at home
b.
outside home
2. Environmental exposures
1. Recreational water exposure in past 7 days
2. Location of exposure- lake, river, beach, pool (Chicago river/lake/lagoon vs other places)
3. Get wet during water-recreational activity
1.
Any part of body wet
2. Face/head wet
2.
Water in mouth
3.
Swallowed water
3. Pre-existing health conditions
1.
Chronic stomach illness/GI illness
i.
Crohn’s
ii.
Gastritis
iii.
Ulcers
iv.
Lactose intolerance
v.
Acid reflux
vi. Inflammatory bowel syndrome
vii. Irritable bowel syndrome
viii. Other: Specify
2.
Asthma/emphysema
3.
diabetes
5. Antibiotic use in past 7 days
4.
prone to infections
5. Average daily bowel movements
7.
Effect modifiers and/or confounders: Post-exposure Non-water related GI factors
Effect Modifiers
B. Post-exposure and health
1. Non-water related GI illness exposures
1.
Animal contact
a.
dog/cat
b.
other animal (petting zoo/farm)
2.
Food exposure
a.
Raw shell fish
b.
Raw meats
c.
raw eggs
d. Store bought cold sandwich
e. Store bought cold salad
d. Fresh produce
e.
Hamburger
3.
Exposure to someone ill
a.
at home
b.
outside home
2. Environmental exposures
1. Recreational water exposure since last contact
2. Location of exposure- lake, river, beach, pool (Chicago river/lake/lagoon vs other places)
3. Get wet during water-recreational activity
1.
Any part of body wet
2. Face/head wet
2.
Water in mouth
3.
Swallowed water
8. Behavioral Predictors of Illness: Activity-based indicators of exposure
Predictors
A. Activity 3 study groups.
1. Water- recreational activity (exposed groups)
1a. Type of water-recreational activity
1b. Location for launch and exit
1c. Duration of activity
1d. Wet while launching OR wade while fishing
2a. Non-water recreational activities (unexposed)
2b. Type of non-water recreational activity
2c.
Get wet at all
1.
Any part of body wet
2. Face/head wet
2.
Water in mouth
3.
Swallowed water
B. Water exposure by activity
Canoeing or Kayaking or Rowing or Rafting or boating or sailing or fishing on a boat or fishing at the pier
a.
Flip over (by accident)
i.
How many times
ii.
How long in water
iii.
Head go under water
b.
Swim (intentionally)
i.
How long in water
ii.
Head go under water
c.
Get wet at all
1.
Any part of body wet
2. Face/head wet
2.
Water in mouth
3.
Swallowed water
e.
Food/water
1.
ate/drank during or after activity on the water
2. washed hands before eating
f.
Rubbing of eyes with hands
g.
For fishermen
i.
Type of bait (for handling exposure)
ii.
No: of fish 0, 1, 2,3, >3
iii. Will they eat the fish they caught
Table of Contents
A. PROJECT MANAGEMENT
Page
1. Distribution List
1
2. Project/Task Organization
1
3. Problem Definition/Background
5
4. Project Task/Description
5
5. Quality Objectives and Criteria
6
6. Special Training/Certification
6
7. Documents and Records
7
B. DATA GENERATION AND ACQUISITION
1. Sampling Process Design (Experimental Design)
8
2. Sampling Methods
12
3. Sample Handling and Custody
12
4. Analytical Methods
13
5. Quality Control
15
6. Instrument/Equipment Testing, Inspection, and Maintenance
16
7. Instrument/Equipment Calibration and Frequency
16
8. Inspection/Acceptance of Supplies and Consumables
16
9. Non-direct Measurements
16
10. Data Management
17
11. Archiving Clinical Specimens
17
12. Disease Reporting
17
C. ASSESSMENT AND OVERSIGHT
1. Assessments and Response Actions
18
2. Reports to Management
18
D. DATA VALIDATION AND USABILITY
1. Data Review, Verification, and Validation
19
2. Verification and Validation Methods
19
3. Reconciliation with User Requirements
19
CHEERS QAPP 3
ii
July 2008
List of Figures
Figure
Description
Page
Figure 1
Overall Project Management Structure for Survey Methods
4
List of Tables
Table
Description
Page
Table 1
Index of Documents and Records, Storage and Distribution
7
Table 2
Index of Analytic Methods for Clinical Specimens
14
CHEERS QAPP 3
iii
July 2008
List of Appendices
Appendix
Description
1
CHEERS Home Clinical Evaluation Form
2
Specimen Collection System
3
Letter to Study Participants
4
Conjunctivitis Rating Scale
5
Positive Culture Letter: Stool
6
Positive Culture Letter: Eye/Wound
7
Fact Sheets
8
Low Cost Clinics in Chicago
9
10
11A-C
Chain of Custody Log
UIH Laboratory: Stool Culture
UIH Laboratory: Stool Viral Culture
12
UIH Laboratory Protocol: Cryptosporidium
13
UIH Laboratory Protocol: Rotavirus
14
UIH Laboratory Protocol: Eye
15
UIH Laboratory Protocol: Wound
16
IDPH Laboratory Protocol: Shigatoxin
17
IDPH Laboratory Protocol: Norovirus
18
UIH Laboratory Details
19
UIH CLIA Certification
20
IDPH CLIA Certification
21
IDPH QA
22
UIH Laboratory Protocol: Archiving Samples and Long-term Storage
23
24
IDPH Laboratory Protocol: Archiving Samples and Long-term Storage
Unusual Occurrence Log
CHEERS QAPP 3
iv
July 2008
Quality Assurance Project Plan 3: Clinical Evaluations and Microbial Analyses
A. PROJECT MANAGEMENT
1. Distribution List
UIC: S. Dorevitch, P. Scheff, M. Javor, P. Pratap, J. Wuellner, S. Wuellner,
T. Schoonover and all clinicians
MWRDGC: T. Granato
2. Project/Task Organization
The key members of the CHEERS study team involved in the clinical
evaluations and microbial analyses include the Study Director, Quality Manager,
Clinical Project Manager as well as the clinical staff. The Study Director will have
overall authority for the development and implementation of the study, hiring of
clinical staff involved in assessments and obtaining biologic samples for analyses,
and communicating with CHEERS study stakeholders. The Quality Manager will
establish overall data quality objectives for the CHEERS study and will be
responsible for reviewing the quality control data of the data collection process. The
detailed organizational structure is provided in the Study Overview.
The Clinical Project Manager (CPM), Jacqueline Wuellner, MPH, RN, is
responsible for maintaining the overall quality of the clinical evaluations and
specimen collection. The Project Manager is responsible for the following project
related tasks:
2.1 Assist in writing the application to University of Illinois at Chicago (UIC)
Institutional Review Board (IRB) for Human Subjects Research approval.
2.2 Develop protocols for obtaining and transporting clinical specimens.
2.3 Work with the University of Illinois Hospital (UIH) Microbiology Laboratory
and the Illinois Department of Public Health (IDPH) Microbiology Laboratory
to ensure proper protocols for specimen chain of custody are followed.
2.4 Ensure all clinical team members performing home visits to obtain culture
specimens are certified (and maintain certification) by the UIC IRB.
CHEERS QAPP 3
- 1 -
July 2008
2.5 Work with the Study Director to develop and conduct the appropriate training
methods for the clinical team.
2.6 Work with the UIC Survey Research laboratory (SRL) to identify all study
participants from whom clinical specimens are required for culture.
2.7 Work with a commercial courier service to ensure the prompt transport of stool
specimens from the home of the study participant to the UIH Microbiology Lab.
2.8 Train staff how to prepare for shipping Fisherbrand Commode Specimen
Collection kits (Fisher Health Catalog # 02-544-208) with instructions for use to
study participants identified during telephone interviews as candidates for stool
sample analysis.
2.9 Ensure that clinical specimens are archived for potential future analysis at the
IDPH microbiology lab.
2.10 Ensure that pathogens isolated in the UIH microbiology lab are archived for
potential future analysis.
2.11 Ensure all clinical team members are trained in assessment techniques and
proper specimen handling techniques before they begin working in the field.
2.12 Ensure availability of stool collection kits for distribution as needed.
2.13 Ensure all study participants are mailed project reminders within 5 days of
enrollment to promote phoning the CHEERS study nurse if gastrointestinal, eye
or skin symptoms develop at anytime during the 21-day active study period.
2.14 Implement adequate quality control methods to ensure accurate documentation
of clinical data and home visit data.
2.15 Work with the Survey Project Manager to ensure smooth flow of participants
through the phases of field, phone and clinical data collection.
2.16 Receive and properly store laboratory reports from the UIH Microbiology
laboratory and IDPH microbiology laboratory.
2.17 Notify the study participant by telephone within 24 hours of receipt of positive
results and provide via U.S. Postal Service health information sheets specific to
the participant’s infection.
2.18 Provide a directory of low-cost clinics to study participants who do not have a
personal health care provider as needed.
CHEERS QAPP 3
- 2 -
July 2008
2.19 Provide timely feedback and reports to the CHEERS Study Quality Manager and
Study Director throughout the clinical data collection process.
The clinical staff will be responsible for completing home visits to collect the appropriate
specimens from those participants reporting eye drainage, wound drainage, or redness.
During the home visit, the clinician will complete an assessment, document findings and
obtain any clinical specimens required. Further staff duties include arranging home visits
with participants as required, transporting specimens to the UIC microbiology laboratory,
and communicating with the Clinical Project Manager daily, following any home visits
completed the previous evening. Also, the clinical staff will maintain an unusual
occurrence log to document and communicate any unexpected events that arise during a
home visit.
CHEERS QAPP 3
- 3 -
July 2008
Study Director
S. Dorevitch, MD, MPH
CHEERS Clinical Staff
Clinical
Project Manager
J. Wuellner, RN, MPH
UIH Microbiology
W. Janda, PhD
IDPH Microbiology
G. Dizikes, PhD
Figure 1:
Overall Project Management Structure for Clinical Evaluations and Microbial
Analyses
CHEERS QAPP 3
- 4 -
July 2008
3. Problem Definition/Background
The overall scientific background and goals of the CHEERS study are outlined in
the Study Overview. The following sections will focus on development and
implementation of the CHEERS clinical data collection and microbial analyses
methods.
Problems specifically related to clinical evaluations and microbial analyses are:
3.1 Prior studies of recreational waterborne illness generally did not confirm
acute gastrointestinal illness and other infectious diseases by culture.
3.2 Prior studies had no basis for identifying the pathogens responsible for illness
among recreators.
4. Project/Task Description
The overall clinical project objectives are:
4.1 Develop CHEERS home visit clinical evaluation forms.
4.2 Ensure all clinicians are licensed health care professionals.
4.3 Develop a system for specimen collection from study participants and document
specific clinical findings at the time the culture specimen is obtained.
4.4 Provide specific training to clinical staff to evaluate conjunctivitis using the
conjunctiva grading scale, and assess as well as measure skin lesions.
4.5 Train all clinical staff how to collect, label and transport a biologic specimen,
maintaining sample integrity at all times.
4.6 Perform follow-up home visits and sample collection as per protocol based on
participants’ answers to the follow-up telephone surveys.
4.7 Contract with courier service to pick up stool specimens from participants’
homes as needed and deliver the samples to UIC microbiology lab.
4.8 Work with the UIH and IDPH labs to develop a system to deliver and record
biologic specimens collected, and maintain a record of all results.
4.8.1 List of microbes of interest that we will be able to identify
CHEERS QAPP 3
- 5 -
July 2008
Bacteria:
Salmonella, Shigella, Yersinia, Plesiomonas,
Campylobacter, Aeromonas, Edwardsiella, E. Coli
0157:H7
Virus: norovirus, rotavirus, enteric adenovirus, enterovirus
Parasites:
Cryptosporidium spp., Giardia labmlia, Cyclospora,
Entamoeba histolytica
5. Quality Objectives and Criteria
5.1 Provide stool specimen collection kits to participants identified on follow-up
survey as needing such an evaluation, within 24 hours.
5.2 Transport 90% of stool samples to the UIH laboratory within 8 hours of sample
collection.
5.3 Make telephone contact with participants potentially requiring home visits for
evaluations of eye and skin symptoms, within 12 hours of notification by SRL
that a home visit may be warranted, based on participants’ symptoms.
5.4 Require 100% accuracy in sample handling/labeling/transport and receipt
in lab. The Clinical Project Manager will train all clinical staff in proper
handling and labeling of specimens and will maintain an unsatisfactory
specimen log to assure immediate correction of problems/inconsistencies.
5.5 Conduct consistent clinical assessments among clinical personnel.
6. Special Training/Certification
All clinical personnel will be licensed health professionals and therefore,
will only require specific training focused on documenting conjunctivitis using the
conjunctiva grading scale, and assessing and measuring skin lesions for accuracy and
consistency. Training will be conducted using didactic and interactive teaching
methods. Members of the clinical team will be required to verbalize standard procedure
protocols and perform return demonstrations indicating an acceptable mastery of the
required techniques.
CHEERS QAPP 3
- 6 -
July 2008
7. Documents and Records
CHEERS Study
Clinical Documents
and Records
Personnel Responsible
for Developing and
Storage
Storage Type
Distribution List
QAPP # 3 with
appendices.
Clinical Project Manager
Computer file
Hard copy
-
All CHEERS study personnel
-
MWRDGC liaison
IRB certification
status for CHEERS
clinical personnel
Clinical project manager
Computer file
Log book
-
Survey Project Manager
-
Clinical Project Manager
SRL training
completion
SRL/ ASD-Q
Computer file Training log
-
Survey Project Manager
Clinical evaluation
forms
ASD- Q
Computer file
-
Field Data Coordinator
Hard copy
-
All CHEERS clinical personnel
-
Clinical Project Manager
UIC Microbiology
Laboratory protocols
UIH Labs
Computer file
Hard copy
-
Clinical Project Manager
Paper-based forms
(logs)
Clinical project manager
Copied and stored in a
secure file folder
-
Clinical Project Manager
-
Study Director
Instructions for use
of stool collection
kit at home
Clinical project manager
Computer file
-
Clinical Project Manager
Hard copy kept in Room 210
-
All CHEERS field personnel
Reminder mailing
Clinical project manager
Computer file
Hard copy kept in Room 210
Table 1: Index of Documents and Records, Storage and Distribution
-
Clinical Project Manager
A copy of the QAPP 3 will be stored in a binder with all other study-related QAPP
documents in the study office: Room 210, School of Public Health West. An additional
print copy will be with the Clinical Project Manager. An electronic copy will be stored
on the UIC Blackboard site, accessible to all clinical personnel.
CHEERS QAPP 3
- 7 -
July 2008
B. DATA GENERATION AND ACQUISITION
1. Sampling Process Design
The study design, sample size calculations, sampling locations and rationale for the
design are outlined in the “Overview” document. The following sections will focus
on the development of sampling methods for the follow-up, home visit clinical
evaluations, and the collection/transport/analyses of biologic specimens.
1.1 Clinical evaluations for skin and eye symptoms
1.1.1 Home visits can be triggered by answers given during the follow-up
telephone surveys indicating the participant has eye crusting or drainage,
or has wound or skin drainage, or by the participant calling the study nurse
anytime during the 21-day active study participation period and reporting
symptoms of eye crusting or drainage, or wound or skin lesions or
drainage. Acute respiratory symptoms will not prompt a home visit, given
the limited value that such a visit would have in either identifying
pathogens or objectively establishing the presence of infection.
1.1.2 The clinician will contact the participant within 12 hours of notification to
determine the best time within the next 24 hours, for the home visit to
occur. The clinician will confirm the participant’s home address at this
time.
1.1.3 One member of the home visit team will confirm the appointment
with the participant, ideally 1 hour prior to the appointment, and
complete the visit at the appointed hour.
1.1.4 Wearing gloves, the clinician will complete an exam of the eyes, ears, and
skin. The clinician will also obtain swabs of any discharge. Culturette
tubes will be labeled and placed in a laboratory transport bag with the
appropriate requisition.
1.1.4.1 Conjunctivitis: Two swabs of the palpebral conjunctiva will be
obtained, one swab for bacterial culture and one swab for viral
culture obtained on a Dacron swab and placed in viral transport
medium.
CHEERS QAPP 3
- 8 -
July 2008
1.1.4.2 Wound: Only wound discharge will be cultured. Scabbed-
over lesions or dry areas (such as cellulitis or a skin abscess) will not
be cultured, nor will study personnel attempt wound drainage.
1.1.5 The clinician will complete the Home Clinical Evaluation Form
(Appendix #1) prior to leaving the participant’s home.
1.1.6 The swabs obtained during the home visit will be delivered to the UIC
laboratory within 12 hours, either by the clinician or by courier.
1.2 Clinical Evaluations for Acute Gastrointestinal Symptoms
1.2.1 Reports of acute gastrointestinal (AGI) symptoms (diarrhea, vomiting,
acute abdominal cramping, unrelated to menstrual cramps), either during
follow-up telephone survey or by participant-initiated phone call will
trigger request for stool sample collection.
1.2.2 If AGI symptoms are reported during a routine follow-up telephone
survey, the participant will be sent (via FedEx) a package containing 3
Fisherbrand Commode Specimen Collection Systems (Appendix #2),
printed instructions for collection, and the courier service telephone
number for the participant to call when there is a sample for delivery to the
UIC laboratory. The interviewer will confirm the participant’s address for
delivery.
1.2.3 If a participant experiences AGI symptoms anytime during the 21-day
active study participation period they are encouraged to report those
symptoms to the Clinical Project Manager who will arrange for overnight
FedEx delivery of the stool collection kits (as noted in 1.2.2 above). All
participants are mailed a magnet with the nurse’s phone number and a
letter encouraging them to report any symptoms immediately (Appendix
#3).
1.2.4 The Survey Research Lab call center will generate a list of participants
reporting AGI symptoms to the Clinical Project Manager for follow-up the
next day.
CHEERS QAPP 3
- 9 -
July 2008
1.2.5 Stool specimens will be collected three times on alternating days over a 5
day period from those reporting AGI symptoms to increase the likelihood
of detecting infection caused by
Giardia
.
1.3 Home Visit Protocol
1.3.1 Overview
Participants who report on telephone follow-up symptoms of eye drainage or
crusting, skin lesion or wound drainage will require a home visit conducted by a
licensed clinician (RN or MD). Additionally, participants may call the Clinical
Project Manager directly if they develop specific symptoms which would trigger a
home visit anytime during the 21 days of active study participation.
Based on the symptoms reported, the staff clinician will obtain specimens for
culture from the participant’s eye(s) or skin discharge. The clinician will also
document relevant physical findings on the paper-based Home Clinical
Evaluation form (Appendix #1).
The steps to be followed before and during a home visit include:
a. Telephone interviewer will inform the study participant that based on the
answers to the survey, a home visit, by a nurse or doctor for a brief follow-up
exam and to obtain eye or wound drainage samples, is required. Interviewer will
confirm the best telephone number to reach the study participant and indicate that
they will be getting a call from a member of the clinical staff within 12 hours.
b. Every two hours, the interviewer will notify the staff clinician via telephone or
e-mail, of any participants requiring a home visit who were identified in the
preceding 2 hours.
c. The staff clinician will consult the on-call list of project team members to
identify the appropriate team member that a home visit will occur within 24
hours, although preferably within 12 hours.
d. Each home visit team member will carry a cell phone and wear UIC
CHEERS study identification. The clinician will bring the home visit bag. The
CHEERS QAPP 3
- 10 -
July 2008
clinician will follow study protocol for examining eyes (Appendix #4): obtaining
necessary samples, conducting a thorough evaluation, and completing required
documentation. Samples will be labeled and specimens will be entered into the
transport log.
e. One member of the home visit team will deliver the specimen to the UIC
Microbiology laboratory at 820 S. Wood Street, Room 215 or will contact the
courier for specimen delivery. .
f. The clinician will fax the Home Clinical Evaluation Form to the office of the
Project Manager - Questionnaire Data or personally deliver the form to that
manager.
g. The Survey Project Manager will be responsible for entering the data into a
desktop computer. Project Manager - Questionnaire Data will work with
CHEERS staff to complete the data entry and upload the files to the SRL server
(see transmittal procedures for all computer files in QAPP #2)
1.3.2 Technique for obtaining swab for culture of wound exudate
•
Wash hands and don gloves.
•
Explain procedure to subject.
•
Cleanse wound with sterile saline rinse.
•
Using aseptic technique, twirl the end of the sterile-tipped applicator stick on
one square centimeter area of the open wound for five seconds.
•
Insert the swab into the gel tube.
•
Label specimen and requisition form. Place in plastic specimen transport bag.
•
Transport immediately to the UIC laboratory for processing.
1.3.3 Technique for obtaining swab culture of eye drainage.
•
Wash hands and don gloves.
•
Explain procedure to subject.
•
Using aseptic technique, pull down the lower eyelid. Roll the swab along the
inside of the lower eyelid. Do not swab over the globe of the eye.
•
Replace the swab in the gel tube.
CHEERS QAPP 3
- 11 -
July 2008
•
Label specimen and requisition form. Place in plastic specimen transport bag.
•
Transport immediately to the UIC laboratory for processing.
1.3.4 Protocol for arranging for courier pick up:
When the call center is notified of a sample ready for delivery, the call center
will either confirm that the participant has the courier contact information or the
call center will call the courier with name, address, and phone number of
participant. Specimen will be picked up and delivered to the UIC microbiology
lab within 3 hours of notification.
1.3.5 Subject notification of positive sample results:
If stool or other specimens collected are culture positive, participants will be
notified of the results via telephone and US mail, along with advice to share
those results with their physician (Appendices #5, #6). CDC fact sheets
pertaining to the particular microbe identified will be included with the
notification letter (Appendix #7). If the participant does not have a physician,
he/she will be given a directory with phone numbers of low cost clinics in the
Chicago area (Appendix #8).
2. Sampling Methods
There is no randomization or statistical approach for obtaining health information
from subsets of study participants. Clinical questions are asked of all study participants in
the field and telephone interviews. Clinical specimen collection is triggered by specific
responses to those questions among all study participants.
3. Sample Handling and Custody
3.1 Before concluding the home visit, the clinician will label the wound and eye
drainage specimens with subject’s Case ID number, date and clinician’s initials.
Afterwards, the clinician will place the samples in a plastic specimen transport
bag with the UIC requisition form.
3.2 The clinician will then complete the specimen chain of custody log (Appendix
#9) and terminate the home visit.
CHEERS QAPP 3
- 12 -
July 2008
3.3 Immediately following the home visit, the clinician will deliver the specimens
to the UIC Microbiology lab, Room 215. The person receiving the specimens in
the lab will sign and date the specimen log. The clinician may elect to use the
courier service to deliver the specimen to the UIC lab. In which case, the courier
service would be called immediately upon termination of the home visit and
have the specimen delivered within 3 hours of notification.
3.4 Lab access – With building “swipe” access, specimens can be delivered
between 7:00 AM and 9:00 PM from Monday through Friday, 7:00 AM and
6:00 PM on Saturday, and 7:00 AM and 3:00 PM Sundays.
3.5 If the subject reports acute gastrointestinal illness and has already obtained and
labeled the stool sample, the courier will pick up the specimen. If
pick-up occurs after normal business hours, lab specimens can be delivered to
the UIH Microbiology laboratory’s loading dock via the “FED EX” bell.
4. Analytical Methods
4.1 Stool samples
Upon receipt of the sample in the UIC microbiology laboratory, an aliquot
of the sample will be removed from the stool sample container, placed in transport
medium, labeled, and then transferred to the IDPH microbiology laboratory by
courier for Norovirus and Shiga toxin analysis. The sample will be received and
tracked in the IDPH laboratory by established protocol, analyzing the samples by
real-time reverse transcriptase polymerase chain reaction.
CHEERS QAPP 3
- 13 -
July 2008
Pathogen/
Specimen source
Lab and Analytical Method
Appendix #
Stool culture
UIC hospital microbiology lab
Stool culture
10
Stool viral culture
UIC hospital microbiology lab
Virus isolation specimens
Virus identification
Virus isolation identification
11-a
11-b
11-c
Cryptosporidium
Giardia
UIC hospital microbiology lab
Enteric protozoa by direct immunofluorescence
12
Rotavirus
UIC hospital microbiology lab
Rotavirus by enzyme immunoassay
13
Eye discharge
UIC hospital microbiology lab
Eye cultures
14
Wound discharge
UIC hospital microbiology lab
Wound Culture
15
Enterovirus
IDPH microbiology lab: Shigatoxin protocol
16
Norovirus
Table 2. Index of Analytic Methods for Clinical Specimens
IDPH microbiology lab: Norovirus by real-time reverse
transcriptase polymerase chain reaction
17
CHEERS QAPP 3
- 14 -
July 2008
At the UIC hospital microbiology laboratory, stool samples will be analyzed for
enteric pathogens and protozoa, including Cryptosporidium and Giardia, following the
laboratory’s standard operating procedures by direct immunofluorescence. Stool
specimens will also be analyzed for rotavirus by enzyme immunoassay. Additionally,
specimens will be analyzed for the presence of enteroviruses by cell culture.
4.2 Eye swabs
Eye swabs will be analyzed by the UIC Hospital Microbiology Laboratory by
culture following the laboratory’s standard protocol (Appendix #14).
4.3 Wound swabs
Wound swabs will be analyzed by the UIC Hospital Microbiology Laboratory
by culture following the laboratory’s standard protocol (Appendix #15).
5. Quality Control
5.1 Quality Control Measures for Clinical Data Analysis
a. The UIC laboratory and IDPH laboratory are fully certified and have their own
internal QA/QC protocols (Appendices 18-21).
b. The laboratory’s QA manager will provide the Clinical Project Manager their
laboratory’s internal QC data on a quarterly basis for review.
5.2 Quality Control for Clinical Data Collection
5.2.1 The Clinical Project Manager will be responsible for ensuring the clinical
staff conforms to the study protocols.
5.2.2 Quality of the health assessment and sampling procedures will be assured
by consistent training of clinical staff and periodic review of home visit
evaluation forms and unusual occurrence logs.
5.2.3 Accuracy of the data entry will be ensured by staff training, before the start
of each year’s recreation season, and also through random quality
assurance checks throughout the season.
5.2.4 The unsatisfactory specimen log will indicate the frequency of incomplete,
poorly prepared, or incompletely identified specimens and the final
disposition of these samples (identified, unable to analyze due to lack of
proper identification, etc.).
CHEERS QAPP 3
- 15 -
July 2008
5.2.5 The Clinical Project Manager will review the log, analyze for problem
areas, and report unsatisfactory outcomes. Clinical staff will be re-trained
as deemed necessary to maintain quality data outcomes.
6. Instrument/Equipment Testing, Inspection, and Maintenance
The UIC Hospital and IDPH laboratories are both certified laboratories with their
own QA/QC protocols. Instrument testing, inspection, and maintenance will be
conducted per their respective protocols. There are no instruments specific to
handling or analyzing CHEERS clinical specimens.
7. Instrument/Equipment Calibration and Frequency
The UIC Hospital and IDPH laboratories are both certified laboratories with their own
QA/QC protocols. Instrument calibration and frequency will be conducted per their
respective protocols. There are no instruments specific to handling or analyzing
CHEERS clinical specimens.
8. Inspection/Acceptance of Supplies and Consumables
The RN on call is responsible for inspection and maintenance of the home visit bag.
Contents will be listed on a checklist, stored in the bag. Missing items can be
replenished from the CHEERS stockroom.
Contents of the “Home Visit Bag”
1. Culturette tubes
2. Latex exam gloves
3. Biohazard (‘red”) bags for disposal
4. Otoscope
5. Disposable measuring tape
6. Clipboard and pen
7. Notebook
8. Home visit data forms
9. Sample ID labels
9. Non-Direct Measurements
Does not apply to this protocol.
CHEERS QAPP 3
- 16 -
July 2008
10. Data Management
Home evaluation data will be uploaded by the Survey Project Manager and sent to
the SRL for analysis. All sample data will remain stripped of personal identifiers.
Logs of laboratory data will be kept by the Clinical Project Manager in a password-
protected computer file. All paper copies will be stored in a locked cabinet.
IDPH provides paper copies of all lab results directly to the Principal Investigator.
UIH provides paper copies of all lab results to the Clinical Project Manager. UIH
lab notifies the Clinical Project Manager by telephone immediately when an
abnormal result is obtained.
11. Archiving Clinical Specimens
When pathogens are identified in stool samples, both the UIH and IDPH
laboratories will freeze an aliquot of the sample in the sub-zero freezer for possible
future investigation. Additionally, IDPH lab will freeze a sample of all raw stool
received (Appendices # 22 and 23).
12. Disease Reporting
11.1 Notifiable Disease Reporting will comply with Illinois requirements.
In the context of this research, it is possible the cases of Giardiasis,
Cryptosporidiosis, Salmonellosis, Shigellosis will be identified. Less likely
cases of Yersiniosis, Shigatoxin-positive enteritis and Cyclosporiasis may be
reported. The Clinical Project manager will contact the Illinois Department of
Public Health and provide any information required by law.
CHEERS QAPP 3
- 17 -
July 2008
C. ASSESSMENT AND OVERSIGHT
1. Assessments and Response Actions
Reviews of the data quality will be performed regularly, as outlined in the
system wide quality management plan (QMP). This project includes several streams
of data quality monitoring and we will use a graded approach to review them.
At weekly reviews, we will note, revise and develop ways to prevent unusual
occurrences that have been reported in the preceding week, which could include:
a. The rejection of samples by the microbiology labs for various reasons.
b. Improper or incomplete data entry during field clinical assessment
c. Incomplete data entry during home visit.
d. Failure to complete home visit within the timeframe prescribed per
protocol.
At monthly quality reviews, summary statistics of laboratory results and UIH
internal laboratory QC data will be reviewed.
At annual quality reviews, all quality data will be reviewed. Emphasis will be
placed on system-wide quality issues with the goal of developing quality
improvement strategies for the next recreation season.
2. Reports to Management
The Clinical Project Manager will receive hard copy reports daily from the UIH and
IDPH microbiology laboratories. All data will be entered a CHEERS clinical
microbiology data file, devoid of all personal identifiers. The study director will be
notified of all positive cultures. A summary data report will be generated monthly
by the Clinical Project Manager and shared with the Study Director and Quality
Manager.
The Clinical Project Manager will also maintain an Unusual Occurrence log to
document any unexpected occurrence with personnel, subjects, data
collection/handling, or data reporting. (Appendix #24)
CHEERS QAPP 3
- 18 -
July 2008
D. DATA VALIDATION AND USABILITY
1. Data Review, Verification, and Validation
Data review, verification, and validation methods have been employed during the
development, programming and implementation of the clinical data gathering process.
These include questionnaire review and validation processes, including internal/external
reviews, IRB clearance, and pilot evaluations of survey questions. Specifically:
•
The computer assisted surveys have been developed with pre-determined
acceptable response options. This prevents the entry of unacceptable or out-of-
range responses.
•
Data will be deemed unusable if a laboratory reports a specimen as unusable.
2. Verification and Validation Methods
Novel methods are not employed for data collection or analysis. Validation of
methods will not be necessary.
3. Reconciliation with User Requirements
The clinical data collected were selected specifically for this research. Clinical
microbiology data reported by laboratories as having been obtained from acceptable
specimens will be used without any reconciliation.
CHEERS QAPP 3
- 19 -
July 2008
CHEERS QAPP 3
Appendix 1: Home clinical evaluation form
UNIVERSITY OF ILLINOIS, CHICAGO
CHEERS HOME VISIT CLINICAL EVALUATION
Clinician Name
: ____________________
1.
You said you had a red, or sore, or draining area on your skin when we last spoke
to you on (fill date of late telephone interview). I will examine your skin now.
(Fill location of skin wound/cut/bruise/bug bite from telephone survey Q 13a).
Observation:
a. Warmth around the wound?
Y N RF DK
b. Is there any tenderness/pain? Y N RF DK
c. Any surrounding erythema (measure diameter in mm)? Y ___mm N RF DK
d. Any discharge from the wound? Y N RF DK
e. Are you currently being treated with any antibiotics or prescription
medications for this? Y N RF DK
f. IF Yes, Is this topical or oral?
Topical/ Oral RF
DK
2.
I will examine your skin for any other similar cuts, bruises, bug bites, or wounds.
Note: Nurse/Physician records observations. Look for infected cuts, bruises, insect bites.
Check all that apply.
Right side
Left side
Head/ Face
Y N RF DK
Head/ Face
Y N RF DK
Neck
Y N RF DK
Neck
Y N RF DK
Upper Arm
Y N RF DK
Upper Arm
Y N RF DK
Fore Arm
Y N RF DK
Fore Arm
Y N RF DK
Hand
Y N RF DK
Hand
Y N RF DK
Thigh
Y N RF DK
Thigh
Y N RF DK
Lower leg
Y N RF DK
Lower leg
Y N RF DK
Ankle/ Foot
Y N RF DK
Ankle/ Foot
Y N RF DK
Note: For each “Yes”- then observe wound-
You have an open cut/wound/insect bite/ scrape. I am going to briefly examine this now.
Observation:
a. Warmth around the wound?
Y N RF DK
b. Is there any tenderness/pain? Y N RF DK
c. Any surrounding erythema (measure diameter in mm)? Y ___mm N RF DK
d. Any discharge from the wound? Y N RF DK
e. Are you currently being treated with any antibiotics or prescription
medications for this? Y N RF DK
f. IF Yes, Is this topical or oral?
Topical/ Oral RF
DK
3.
You said you had eye irritation or draining and crusting in your eyes when we last
spoke to you on (fill date of late telephone interview). I am going to examine your
eyes now.
0-None
1-Mild
2-Severe
7 - RF
8 - DK
a. Discharge
Left eye
Right eye
b. Injection of
conjunctiva
Left eye
Right eye
c. Chemosis
Left eye
Right eye
3d. Have you been diagnosed with an eye infection since we last spoke to you on (fill
Date of survey or last telephone interview)? Y N RF DK
3e. If YES, was it the ….
Left eye,
Right eye
Both eyes
RF DK
3f. Are you currently being treated with any antibiotics or prescription eye drops? Y N
RF DK
4.
You said you ear ache or an ear infection when we last spoke to you on (fill date of late
telephone interview). I am going to examine your ears now.
0- None
1- Mild
2- Severe 7 – RF
8 - DK
a.
Discharge
Left
ear
Right
ear
b. Canal
edema
Left
ear
Right
ear
c. Canal
erythema
Left
ear
Right
ear
d. Pain
with
traction
Left
ear
Right
ear
4e. Have you been diagnosed with an ear infection since we last spoke to you on (fill
Date of survey or last telephone interview)? Y N RF DK
4f. If yes, was it the …
Left ear,
Right ear
Both ears
RF DK
4g. Are you currently being treated with any antibiotics or prescription ear drops? Y N
RF DK
4h. If Yes, is this oral or drops? Oral/ Drops RF DK
5. Specimen collection
Y
N
RF
5a. If YES-
Stool sample Day 1 / 2 / 3
Skin discharge
Eye discharge
CHEERS QAPP 3
Appendix 2: Specimen Collection System
Fisherbrand* Commode Specimen Collection System
Fisher Health Catalog # 02-544-208
.Stool need not be handled
.Sturdy frame enables container to fit securely under any toilet seat
.Lid is snapped on, and support frame is easily removed by snapping it down for transportation
to lab
.Doubles for female urine output
.Easy-to-read graduations on container
.Directions are printed on container lid in both English and Spanish
CHEERS QAPP 3
Appendix 3: Letter to study participants
CHEERS Thank You letter
v1. 04/03/2008
Thank you for participating in the
CHEERS study.
Remember, we want to know about your
health over the next 21 days of the study
period.
If you have any:
abdominal pain,
vomiting, or diarrhea
-
1. Please collect a stool sample using the stool kit provided.
2. Call the courier service at
1.888.333.9112
to schedule a pick-
up of your specimen (just tell them that you’re in the UIC
CHEERS project).
3. Be sure to have the gold lab requisition form with your ID
number in the bag with the collected specimen.
4. Call the
CHEERS call center at 1.800.688.0582
to report
your symptoms.
If you have any:
eye crusting or drainage, or any skin wounds
with drainage
-
Please call the
CHEERS call center at 1.800.688.0582
to
report your symptoms.
If you need a stool kit or a gold requisition form -
Please call the
CHEERS call center at 1.800.688.0582
.
If you have any other questions or concerns regarding the stool
collection procedure or the home visit-
Please call the
CHEERS nurse at 312-996-3395
.
Note: You will receive an additional $ 75 for providing any of the
above specimens.
Thanks.
Jackee Wuellner, RN
Phone 312-996-3395
CHEERS Thank You letter
v1. 04/03/2008
CHEERS QAPP 3
Appendix 4: Conjunctivitis Rating Scale
Conjunctivitis Rating Scale of Lichtenstein, et. J AOPPS, 2003
CHEERS QAPP 3
Appendix 5: Positive Culture Letter
Stool
Date, Month, 2008
Dear First, Last Name,
On (Month, Date,) 2008 we obtained a stool sample from you. Testing
showed that you have an infection known as (pathogen). Please contact
your doctor and let him/her know that you have this infection. Your doctor
can contact the CHEERS study clinical director for further details. Jackee
Wuellner, RN can be reached at (312) 996-3395.
Enclosed is an information sheet for you about this condition. Be sure
to follow-up soon with your doctor, and if your condition is worsening, be
sure to seek treatment right away.
Thank you for your participation in CHEERS.
Sam Dorevitch, MD, MPH
Study Director
UIC School of Public Health
CHEERS QAPP 3
Appendix 6: Positive Culture Letter
Eye/Wound
Date
Dear ______________,
On _____________ we obtained a (wound/eye) swab from you.
Testing showed that you have an infection known as ___________. Please
contact your doctor and let him/her know that you have this infection.
Your doctor can contact the CHEERS study clinical director for further
details by calling the Clinical Director of the CHEERS study, Jackee
Wuellner, at (312) 996-3395.
Enclosed is an information sheet for you about this condition. Be sure
to follow-up soon with your doctor, and if your condition is worsening, be
sure to seek treatment right away.
Thank you for your participation in CHEERS.
Sam Dorevitch, MD, MPH
Study Director
UIC School of Public Health
CHEERS QAPP 3
Appendix 7: Fact Sheets
Fact Sheet
for the general public
Division of Parasitic Diseases 1 of 4
Cryptosporidium
Infection
Cryptosporidiosis (KRIP-toe-spo-rid-ee-OH-sis)
What is cryptosporidiosis?
Cryptosporidiosis is a diarrheal disease caused by microscopic parasites of the genus
Cryptosporidium
. Once an animal or person is infected, the parasite lives in the
intestine and passes in the stool. The parasite is protected by an outer shell that
allows
it to survive outside the body for long periods of time and makes it very resistant to
chlorine-based disinfectants. Both the disease and the parasite are commonly known
as "crypto."
During the past two decades, crypto has become recognized as one of the most
common causes of waterborne disease within humans in the United States. The
parasite may be found in drinking water and recreational water in every region of the
United States and throughout the world.
How is cryptosporidiosis spread?
Cryptosporidium
lives in the intestine of infected humans or animals. Millions of
crypto
germs can be released in a bowel movement from an infected human or animal.
Consequently,
Cryptosporidium
is found in soil, food, water, or surfaces that have
been
contaminated with infected human or animal feces. If a person swallows the parasite
they become infected. You
cannot
become infected through contact with blood. The
parasite can be spread by
• Accidentally putting something into your mouth or swallowing something that has
come into contact with feces of a person or animal infected with
Cryptosporidium
.
• Swallowing recreational water contaminated with
Cryptosporidium
(Recreational
water includes water in swimming pools, hot tubs, jacuzzis, fountains, lakes, rivers,
springs, ponds, or streams that can be contaminated with sewage or feces from
humans or animals.)
Note:
Cryptosporidium
can survive for days in swimming
pools with adequate chlorine levels.
• Eating uncooked food contaminated with
Cryptosporidium
. Thoroughly wash with
clean, safe water all vegetables and fruits you plan to eat raw. See below for
information on making water safe.
• Accidentally swallowing
Cryptosporidium
picked up from surfaces (such as
bathroom fixtures, changing tables, diaper pails, or toys) contaminated with feces
from an infected person.
What are the symptoms of cryptosporidiosis?
2 of 4
The most common symptom of cryptosporidiosis is watery diarrhea. Other symptoms
include:
• Dehydration
• Weight loss
• Stomach cramps or pain
• Fever
• Nausea
• Vomiting
Some people with crypto will have no symptoms at all. While the small intestine is
the site most commonly affected,
Cryptosporidium
infections could possibly affect
other areas of the digestive or the respiratory tract.
How long after infection do symptoms appear?
Symptoms of cryptosporidiosis generally begin 2 to 10 days (average 7 days) after
becoming infected with the parasite.
How long will symptoms last?
In persons with healthy immune systems, symptoms usually last about 1 to 2 weeks.
The symptoms may go in cycles in which you may seem to get better for a few days,
then feel worse again before the illness ends.
If I have been diagnosed with
Cryptosporidium
, should I worry about
spreading the infection to others?
Yes,
Cryptosporidium
can be very contagious. Follow these guidelines to avoid
spreading the disease to others:
1. Wash your hands with soap and water after using the toilet, changing diapers,
and before eating or preparing food.
2. Do not swim in recreational water (pools, hot tubs, lakes or rivers, the ocean,
etc.) if you have cryptosporidiosis and for at least 2 weeks after diarrhea stops.
You can pass
Cryptosporidium
in your stool and contaminate water for several
weeks after your symptoms have ended. This has resulted in outbreaks of
cryptosporidiosis among recreational water users.
Note:
Cryptosporidium can be
spread in a chlorinated pool because it is resistant to chlorine and, therefore, can
live for days in chlorine-treated swimming pools.
3. Avoid fecal exposure during sexual activity.
Who is most at risk for cryptosporidiosis?
People who are most likely to become infected with
Cryptosporidium
include:
• Children who attend day care centers, including diaper-aged children
• Child care workers
• Parents of infected children
• International travelers
• Backpackers, hikers, and campers who drink unfiltered, untreated water
• Swimmers who swallow water while swimming in swimming pools, lakes, rivers,
ponds, and streams
• People who drink from shallow, unprotected wells
• People who swallow water from contaminated sources.
Contaminated water includes water that has not been boiled or filtered. Several
community-wide outbreaks of cryptosporidiosis have been linked to drinking
municipal
water or recreational water contaminated with
Cryptosporidium.
Who is most at risk for getting seriously ill with
cryptosporidiosis?
3 of 4
Although Crypto can infect all people, some groups are more likely to develop more
serious illness.
• Young children and pregnant women may be more susceptible to the dehydration
resulting from diarrhea and should drink plenty of fluids while ill.
• If you have a severely weakened immune system, you are at risk for more serious
disease. Your symptoms may be more severe and could lead to serious or
lifethreatening illness. Examples of persons with weakened immune systems include
those with HIV/AIDS; cancer and transplant patients who are taking certain
immunosuppressive drugs; and those with inherited diseases that affect the immune
system.
If you have a severely weakened immune system, talk to your health
care provider for additional guidance.
You can also call the CDC AIDS HOTLINE toll-free at 1-800- 342-2437. Ask for more information
on cryptosporidiosis, or go to the CDC fact sheet
Preventing Cryptosporidiosis:
A Guide for People with Compromised Immune Systems
available by visiting
What should I do if I think I may have cryptosporidiosis?
If you suspect that you have cryptosporidiosis, see your health care provider.
How is a cryptosporidiosis diagnosed?
Your health care provider will ask you to submit stool samples to see if you are
infected.
Because testing for Crypto can be difficult, you may be asked to submit several stool
specimens over several days. Tests for Crypto are not routinely done in most
laboratories;therefore, your health care provider should specifically request testing
for the parasite.
What is the treatment for cryptosporidiosis?
A new drug, nitazoxanide, has been approved for treatment of diarrhea caused by
Cryptosporidium
in people with healthy immune systems. Consult with your health
care provider for more information. Most people who have a healthy immune system
will recover without treatment. The symptoms of diarrhea can be treated. If you
have diarrhea, drink plenty of fluids to prevent dehydration. Rapid loss of fluids from
diarrhea may be especially life threatening to babies; therefore, parents should talk
to their health care provider about fluid replacement therapy options for infants.
Anti-diarrheal medicine may help slow down diarrhea, but talk to your health care
provider before taking it.
People who are in poor health or who have a weakened immune system are at
higher risk for more severe and more prolonged illness. The effectiveness of
nitazoxanide in immunosuppressed individuals is unclear. For persons with AIDS,
anti-retroviral therapy that improves immune status will also decrease or eliminate
symptoms of Crypto. However, even if symptoms disappear, cryptosporidiosis is
usually not curable and the symptoms may return if the immune status worsens. See
your health care provider to discuss anti-retroviral therapy used to improve your
immune status.
How can I prevent cryptosporidiosis?
Practice good hygiene.
1. Wash hands thoroughly with soap and water. a. Wash hands after using the toilet
and before handling or eating food (especially for persons with diarrhea). b. Wash
hands after every diaper change, especially if you work with diaper-aged children,
even if you are wearing gloves.
4 of 4
2.
Protect others by not swimming if you are experiencing diarrhea (essential for
children in diapers).
Avoid water that might be contaminated.
1. Do not swallow recreational water.
2. Do not drink untreated water from shallow wells, lakes, rivers, springs, ponds,
and streams.
3. Do not drink untreated water during community-wide outbreaks of disease caused
by contaminated drinking water.
4. Do not use untreated ice or drinking water when traveling in countries where the
water supply might be unsafe. In the United States, nationally distributed brands of
bottled or canned carbonated soft drinks are safe to drink. Commercially packaged
non-carbonated soft drinks and fruit juices that do not require refrigeration until
after they are opened (those that are stored unrefrigerated on grocery shelves) also
are safe. If you are unable to avoid using or drinking water that might be
contaminated, then you can make the water safe to drink by doing one of the
following:
• Heat the water to a rolling boil for at least 1 minute. OR
• Use a filter that has an absolute pore size of at least 1 micron or one that has been
NSF rated for "cyst removal."
Do not rely on chemicals to disinfect water and kill
Cryptosporidium.
Because it has a
thick outer shell, this particular parasite is highly resistant to disinfectants such as
chlorine and iodine.
Avoid food that might be contaminated.
1. Wash and/or peel all raw vegetables and fruits before eating.
2. Use safe, uncontaminated water to wash all food that is to be eaten raw.
3. Avoid eating uncooked foods when traveling in countries with minimal water
treatment and sanitation systems.
Take extra care when traveling.
If you travel to developing nations, you may be at a greater risk for
Cryptosporidium
infection because of poorer water treatment and food sanitation.
Warnings about food, drinks, and swimming are even more important when
visiting developing countries. Avoid foods and drinks, in particular raw fruits and
vegetables, tap water, or ice made from tap water, unpasteurized milk or dairy
products, and items purchased from street vendors. These items may be
contaminated with
Cryptosporidium.
Steaming-hot foods, fruits you peel yourself,
bottled and canned processed drinks, and hot coffee or hot tea are probably safe.
Talk with your health care provider about other guidelines for travel abroad.
Avoid fecal exposure during sexual activity.
This fact sheet is for information only and is not meant to be used for self-diagnosis or as a substitute for
consultation with a health care provider.
For information on choosing safe bottled water, see the CDC fact sheet entitled
“Preventing Cryptosporidiosis: A Guide to Water Filters and Bottled Water,”
available by visiting http://www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/
For information on choosing a water filter, see the CDC fact sheet entitled “Preventing Cryptosporidiosis:
A Guide to Water Filters and Bottled Water,” available by visiting
http://www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/
Salmonella
What is salmonellosis?
Salmonellosis is an infection with a bacteria called Salmonella. Most persons infected
with Salmonella develop diarrhea, fever, and abdominal cramps 12 to 72 hours after
infection. The illness usually lasts 4 to 7 days, and most persons recover without
treatment. However, in some persons the diarrhea may be so severe that the patient needs
to be hospitalized. In these patients, the Salmonella infection may spread from the
intestines to the blood stream, and then to other body sites and can cause death unless the
person is treated promptly with antibiotics. The elderly, infants, and those with impaired
immune systems are more likely to have a severe illness.
What sort of germ is Salmonella?
The Salmonella germ is actually a group of bacteria that can cause diarrheal illness in
humans. They are microscopic living creatures that pass from the feces of people or
animals, to other people or other animals. There are many different kinds of Salmonella
bacteria. Salmonella serotype Typhimurium and Salmonella serotype Enteritidis are the
most common in the United States. Salmonella has been known to cause illness for over
100 years. They were discovered by a American scientist named Salmon, for whom they
are named.
How can Salmonella infections be diagnosed?
Many different kinds of illnesses can cause diarrhea, fever, or abdominal cramps.
Determining that Salmonella is the cause of the illness depends on laboratory tests that
identify Salmonella in the stools of an infected person. These tests are sometimes not
performed unless the laboratory is instructed specifically to look for the organism. Once
Salmonella has been identified, further testing can determine its specific type, and which
antibiotics could be used to treat it.
How can Salmonella infections be treated?
Salmonella infections usually resolve in 5-7 days and often do not require treatment
unless the patient becomes severely dehydrated or the infection spreads from the
intestines. Persons with severe diarrhea may require rehydration, often with intravenous
fluids. Antibiotics are not usually necessary unless the infection spreads from the
intestines, then it can be treated with ampicillin, gentamicin,
trimethoprim/sulfamethoxazole, or ciprofloxacin. Unfortunately, some Salmonella
Salmonella p. 2
bacteria have become resistant to antibiotics, largely as a result of the use of antibiotics to
promote the growth of feed animals.
Are there long term consequences to a Salmonella infection?
Persons with diarrhea usually recover completely, although it may be several months
before their bowel habits are entirely normal. A small number of persons who are
infected with Salmonella, will go on to develop pains in their joints, irritation of the eyes,
and painful urination. This is called Reiter's syndrome. It can last for months or years,
and can lead to chronic arthritis which is difficult to treat. Antibiotic treatment does not
make a difference in whether or not the person later develops arthritis.
How do people catch Salmonella?
Salmonella live in the intestinal tracts of humans and other animals, including birds.
Salmonella are usually transmitted to humans by eating foods contaminated with animal
feces. Contaminated foods usually look and smell normal. Contaminated foods are often
of animal origin, such as beef, poultry, milk, or eggs, but all foods, including vegetables
may become contaminated. Many raw foods of animal origin are frequently
contaminated, but fortunately, thorough cooking kills Salmonella. Food may also become
contaminated by the unwashed hands of an infected food handler, who forgot to wash his
or her hands with soap after using the bathroom.
Salmonella may also be found in the feces of some pets, especially those with diarrhea,
and people can become infected if they do not wash their hands after contact with these
feces. Reptiles are particularly likely to harbor Salmonella and people should always
wash their hands immediately after handling a reptile, even if the reptile is healthy.
Adults should also be careful that children wash their hands after handling a reptile.
What can I do to prevent salmonellosis?
•
Cook poultry, ground beef, and eggs thoroughly before eating. Do not eat or drink
foods containing raw eggs, or raw unpasteurized milk.
•
If you are served undercooked meat, poultry or eggs in a restaurant, don't hesitate
to send it back to the kitchen for further cooking.
•
Wash hands, kitchen work surfaces, and utensils with soap and water immediately
after they have been in contact with raw meat or poultry.
•
Be particularly careful with foods prepared for infants, the elderly, and the
immunocompromised.
•
Wash hands with soap after handling reptiles or birds, or after contact with pet
feces.
•
Avoid direct or even indirect contact between reptiles (turtles, iguanas, other
lizards, snakes) and infants or immunocompromised persons.
Salmonella p. 3
•
Don't work with raw poultry or meat, and an infant (e.g., feed, change diaper) at
the same time.
•
Mother's milk is the safest food for young infants. Breast-feeding prevents
salmonellosis and many other health problems.
Date: November 4, 2006
Content source: Coordinating Center for Infectious Diseases / Division of Bacterial and
Mycotic Diseases
Adapted from: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/salmonellosis_g.htm
CDC Answers Your Questions About
Noroviruses:
What are noroviruses?
Noroviruses are a group of viruses that cause the “stomach flu,” or gastroenteritis (GAS-tro-
enter- I-tis), in people. The term norovirus was recently approved as the official name for this
group of viruses. Several other names have been used for noroviruses, including:
•
Norwalk-like viruses (NLVs)
•
caliciviruses (because they belong to the virus family
Caliciviridae
)
•
small round structured viruses.
Viruses are very different from bacteria and parasites, some of which can cause illnesses
similar to norvirus infection. Viruses are much smaller, are not affected by treatment with
antibiotics, and cannot grow outside of a person’s body.
What are the symptoms of illness caused by noroviruses?
The symptoms of norovirus illness usually include nausea, vomiting, diarrhea, and some
stomach cramping. Sometimes people additionally have a low-grade fever, chills, headache,
muscle aches, and a general sense of tiredness. The illness often begins suddenly, and the
infected person may feel very sick. The illness is usually brief, with symptoms lasting only
about 1 or 2 days. In general, children experience more vomiting than adults. Most people
with norovirus illness have both of these symptoms.
What is the name of the illness caused by noroviruses?
Illness caused by norovirus infection has several names, including:
•
stomach flu – this “stomach flu” is
not
related to the flu (or influenza), which is a
respiratory illness caused by influenza virus.
•
viral gastroenteritis – the most common name for illness caused by norovirus.
Gastroenteritis refers to an inflammation of the stomach and intestines.
•
acute gastroenteritis
•
non-bacterial gastroenteritis
•
food poisoning (although there are other causes of food poisoning)
•
calicivirus infection
How serious is norovirus disease?
Norovirus disease is usually not serious, although people may feel very sick and vomit many
times a day. Most people get better within 1 or 2 days, and they have no long-term health
effects related to their illness. However, sometimes people are unable to drink enough liquids
to replace the liquids they lost because of vomiting and diarrhea. These persons can become
dehydrated and may need special medical attention. This problem with dehydration is usually
only seen among the very young, the elderly, and persons with weakened immune systems.
There is no evidence to suggest that an infected person can become a long-term carrier of
norovirus.
How do people become infected with noroviruses?
Noroviruses are found in the stool or vomit of infected people. People can become infected
with the virus in several ways, including:
CDC Answers Your Questions About Norovirus
•
eating food (see food handler fact sheet) or drinking liquids that are contaminated with
norovirus;
•
touching surfaces or objects contaminated with norovirus, and then placing their hand in
their mouth;
•
having direct contact with another person who is infected and showing symptoms (for
example, when caring for someone with illness, or sharing foods or eating utensils with
someone who is ill).
Persons working in day-care centers or nursing homes should pay special attention to
children or residents who have norovirus illness. This virus is very contagious and can spread
rapidly throughout such environments.
When do symptoms appear?
Symptoms of norovirus illness usually begin about 24 to 48 hours after ingestion of the virus,
but they can appear as early as 12 hours after exposure.
Are noroviruses contagious?
Noroviruses are very contagious and can spread easily from person to person. Both stool and
vomit are infectious. Particular care should be taken with young children in diapers who may
have diarrhea.
How long are people contagious?
People infected with norovirus are contagious from the moment they begin feeling ill to at
least 3 days after recovery. Some people may be contagious for as long as 2 weeks after
recovery. Therefore, it is particularly important for people to use good handwashing and
other hygienic practices after they have recently recovered from norovirus illness.
Who gets norovirus infection?
Anyone can become infected with these viruses. There are many different strains of
norovirus, which makes it difficult for a person’s body to develop long-lasting immunity.
Therefore, norovirus illness can recur throughout a person’s lifetime. In addition, because of
differences in genetic factors, some people are more likely to become infected and develop
more severe illness than others.
What treatment is available for people with norovirus infection?
Currently, there is no antiviral medication that works against norovirus and there is no
vaccine to prevent infection. Norovirus infection cannot be treated with antibiotics. This is
because antibiotics work to fight bacteria and not viruses. Norovirus illness is usually brief in
healthy individuals. When people are ill with vomiting and diarrhea, they should drink plenty
of fluids to prevent dehydration. Dehydration among young children, the elderly, the sick,
can be common, and it is the most serious health effect that can result from norovirus
infection. By drinking oral rehydration fluids (ORF), juice, or water, people can reduce their
chance of becoming dehydrated. Sports drinks do not replace the nutrients and minerals lost
during this illness.
Can norovirus infections be prevented?
Yes. You can decrease your chance of coming in contact with noroviruses by following these
CDC Answers Your Questions About Norovirus
preventive steps:
•
Frequently wash your hands, especially after toilet visits and changing diapers and before
eating or preparing food.
•
Carefully wash fruits and vegetables, and steam oysters before eating them.
•
Thoroughly clean and disinfect contaminated surfaces immediately after an episode of
illness by using a bleach-based household cleaner.
•
Immediately remove and wash clothing or linens that may be contaminated with virus
after an episode of illness (use hot water and soap).
•
Flush or discard any vomitus and/or stool in the toilet and make sure that the surrounding
area is kept clean.
Persons who are infected with norovirus should not prepare food while they have symptoms
and for 3 days after they recover from their illness (see food handler information sheet)
. Food
that may have been contaminated by an ill person should be disposed of properly.
Diarrhea
Alternative names
Stools - watery; Frequent bowel movements; Loose bowel movements
Definition
Diarrhea is loose, watery, and frequent stool. Diarrhea is considered chronic (long-term)
when you have had loose or frequent stools for more than 4 weeks.
Considerations
Diarrhea in adults is usually mild and goes away quickly without complications. In
infants and children (especially under age 3), diarrhea can cause dehydration fairly
quickly.
Common Causes
The most common cause of diarrhea is viral gastroenteritis, a mild viral infection that
goes away on its own within a few days. This condition is often called the stomach flu.
Viral gastroenteritis often occurs in mini-epidemics in schools, neighborhoods, or
families.
Food poisoning and traveler's diarrhea are two other common causes of diarrhea. They
occur as a result of eating food or drinking water contaminated with bacteria or parasites.
Medications, especially antibiotics, laxatives containing magnesium, and chemotherapy
for cancer treatment, can also cause diarrhea.
The following medical conditions can also lead to diarrhea:
•
Malabsorption syndromes such as lactose intolerance
•
Inflammatory bowel diseases (Crohn's disease and ulcerative colitis)
•
Irritable bowel syndrome (IBS)
•
Celiac disease
Other less common causes of diarrhea include:
•
Zollinger-Ellison syndrome
•
Nerve disorders like autonomic neuropathy or diabetic neuropathy
•
Carcinoid syndrome
•
Gastrectomy (partial removal of the stomach)
•
High dose radiation therapy
Diarrhea p. 2
Home Care
•
Drink plenty of fluid to avoid becoming dehydrated. Start with sips of any fluid
other than caffeinated beverages. Milk may prolong loose stools, but also
provides needed fluids and nourishment. Drinking milk may be fine for mild
diarrhea. For moderate and severe diarrhea, electrolyte solutions available in
drugstores are usually best.
•
Active cultures of beneficial bacteria (probiotics) make diarrhea less severe and
shorten its duration. Probiotics can be found in yogurt with active or live cultures
and in supplements.
•
Foods like rice, dry toast, and bananas can sometimes help with diarrhea.
•
Avoid over-the-counter anti-diarrhea medications unless specifically instructed to
use one by your doctor. Certain infections can be made worse by these drugs.
When you have diarrhea, your body is trying to get rid of whatever food, virus, or
other bug is causing it. The medicine interferes with this process.
•
Rest.
If you have a chronic form of diarrhea, like the one caused by irritable bowel syndrome,
try adding bulk to your diet -- to thicken the stool and regulate bowel movements. Such
foods include rice, bananas, and fiber from whole-wheat grains and bran. Psyllium-
containing products such as Metamucil or similar products can also add bulk to stools.
Call your health care provider if :
•
You have blood or pus in your stools or your stool is black
•
You have abdominal pain that is not relieved by a bowel movement
•
You have symptoms of dehydration
•
You have a fever above 101°F, or your child has a fever above 100.4°F, along
with diarrhea
•
You have foul smelling or oily-looking stools
•
You have recently traveled to a foreign country
•
You have eaten with other people who also have diarrhea
•
You have started on a new medication
•
Your diarrhea does not get better in 5 days (2 days for an infant or child), or
worsens before that
•
Your child has been vomiting for more than 12 hours (in a newborn under 3
months you should call as soon as vomiting or diarrhea begins)
Prevention
•
Wash your hands often, especially after going to the bathroom and before eating.
•
Teach children to not put objects in their mouth.
Diarrhea p. 3
•
When taking antibiotics, try eating food with Lactobacillus acidophilus, a healthy
bacteria. This helps replenish the good bacteria that antibiotics can kill. Yogurt
with active or live cultures is a good source of this good bacteria.
•
Use alcohol-based hand gel frequently
References
Yates J. Traveler's diarrhea. Am Fam Physician. 2005; 71(11): 2095-2100.
Guerrant RL. Practice guidelines for the management of infectious diarrhea. Clin Infect
Dis. 2001; 32(3): 331-351.
Update Date: 5/8/2006
Updated by: Jenifer K. Lehrer, MD, Department of Gastroenterology, Frankford-
Torresdale Hospital, Jefferson Health System, Philadelphia, PA. Review provided by
VeriMed Healthcare Network.
Swimmer's ear
Alternative names
Ear infection - outer ear - acute; Otitis externa - acute
Definition
Swimmer's ear is an inflammation, irritation, or infection of the outer ear and ear canal.
Causes, incidence, and risk factors
Swimmer's ear (otitis externa) is fairly common, especially among teenagers and young
adults. Swimming in polluted water is one way to contract swimmer's ear. The condition
also can be caused by scratching (in) the ear or by an object stuck in it. Trying to clean
wax from the ear canal, especially with cotton swabs or small objects, can irritate or
damage the skin.
Swimmer's ear is occasionally associated with middle ear infection (otitis media) or
upper respiratory infections such as colds. Moisture in the ear makes the ear susceptible
to infection from water-loving bacteria such as Pseudomonas. Other bacteria, and rarely,
fungus, can also cause infection.
Symptoms
•
Ear pain -- may worsen when pulling the outer ear
•
Itching of the ear or ear canal
•
Drainage from the ear -- yellow, yellow-green, pus-like, or foul smelling
Signs and tests
When the doctor looks in the ear, it appears red and swollen, including the ear canal. The
ear canal may appear eczema-like, with scaly shedding of skin. Touching or moving the
outer ear increases the pain. The eardrum may be difficult for the doctor to see with an
otoscope because of the swollen outer canal. Taking some of the ear's drainage and doing
a culture on it may identify bacteria or fungus.
Treatment
The goal of treatment is to cure the infection. The ear canal should be cleaned of drainage
to allow topical medications to work effectively.
Swimmer's ear p. 2
Effective medications include ear drops containing antibiotics to fight infection, and
corticosteroids to reduce itching and inflammation. Ear drops should be used abundantly
(four or five drops at a time) in order to penetrate the end of the ear canal. If the ear canal
is very swollen, a wick may be applied in the ear to allow the drops to travel to the end of
the canal.
Occasionally, pills may be used in addition to the topical medications. Analgesics may be
used if pain is severe. Putting something warm against the ears may reduce pain.
Protect ears from further damage. Do not scratch the ears or insert cotton swabs or other
objects in the ears. Keep ears clean and dry, and do not let water enter the ears when
showering, shampooing, or bathing.
Expectations (prognosis)
Swimmer's ear responds well to treatment, but complications may occur if it is not
treated. Some individuals with underlying medical problems, such as diabetes, may be
more likely to get complications such as malignant otitis externa.
Complications
•
Chronic otitis externa
•
Malignant otitis externa
•
Spread of infection to other areas of the body
Calling your health care provider
Call for an appointment with your doctor if you develop any symptoms of swimmer's ear.
Call your doctor if the symptoms worsen or persist despite treatment, or if new symptoms
appear, including pain and redness of the skull behind the ear or persistent fever.
Prevention
•
Dry the ear thoroughly after exposure to moisture.
•
Avoid swimming in polluted water.
•
Use earplugs when swimming.
•
Consider putting a few drops of a 1:1 mixture of alcohol and white vinegar in the
ears after they get wet. The alcohol and acetic acid prevent bacterial growth.
Update Date: 6/15/2005
Updated by: Monica Gandhi, MD, MPH, Assistant Professor, Division of Infectious Diseases, UCSF, San
Francisco, CA. Review provided by VeriMed Healthcare Network.
http://www.nlm.nih.gov/medlineplus/ency/article/000622.htm
Fact Sheet
for the general public
Division of Parasitic Diseases
Giardia
Infection
Giardiasis (GEE-are-DYE-uh-sis)
1 of 4
What is giardiasis?
Giardiasis (GEE-are-DYE-uh-sis) is a diarrheal illness caused by a one-celled,
microscopic parasite,
Giardia intestinalis
(also known as
Giardia lamblia
). Once an
animal or person has been infected with
Giardia intestinalis
, the parasite lives in the
intestine and is passed in the stool. Because the parasite is protected by an outer
shell, it can survive outside the body and in the environment for long periods of
time.
During the past 2 decades,
Giardia
infection has become recognized as one of the
most common causes of waterborne disease (found in both drinking and recreational
water) in humans in the United States.
Giardia
are found worldwide and within every
region of the United States.
How do you get giardiasis and how is it spread?
The
Giardia
parasite lives in the intestine of infected humans or animals. Millions of
germs can be released in a bowel movement from an infected human or animal.
Giardia
is found in soil, food, water, or surfaces that have been contaminated with
the feces from infected humans or animals. You
can
become infected after
accidentally swallowing the parasite; you
cannot
become infected through contact
with blood.
Giardia
can be spread by:
•
Accidentally putting something into your mouth or swallowing something that has
come into contact with feces of a person or animal infected with
Giardia
.
•
Swallowing recreational water contaminated with
Giardia
. Recreational water
includes water in swimming pools, hot tubs, jacuzzis, fountains, lakes, rivers,
springs, ponds, or streams that can be contaminated with sewage or feces from
humans or animals.
•
Eating uncooked food contaminated with
Giardia
.
•
Accidentally swallowing
Giardia
picked up from surfaces (such as bathroom
fixtures, changing tables, diaper pails, or toys) contaminated with feces from an
infected person.
What are the symptoms of giardiasis?
Giardia
infection can cause a variety of intestinal symptoms, which include
•
Diarrhea
•
Gas or flatulence
•
Greasy stools that tend to float
•
Stomach cramps
•
Upset stomach or nausea.
These symptoms may lead to weight loss and dehydration. Some people with
giardiasis have no symptoms at all.
How long after infection do symptoms appear?
Symptoms of giardiasis normally begin 1 to 2 weeks (average 7 days) after
becoming infected.
D i v i s i o n o f P a r a s i t i c
D i s e a s e s
2 of 4
How long will symptoms last?
In otherwise healthy persons, symptoms of giardiasis may last 2 to 6 weeks.
Occasionally, symptoms last longer.
Who is most likely to get giardiasis?
Anyone can get giardiasis. Persons more likely to become infected include
•
Children who attend day care centers, including diaper-aged children
•
Child care workers
•
Parents of infected children
•
International travelers
•
People who swallow water from contaminated sources.
•
Backpackers, hikers, and campers who drink unfiltered, untreated water
•
Swimmers who swallow water while swimming in lakes, rivers, ponds, and streams
•
People who drink from shallow wells
Contaminated water includes water that has not been boiled, filtered, or disinfected
with chemicals. Several community-wide outbreaks of giardiasis have been linked to
drinking municipal water or recreational water contaminated with
Giardia
.
What should I do if I think I may have giardiasis?
See your health care provider.
How is a
Giardia
infection diagnosed?
Your health care provider will likely ask you to submit stool samples to check for the
parasite. Because
Giardia
can be difficult to diagnose, your provider may ask you to
submit several stool specimens over several days.
What is the treatment for giardiasis?
Several prescription drugs are available to treat
Giardia
. Although
Giardia
can infect
all people, young children and pregnant women may be more susceptible to
dehydration resulting from diarrhea and should, therefore, drink plenty of fluids while
ill.
My child does not have diarrhea, but was recently diagnosed as
having giardiasis. My health care provider says treatment is not
necessary. Is this true?
Treatment is not necessary when the child has no symptoms. However, there are a
few exceptions. If your child does not have diarrhea, but is having nausea, fatigue
(very tired), weight loss, or a poor appetite, you and your health care provider may
wish to consider treatment. If your child attends a day care center where an
outbreak is continuing to occur despite efforts to control it, screening and treating
children who have no obvious symptoms may be a good idea. The same is true if
several family members are ill, or if a family member is pregnant and therefore not
able to take the most effective anti-
Giardia
medications.
If I have been diagnosed with giardiasis, should I worry about
spreading the infection to others?
D i v i s i o n o f P a r a s i t i c
D i s e a s e s
3 of 4
Yes, a
Giardia
infection can be very contagious. Follow these guidelines to avoid
spreading giardiasis to others:
1. Wash your hands with soap and water after using the toilet, changing diapers, and
before eating or preparing food.
2. Do not swim in recreational water (pools, hot tubs, lakes or rivers, the ocean, etc.)
if you have
Giardia
and for at least 2 weeks after diarrhea stops. You can pass
Giardia
in your stool and contaminate water for several weeks after your symptoms
have ended. This has resulted in outbreaks of
Giardia
among recreational water
users.
3. Avoid fecal exposure during sexual activity.
How can I prevent a
Giardia
infection?
Practice good hygiene.
1. Wash hands thoroughly with soap and water.
a. Wash hands after using the toilet and before handling or eating food
(especially for persons with diarrhea).
b. Wash hands after every diaper change, especially if you work with diaper-
aged children, even if you are wearing gloves.
2. Protect others by not swimming if you are experiencing diarrhea (essential for
children in diapers).
Avoid water that might be contaminated.
1. Do not swallow recreational water.
2. Do not drink untreated water from shallow wells, lakes, rivers, springs, ponds, and
streams.
3. Do not drink untreated water during community-wide outbreaks of disease
caused by contaminated drinking water.
4. Do not use untreated ice or drinking water when traveling in countries where
the water supply might be unsafe.
In the United States, nationally distributed brands of bottled or canned carbonated
soft drinks are safe to drink. Commercially packaged non-carbonated soft drinks and
fruit juices that do not require refrigeration until after they are opened (those that
are stored unrefrigerated on grocery shelves) also are safe.
If you are unable to avoid using or drinking water that might be
contaminated, then you can make the water safe to drink by doing one of
the following:
•
Heat the water to a rolling boil for at least 1 minute, OR
•
Use a filter that has an absolute pore size of at least 1 micron or one that has been
NSF rated for "cyst removal."
•
If you cannot heat the water to a rolling boil or use a recommended filter, then try
chemically treating the water by chlorination or iodination.
For information on recreational waterrelated illnesses, visit CDC’s Healthy Swimming website at http://
www.cdc.gov/healthyswimming.
For information on choosing safe bottled water, see the CDC fact sheet entitled
“Preventing Cryptosporidiosis: A Guide to Water Filters and Bottled Water,”
available by visiting http://
www.cdc.gov/ncidod/dpd/parasites/ cryptosporidiosis/
D i v i s i o n o f P a r a s i t i c D i
s e a s e s
4 of 4
Using chemicals may be less effective than boiling or filtering because the amount of
chemical required to make the water safe is highly dependent on the temperature,
pH, and cloudiness of the water.
Avoid food that might be contaminated.
1. Wash and/or peel all raw vegetables and fruits before eating.
2. Use safe, uncontaminated water to wash all food that is to be eaten raw.
3. Avoid eating uncooked foods when traveling in countries with minimal water
treatment and sanitation systems.
Avoid fecal exposure during sexual activity.
If my water comes from a well, should I have my well water tested?
You should consider having your well water tested if you can answer “yes” to any of
the following questions:
•
Are members of your family or others who use your well water becoming
ill?
If yes, your well may be the source of infection.
•
Is your well located at the bottom of a hill or is it considered shallow?
If so, runoff from rain or flood water may be draining directly into your well causing
contamination.
•
Is your well in a rural area where animals graze?
Well water can become
contaminated with feces if animal waste seepage contaminates the ground water.
This can occur if your well has cracked casings, is poorly constructed, or is too
shallow. Tests used to specifically identify
Giardia
are often expensive, difficult, and
usually require hundreds of gallons of water to be pumped through a filter. If you
answered “yes” to the above questions, consider generally testing your well for fecal
contamination by testing it for the presence of coliforms or
E. coli
instead of
Giardia
.
Although tests for fecal coliforms or
E. coli
do not specifically tell you whether
Giardia
is present, these tests will show whether your well water has been contaminated by
fecal matter. These tests are only useful if your well is not routinely disinfected with
chlorine, since chlorine kills fecal coliforms and
E. coli
. If the tests are positive, it is
possible that the water may also be contaminated with
Giardia
or other harmful
bacteria and viruses.
Contact your county health department, your county cooperative extension service,
or a local laboratory to find out who offers water testing in your area. If the fecal
coliform test comes back positive, indicating that your well is fecally contaminated,
discontinue drinking the well water and contact your local water authority for
instructions on how to disinfect your well.
This fact sheet is for information only and is not meant to be used for self-diagnosis or as a substitute for
consultation with a health care provider. If you have any questions about the disease described above or
think that you may have a parasitic infection, consult a health care provider.
For information on choosing a water filter, see the CDC fact sheet entitled
“Preventing Cryptosporidiosis: A Guide to Water Filters and Bottled Water,” available by visiting
http://www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm.
CHEERS QAPP 3
Appendix 8: Low Cost Clinics in Chicago
Low Cost Health-Care Providers
Chicago, IL
Name
Location
Phone #
South/Southwest Chicago
Access Ashland Family Health Center
5256 S. Ashland Avenue
773-434-9216
Access Auburn-Gresham Family
Health Center
8234 S. Ashland Avenue
773-874-1400
Access Booker Family Health Center
747 E. 47th Street
773-624-4800
Access Brandon Family Health Center
8300 S. Brandon Avenue
773-721-7600
Access Grand Family Health Center
5401 S. Wentworth Avenue
773-288-6900
Access Ideal Family Health Center
2413 S. State Street
312-225-6800
Access South State Family Health
Center
5050 S. State Street
773-624-2700
Access Southwest Family Health
Center
4839 W. 47th Street
773-735-2345
Access Taylor Family Health Center
4501 S. State Street
773-548-0800
Access Wells Family Health Center
3747 S. Cottage Grove
773-536-1000
CDPH Englewood Family Health
Center
641 W. 63rd Street
312-747-7831
CDPH Roseland Neighborhood Health
Center
200 E. 115th Street
312-747-9500
CDPH South Chicago
2938 E. 89th Street
312-747-5285
Chicago Family Health Center -
Roseland
556 E. 115th Street
773-785-6800
Chicago Family Health Center- South
Chicago
9119 S. Exchange Avenue
773-768-5000
Christian Community Health Center
9718 S. Halsted Street
773-233-4100
Cook County Woodlawn Health Center 6337 S. Woodlawn
312-747-7700
Cook County Englewood Health Center 1135 W. 69th Street
773-483-5090
Friend Family Health Center - East
800 E. 55th Street
773-702-0660
Friend Family Health Center - West
5843 S. Western Avenue
773-434-8600
Holy Cross Family Medical Center
2701 W. 68th Street
773-884-3400
John Sengstacke Health Center
450 E. 51st
312-572-2900
Komed/Holman Health Center
4259 S. Berkeley Avenue
773-268-7600
TCA Health Center
1029 E. 130th Street
773-995-6300
University of Chicago Friend & Family
Center
800 E. 55th Street
773-702-0660
West Chicago
Access Armitage Family Health Center 2957 W. Armitage Avenue
773-772-4319
Access Austin Family Health Center
5835 W. North Avenue
773-745-1200
Access Cabrini Family Health Center
1858 W. 35th Street
773-523-1000
Access Centro Medico
3700 W. 26th Street
773-542-5203
Access Centro Medico San Rafael
3204 W. 26th Street
773-927-3100
Access Doctors Medical Group
6240 W. 55th Street
773-284-2200
Access Dr. James West Clinic at
Haymarket Center
120 N. Sangamon Street
312-226-7984
x408
Access Humboldt Park Family Health
Center
3202 W. North Avenue
773-489-6333
Access Jackson Family Health Center
2450 W. Jackson Boulevard
773-829-7650
Access Kedzie Family Health Center
3213-21 W. 47th Place
773-254-6044
Access Lawndale Family Health Center 1108 S. Kedzie Avenue
773-722-2712
Access Madison Family Health Center
3800 W. Madison
773-826-6600
Access Near West Family Health
Center
1158 W. Taylor Street
312-455-8640
Access Pilsen Family Health
1817 S. Loomis Street
312-666-6511
Access Plaza Medical Center
2507 W. Cermak Road
773-523-0900
Access Servicios Medicos La Villita
3303 W. 26th Street
773-277-6589
Access Warren Family Health Center
2409 W. Warren Boulevard
312-733-4475
Access West Division Family Health
Center
4401 W. Division Street
773-252-3122
Access Westside Family Health Center
3606 W. 16th Street
773-762-2435
Alivio Medical Center
966 W. 21st Street
312-829-6304
Alivio Medical Center
2355 S. Western Avenue
773- 254-1400
Logan Square Health Center
1108 S. Kedzie
773-722-2712
CDPH Lower West Side
1713 S. Ashland
312-746-5157
CDPH South Lawndale
3059 W. 26th St.
312-747-0066
Circle Family Care
4909 W. Division Street
773-921-8100
Cook County Austin Health Center
4800 W. Chicago Ave.
773-826-9600
Cook County Cicero Health Center
5912 W. Cermak
708-783-9800
Cook County Fantus Health Center
621 S. Winchester
312-864-6224
Erie West Side Family Health Center
646 N. Lawndale Avenue
312-666-3494
Lawndale Christian Health Center -
Arthington
3517 W. Arthington Street
773-843-3000
Lawndale Christian Health Center -
Farragut
2345 S. Christiana Avenue
773-843-3000
Lawndale Christian Health Center -
Ogden
3860 W. Ogden Avenue
773-843-3000
Louise Landau Health Center
3645 W. Chicago Avenue
773-826-3450
North/Northwest Chicago
Access Anixter Center
2020 N. Clybourn Avenue
773-404-5277
Access Evanston-Rogers Park Family
Health Center
1555 W. Howard Street
773-764-3425
Access Humboldt Park Family Health
Center
3202 W. North Avenue
773-278-1880
Access Near North Family Health
Center
361 W. Chestnut Street
312-751-4080
Access Peterson Family Health Center
2655 W. Peterson Avenue
773-271-8880
CDPH Lakeview Neighborhood Health
Center
2849 N. Clark Street
(773) 528-1188
CDPH Uptown Neighborhood Health
Center
845 W. Wilson
312-744-1938
CDPH West Town Neighborhood
Health Center
2418 W. Division Street
312-744-0943
Chicago Health Outreach
1015 W. Lawrence Avenue
773-275-2586
Circle Family Care
118 N. Central Avenue
773-921-1446
Erie Helping Hands Health Center
4759 N. Kedzie
773-588-9640
Erie Humboldt Park Family Health
Center
2750 W. North Avenue
312-666-3494
Erie West Town Family Health Center
1701 W. Superior Street
312-666-3494
Health Service Center
4909 W. Division Street
773-921-8100
James Jordan Family Life Center
2102 W. Madison Street
312-226-2323
Miles Square Health Center
2045 W. Washington
Boulevard
312-413-7810
Near West Family Health Center
2310 W. Roosevelt Road
773-432-4087
PCC Austin Family Health Center
355 N. Mason Avenue
773-378-3347
PCC Lake Street Family Health Center
14 Lake Street
773-921-8100
PCC Salud Family Health Center
5359 W. Fullerton Avenue
773-836-2785
PrimeCare Community Wellness -
Northwest
4235 W. North Avenue
773-278-6868
PrimeCare Community Wellness -
West Logan Square
3924 W. Fullerton Avenue
773-276-2229
PrimeCare Community Wellness -
West Town
1431 N. Western Avenue
312-633-5841
Winfield Moody Health Center
1276 N. Clybourn Avenue
312-337-1073
Chicago Suburbs
Access Alma Family Health Center
318 Madison Street
Maywood, IL 60153
708-344-5300
Access Blue Island Family Health
Center
13000 Maple Avenue
Blue Island, IL 60406
708-385-6100
Access Cicero Family Health Center
5817 W. Cermak Road
Cicero, IL 60804
708-458-0757
Access Des Plaines Valley Health
Center
7450 W. 63rd Street
Summit, IL 60501
708-458-0757
Access Family Health Society
152 W. Lincoln Highway
Chicago Heights, IL 60411
708-754-9687
Access Genesis
1 N. Broadway Street
Des Plaines, IL 60440
847-298-3150
Access Hawthorne Family Health
Center
2307-09 S. Cicero Avenue
Cicero, IL 60804
708-780-9777
Access Maywood Family Health
Center
318 West Madison St.
Maywood, IL 60153
708-344-5300
Access Melrose Park Family Health
Center
8321 W. North Avenue
Melrose Park, IL 60160
708-681-2298
Belvidere Medical Building
2400 Belvidere Road
Waukegan, IL 60085
847-360-6500
Medical Services Northeast Satellite
1819 27th Street
Zion, IL 60099
847-872-1918
Vincennes Health Center
1644 Vincennes Avenue
Chicago Heights, IL 60411
708-756-2001
CHEERS QAPP 3
Appendix 9: Specimen Chain of Custody Log
SPECIMEN TRACKING LOG/ CHAIN of CUSTODY
DATE of
PICK-UP
ID Number
TYPE
CHEERS TEAM
MEMBER SIGNATURE
LABORATORY PERSONNEL SIGNATURE & DATE
CHEERS QAPP 3
Appendix 10: UIC Laboratory: Stool Culture
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 11
STOOL CULTURE
PRINCIPLE:
Acute infectious diarrhea is caused by a number of different agents: bacteria, viruses, and
protozoa. The laboratory routinely searches for the bacteria that are most likely to cause diarrhea.
Stool specimens are cultured in order to isolate one or more of the following pathogens:
Salmonella, Shigella, Edwardsiella, Yersinia, Aeromonas, Plesiomonas, Vibrio, Campylobacter,
and
E. coli
0157:H7.
Campylobacter jejuni
is the most important species of
Campylobacter
in
terms of human disease, usually causing diarrhea (sometimes with blood in the stool), abdominal
pain, nausea, fever, and sometimes vomiting. The most common sources of infection are
unpasteurized milk, and rare or partially cooked poultry. Several new
Campylobacter
species have
been discovered and are associated with new clinical syndromes. In addition, a predominance of
yeast,
Staphylococcus aureus
, beta streptococci, or
Pseudomonas aeruginosa
may be clinically
significant.
Normal stool flora consists of a variety of gram positive and gram negative organisms. In
order to isolate and identify pathogens, a combination of selective and differential media,
biochemical tests, and serological typing are employed. Isolation of
Campylobacter jejuni/coli
depends on the use of selective media, a microaerophilic atmosphere (5% O
2
, 10% CO
2
, 85% N
2
)
and growth optimally at 42
o
C. One selective media used is Campy BAP which is a brucella agar
base with 10 % sheep blood. The agar contains a variety of antibiotics which prevent or retard the
growth of competing enteric flora. A selective enrichment broth is also used to recover low
numbers of organisms. The organisms are slender, spirally curved gram negative rods which
appear as non-hemolytic clear or gray colonies on the media. Colonies may appear at 24 to 72
hours of incubation.
SPECIMEN:
Acceptable Specimens:
1.
Freshly passed stool. For optimal recovery of organisms, collect specimen during
the
Acute stage (first 3 days) of diahrreal disease, prior to antibiotic therapy.
2.
Rectal swabs. Swabs will not yield maximal number or positive cultures. Should be
primarily used to sample feces flora from persons ill in an epidemic or from infants.
Single rectal swabs are of little value in examination of convalescent patients.
3.
Acceptable containers: Plastic stool container, Cairy Blair Transport, Culturette
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 11
Unacceptable Specimens:
1.
More than one specimen per day
2
Specimen collected after 3 hospital day
3.
Specimens received in diapers
4.
Specimens received >24 in transit. Specimens received in Cary Blair can be held
for up to 3 days at room temperature.
5.
Dried specimens
EQUIPMENT AND MATERIALS:
Equipment:
Campy jar
Campy gas generator
42
o
C incubator
35
o
C incubator
Materials:
Blood Agar Plate (BAP)
Hektoen Enteric Agar Plate (HE)
MacConkey Agar Plate (MAC)
Campylobacter BAP (CVA)
Campylobacter Thioglycollate
QUALITY CONTROL:
Materials:
3 CVA agar plates
2 Campylobacter Thioglycollate
Tryptic Soy Broth (TSB)
Sterile swabs
Sterile tubes
Sterile saline
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 3 of 11
10 ul loops
Quality control is to be performed on each new shipment of CVA and Campylobacter
Thioglycollate. The organisms to be used for Quality Control testing are the following:
Campylobacter jejuni
ATCC 33291
Staphylococcus epidermidis
ATCC 14990
Candida albicans
ATCC 10231
Escherichia coli
ATCC 25922
These isolates are frozen at -70
o
C. These organisms are subcultured to a blood agar plate
when quality control testing is to be performed. The organisms are streaked for isolation
and incubated at 35 - 37
o
C or 42
o
C. The organisms are to be subcultured at least three times
prior to testing. The organisms may then be set up for testing using the following
instructions:
1.
Designate plates and inoculate as follows:
CVA :
(# 1)
l/2
C. jejuni
1/2
E. coli
(# 2)
1/2
S. epidermidis
1/2
C. albicans
(# 3)
Sterility
Campy Thio:
(#1)
C. jejuni
(#2)
Sterility
2.
To perform QC for the CVA prepare the following suspension:
a.
Obtain fresh stock cultures. Suspend several isolated colonies in TSB to match the
turbidity of a 0.5 McFarland standard.
b.
Dilute the basic cell suspension 1:10 in sterile normal saline (9 ml saline with 1 ml
of the cell suspension added).
c.
Inoculate each organism suspension to the CVA using a 10 ul loop (0.01 ml
disposable blue loop) or 10 ul MLA pipette.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 11
d.
Incubate as described in Campy gas at 42
o
C.
e.
Examine plates at 24 and 48 hours. Record reactions in the appropriate log sheets,
initial and date.
3.
To perform QC for the
Campylobacter
Thioglycollate prepare the following suspension:
a.
Obtain a fresh subculture of
C. jejuni
. Suspend several isolated colonies in TSB to
match the turbidity of a 0.5 McFarland Standard.
b.
Dilute the basic cell suspension 1:10 in sterile normal saline (9 ml saline with 1 ml
cell suspension added).
***If QC is performed in conjunction with the CVA procedure, the same
C. jejuni
suspension can be used ***
c.
Inoculate the suspension to the Campylobacter Thioglycollate using a 10 ul loop or
10 ul MLA pipette.
d.
Refrigerate at 2 – 8
o
C for 18 to 24 hours.
e.
After 18-24 hours refrigeration, subculture to a CVA, as described in
PROCEDURE
:
Subculture of Campy Thioglycollate
.
f.
Examine subcultured plates after 24 and 48 hours. Record reactions in appropriate
log sheets, initial and date.
4.
Expected Reactions for CVA and
Campylobacter
Thioglycollate are as follows:
ORGANISMS
EXPECTED REACTIONS
C. jejuni
Vigorous growth
E. coli
Weak or no growth
S. epidermidis
Weak or no growth
C. albicans
Weak or no growth
Sterility
No growth
If any deficiencies are observed, notify supervisor for appropriate action. Any corrective action
taken should be noted on the QC worksheet.
STORAGE REQUIREMENTS:
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 11
All media is to be stored at 2 to 8
o
C.
PROCEDURE - STEPWISE:
A.
Culture Examination and Interpretation of Aerobic Cultures (non-campylobacter)
1.
Examine original BAP, MAC, and HE plates at both 24 and 48 hours incubation.
2.
Look for "suspicious colonies" on each plate as follows:
PLATE
SUSPICIOUS COLONIES
BAP
Predominance of yeast,
Staphylococcus aureus
, beta streptococci, or
Pseudomonas aeruginosa,
β
hemolytic gram negative rods (screen
with oxidase)
MAC
Lactose negative colonies (clear to pink colonies)
HE
Lactose negative and/or H
2
S positive colonies (clear colonies with
or
without black centers, or black colonies)
3.
Work up suspicious colonies as follows:
a.
Yeast - gram stain only
b.
S. aureus
- staphaurex
c.
Beta streptococci - slidex
d.
Lactose negative - inoculate Kliglers and LIA
f.
H
2
S positive - inoculate Kliglers and LIA
Refer to appropriate procedure for instructions on these tests.
4.
Interpretation of Kligler’s iron agar and LIA reactions for stool cultures
a.
Read reactions of Kliglers and LIA as described in their procedures.
b.
Using Stool Cultures Flow Chart, incorporate KL and LIA reactions. Follow
directions on flowchart, and perform tests indicated. (Refer to appropriate
procedures for information on performing tests.)
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 6 of 11
5.
Cultures positive for
Salmonella
and
Shigella
a.
All isolates must be sent to IDPH for further serological differentiation.
Streak organisms to a trypic soy agar slant and refer to procedure for
sending specimens to IDPH.
b.
It is not necessary to retype multiple identical isolates on the same culture if
one has already typed positive. Do not send multiple isolates to IDPH.
B.
Culture Examination and Interpretation of
Campylobacter
Cultures
Examination of Direct Plates:
1.
Examine direct plates at 24, 48, and 72 hours of incubation. Examine the Campy
thioglycollate subculture plates at 24 hours and 48 hours. Look for gray or
colorless, nonhemolytic colonies that may be either flat and watery with irregular
edges, or round and convex with entire edges. The colonies may range in size from
pinpoint to large and spready.
1
Treat these organisms like anaerobes. Examine plates quickly and place them back
into special gas mixture.
2.
Gram stain any suspicious colonies, leave safranin on slide for about 3 minutes.
Campylobacter
organisms may appear as:
a)
comma shaped
b)
s-shaped
c)
long spirals
d)
gull winged shaped
3.
If gram stain correlates, perform oxidase and catalase test on suspicious colony.
Campylobacter jejuni/coli
will be oxidase and catalase positive (reactions may be
weak).
4.
If colony has typical morphology on plate, appropriate gram stain, and is oxidase
and catalase positive, report as presumptive
Campylobacter
species. Notify floor or
clinic.
5.
Obtain a blood agar plate. Inoculate plate and streak for isolation.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 7 of 11
6.
Place a Nalidixic acid disk on the first quadrant of the plate.
7.
Place the plate a Campy jar. Open Campy generator and place inside jar. Quickly
close top.
8.
Incubate plates at 42
o
C.
9.
Examine plate at 24 and 48 hours.
10.
From the subculture plate, perform a rapid Sodium Hippurate (See Aerobic
Procedure Manual for procedure).
Subculture of Campy Thioglycollate:
MATERIALS:
Sterile plastic pasteur pipette (with bulb attached)
CVA
Campy jar
Campy generator
Inoculating loop
1.
After 18-24 hours refrigeration at 2 - 8
o
C, remove Campylobacter Thioglycollate.
2.
Place the tip of a sterile plastic pasteur pipette approximately one inch below the
surface of thioglycollate
3.
Withdraw an aliquot towards the surface the tube.
4.
Invert pipette and mix contents in bulb.
5.
Place 2-3 drops of specimen on CVA and streak for isolation.
6.
Place plate in Campy Jar. Place Campy generator in jar and quickly close the top.
Incubate at 42
o
C.
7.
Place only 6 - 8 plates per jar.
Interpretation of Positive Campylobacter Cultures
:
Campylobacter jejuni
will produce the following reactions:
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 8 of 11
TEST
REACTION
42
o
growth
( + )
Nalidixic Acid Disk
S
Sodium Hippurate
( + )
Campylobacter coli
will produce the following reactions:
TEST
REACTION
42
o
growth
( + )
Nalidixic Acid Disk
S
Sodium Hippurate
( - )
If any other results occur or the organism grows at 35
o
C
only
, bring to the attention of the
supervisor or lab rounds.
REPORTING RESULTS:
The workup and reporting of Stool Cultures is to include the reporting of (or absence of) normal
stool flora, reporting of no growth, reporting of the absence of coliforms as part of sparse normal
stool flora, and the exclusion of workup for gram positive stool flora. When reporting stool flora
and negative for enteric pathogens, use to precoded statements in Mysis.
The precoded results are:
1.
Normal Stool Flora, Negative for Enteric Pathogens including Campylobacter, Salmonella,
Shigella, Yersinia, Edwardsiella, Aeromonas, and Pleiomonas
. Use this result when the
following conditions have been met:
a.
absence of pathogens
b.
moderate to many lactose positive gram negative rods
c.
no beta hemolytic oxidase positive gram negative rods
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 9 of 11
d.
Presence or absence of any one of the following: rare to few yeast, rare to few
Staphylococcus aureus
, any amount of gram positive organisms (not yeast or
S.
aureus
).
2.
No growth.
Use this result for no growth cultures.
3.
Sparse Normal Stool Flora, Negative for Enteric Pathogens including Campylobacter,
Salmonella, Shigella, Yersinia, Edwardsiella, Aeromonas, and Pleiomonas
. Use this result
when the following conditions have been met:
a.
absence of pathogens
b.
rare to few lactose positive gram negative rods rare to few yeast, rare to few
Staphylococcus aureus
c.
no beta hemolytic oxidase positive gram negative rods
d.
Presence or absence of any one of the following: rare to few yeast, rare to few
Staphylococcus aureus
, any amount of gram positive organisms (not yeast or
S.
aureus
).
4.
Sparse Normal Stool Flora, No Coliforms Present, Negative for Enteric Pathogens
including Campylobacter, Salmonella, Shigella, Yersinia, Edwardsiella, Aeromonas, and
Pleisiomonas
. Use this result when the following conditions have been met:
a.
absence of pathogens
b.
absence of fermenting gram negative rods
c.
no beta hemolytic oxidase positive gram negative rods
d.
Presence or absence of any one of the following: rare to few yeast, rare to few
Staphylococcus aureus,
any amount of gram positive organisms (not yeast or
S.
aureus
).
5. Yeast and
Staphylococcus aureus
are part of stool flora and will be reported using the
criteria:
Yeast will be reported if it is found to be in pure culture or the predominating organism. No
germ tube or further workup is required unless a physician requests identification. If yeast
is found in pure culture, none of the precoded results can be used. Report the absence of
stool flora, the quantity of yeast, and negative for enteric pathogens. If yeast is the
predominant organism, use one of the precoded results to report the type of normal flora,
then report the quantity of yeast.
6.
Staphylococcus aureus
will be reported if it is found to be in pure culture or the
predominating organism. No susceptibility is required. If
S. aureus
is found in pure culture,
none of the precoded results can be used. Report the absence of stool flora, quantity of
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 10 of 11
staph, and negative for enteric pathogens. If
S. aureus
is the predominant organism, use one
of the precoded results to report the type of normal flora, then report the quantity of staph.
7.
Pseudomonas aeruginosa
is identified when found to be the predominant gram negative
rod. No susceptibility is required. When reporting, use one of the precoded results to report
the type of normal flora, then report the quantity of pseudo.
8. When reporting a stool pathogen, DO NOT use any of the precoded results. There is no
reason to report the presence of stool flora.
9.
Organism identifications done by IDPH or CDC must be stated on the CULTURE
report
. There are precoded comments for IDPH and CDC in Mysis. These precoded
comments must be used along with the confirmed identification of an organism. Example:
Salmonella serotype enteritidis
. Identification confirmed by the Illinois Department of
Health Lab, Chicago, Illinois.
REFERENCES:
Murray et al. 2007.
Manual of Clinical Microbiology
, 9th ed. American Society for Microbiology,
Washington D.C.
Isenberg, Henry D. 1992.
Clinical Microbiology Procedures Handbook
. American Society for
Microbiology, Washington, D.C.
Winn et al. 2006. Koneman’s
Color Atlas and Textbook of Diagnostic Microbiology
, 6th ed.
Lippincott-Raven, Philadelphia, PA.
NCCLS: Quality Assurance for Commercially Prepared Microbiological Culture Media.
(Approved Standard, Third Edition, M22-A3). NCCLS, Villanova, Pa, 2004.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 11 of 11
LIA
R/Y
P/P OR P/Y
Proteus
Providencia
DISCARD
H
2
S Pos
(Kliglers)
NEG
Oxidase
Possible
Salmonella
Vitek Neg
DISCARD
Vitek Pos
H
2
S Neg
(Kliglers)
Call Dr if Pos.
Rounds
.
Oxi Pos
Glucose Pos
(Kliglers)
R/O
Plesiomonas,
Aeromonas,
Vibrio
R/O
Pseudomonas
aeruginosa
Vitek
ID & report ONLY
if predominant.
(No sens.)
Glucose Neg
(Kliglers)
Glucose Neg
(Kliglers)
DISCARD
Lactose 24hr
Kligler
Negativ
Rounds
e
.
Call Dr
. (Send to IDPH. Save all plates
on desk until report comes back from IDPH.)
Positive
Cont’d from Page 1, H2S neg, Oxidase neg, Glucose pos or weak
DISCARD
Gas (Kliglers)
Gas Positive
Gas Negative
LIA
P/P
Indole (LIA) Pos
Indole (LIA) Neg
P/Y
P/P
DISCARD
VITEK
LIA
DISCARD
No pathogen ID-
DISCARD
Salmonella ID
Shigella ID
Yersinia ID
Rounds
, Call Dr, Send to IDPH. Save all plates on desk until report comes bac
CHEERS QAPP 3
Appendix 11: UIC Laboratory: Stool Viral
Culture
A: Virus isolation and identification procedures
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 5
VIRUS ISOLATION AND IDENTIFICATION
PROCEDURES
INTRODUCTION:
A variety of methods are used for the laboratory diagnosis of viral infections. These include
isolation in cell culture; direct examination of clinical material to detect viral particles, antigens,
or nucleic acids; cytohistological evidence of infection; and serologic assays to assess the
individual’s antibody response to infection. No single approach can meet the needs of the
diagnostic virology laboratory. Instead, a combination of methods must be implemented. The
choice of methods involves several factors, including knowledge of the pathogenesis of the
suspected agent(s), the stage of illness, and the availability and utility of various methods for the
particular infection in question.
The field of clinical virology is changing rapidly and a wide variety of testing methodologies are
now available for virus detection. Cell culture isolation, the backbone of the virology laboratory,
has also changed significantly in the past 10 years. The introduction of the centrifugation-
enhanced (shell vial) technique has for the first time, allowed laboratories to provide culture
confirmation within a clinically relevant time frame for many slow-growing viruses.
Rapid identification of viral agents is becoming increasingly important because of the
development and use of antiviral agents. Unfortunately, the conventional method of virus
isolation in standard tube culture can take up to 21 days. The shell vial technique which
combines centrifugation with monoclonal antibody staining has further reduced the time for virus
detection. However, due to the limited optimum sensitivity of a single cell line preparation for
different viral pathogens, several cell lines along with primary monkey kidney cells are often
included for conventional tube or shell vial culture. This practice is both costly and time
consuming. Recently mixed cell cultures have come on the market that ultimately can save time
and money.
PRINCIPLE:
Viruses are obligate intracellular parasites and require a suitable cell culture substrate for
propagation. Since there is no one cell type that will support the replication of all viruses, a
spectrum of different cell types is used to cultivate the medically important viruses. Certain
viruses, including Ebstein-Barr virus and human immunodeficiency virus type 1 are not
culturable in traditional mono-layered cell cultures and require the use of blood WBCs. Several
other viruses remain unculturable or require specialized cell cultures or alternative hosts that are
not generally available in the diagnostic setting. Several Coxsackie A viruses, rabies virus, and
arbovirus are best isolated in mice. In some instances, noninfectious subviral particles may be
present in cells. These are not recoverable by conventional isolation procedures. For example,
the recovery of measles virus from the brain tissue of patients with subacute sclerosing
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 5
panencephalitis was achieved by co-cultivation involving the fusion of brain cells with cell
cultures.
Once a specimen is received in the laboratory, it is processed to yield material suitable for the
inoculation into shell vial cultures. Specimen preparation depends on the specimen type. Swabs
are vortexed in viral transport medium, tissue samples are homogenized, and fluid specimens
usually require only the addition of antimicrobial agents. After specimens are inoculated into the
appropriate cell cultures, the cultures are incubated at 35 – 37ºC. A major emphasis in recent
years has been on providing culture results within the shortest possible time.
SPECIMEN COLLECTION:
Successful laboratory diagnosis of viral infections requires an understanding of the pathogenesis
of the suspected organism and knowledge regarding the stage of infection and the age and
immunocompetence of the infected individual. The success of culture depends on several factors
including the proper choice of specimens, careful collection to optimize recovery of the agent,
and transport of specimens in a manner that maintains viability and minimizes overgrowth with
contaminating organisms. For best correlation with a particular disease, the specimen should
reflect the target organ whenever possible.
A) Special transport media is available for specimen collection.
B) All specimens are processed immediately upon receipt.
C) Those specimens received outside normal working hours are kept at 2 – 8ºC until
processed.
D) All handling and processing of virology specimens must be done under a class II
biological safety cabinet in accordance with the universal precautions for blood-borne
pathogens. Refer to the Laboratory Safety Manual for specific details.
E) Specimen Rejection Criteria
1. All specimens received without an attached identifying label, or label that does
not correspond with the information provided on the accompanying requisition
or transmittal form, will not be tested until the requesting physician is contacted
and asked to either personally identify and label the specimen or submit a new
specimen.
If the physician requests that the test be performed on the
improperly labeled specimen, all subsequent reports will include a comment to
the effect.
2. All specimens submitted for viral isolation must be collected according to
instructions outlined in the Laboratory Users Guide
. All requests for viral
isolation must include the source of specimen and if possible the type of
infection and/or the virus expected. Dry swabs will not be accepted by the
virology lab. All virology specimen transport kits contain detailed instructions
for each type of patient specimen that needs to be collected. The clinician is
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 3 of 5
also encouraged to contact the laboratory if any additional instruction is needed.
The requesting physician will be notified and asked to submit a properly
collected specimen if necessary.
3. Grossly contaminated specimen containers and/or soiled requisition will not be
accepted.
Any spillage is treated with the proper disinfectant and all
contaminated materials disposed in an appropriate biohazard bag. If possible,
another specimen should be submitted following notification of the requesting
physician. The head nurse on the patient’s unit will be notified of all
improperly collected and/or transported specimens.
4. “QNS” specimens: The requesting physician will be notified by telephone and
a canceled test report will be sent out in the computer. If more than one test is
requested, the tests to be performed will be prioritized in consultation with the
requesting physician.
PROCEDURE:
A) Collection and Processing of Clinical Specimens—See Specimen Collection section.
B) Inoculation and Examination of Cell Cultures
1) All routine specimens are inoculated into a cell spectrum of at least two (unless
otherwise specified) cell types to ensure cell susceptibility and virus recovery.
2) Uninoculated cell culture controls from each new lot are incubated along with the
patients. When new cells are checked into the laboratory, a record is kept of cell
type, passage number, and source. Observe the gross and microscopic conditions
of the cultures.
Inspect the cultures for leaking, breakage, pH extremes,
contamination, etc. Microscopically examine representative monolayer to assess
the quality and extent of development (sparse, sub confluent, confluent,
overgrown). Record any problems and if necessary, notify the technical service
of the manufacturer.
3) Patient and control cell cultures are incubated at 35 – 37ºC in exactly the same
manner as patients.
4)
All cultures, including controls, are examined every working day for the
appearance of cytopathic effects (CPE).
The cultures are held 1 – 7 days prior
to issuing a negative report. Preliminary positive results are entered in the
computer and are also reported by telephone (except for HSV request from the
clinics) to the requesting physician. Final computer results are submitted after
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 5
definitive identification has been completed and reviewed and signed out by the
section supervisor.
5) All viral identifications are confirmed with monoclonal antibodies when
available
.
Probable Enteroviruses are sent to IDPH for confirmation.
6) Viral isolates are stored at -70ºC for future reference.
C) Virus Detection and Identification
1) Viral Cytopathogenic Effects
a) A table describing typical viral CPE, inclusion body formation, viral
hemadsorption, virus susceptibility, cell spectrum and other
characteristics helpful in identification procedures is available (Table I).
b) A pictorial atlas of typical virus CPE in stained and unstained cell
cultures has been compiled for the laboratory staff as an additional aid.
c) Representative virus cultures (formalin preserved monolayer)
demonstrating viral CPE is also available for reference.
2) Immunological Methods
a) Neutralization—Enteroviruses. Sent to the state lab per M.D.’s request.
b) Fluorescent Antibody—CMV, HSV, VZV, Adeno group, RSV,
Influenza A/B, and Parainfluenza 1, 2, 3.
INTERPRETATION:
The occurrence of viruses in various clinical specimens is shown in Table II. The significance of
a viral isolate depends upon the nature of the illness and pathogenesis of the virus. For example:
Isolation of a viral agent from the nasopharynx and stool specimens may not be related to the
suspected clinical syndrome and must be interpreted with caution. However, isolation of a viral
agent from the blood, CSF, and/or vesicle fluid provides direct evidence of its involvement in the
disease process.
QUALITY CONTROL:
A) Media and Media Components
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 5
1) Only reagents quality control tested by suppliers to ensure freedom from
Mycoplasma, endogenous viruses, bacteria and other extraneous agents are
used in isolation procedures. Sufficient quantities of the same lot number are
kept in stock supply to ensure uniform and reproducible results.
2) All new lots of media are labeled with receipt date and used within their
specified expiration time.
B) Cell culture Substrates
1) Primary cell cultures (PRMK) and continuous cell cultures (MRC-5, A549) are
obtained from reliable commercial sources and certified to be free of
mycoplasma and endemic viruses.
2) The laboratory staff is trained to be aware and to recognize latent virus
contaminants which may be present in “normal” cell cultures, especially
primary cells of Simian origin.
REFERENCES:
Clark, L.M. “Viruses, Rickettsiae, Chlamydiae, and Mycoplasmas.” ASM Clinical Microbiology
Handbook. Washington, D.C.: ASM Press, 1992.
Evans, Alfred S. Viral Infections of Humans: Epidemiology and Control. New Haven: Plenum
Medical Book co., 1991.
Winn, W. et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6
th
edition.
Philadelphia: J.B. Lippincott Co., 2006.
Murray, Patrick R. Manual of Clinical Microbiology,9
th
ed. Washington D.C.: ASM Press, 2007.
CHEERS QAPP 3
Appendix 11: UIC Laboratory: Stool Viral
Culture
B: Specimen collection for virus isolation
SPECIMEN COLLECTION FOR VIRUS ISOLATION
Please use the swab provided in the kit and break it so that it fits into the tube
containing the transport media. Then securely fasten the cap to prevent unwanted media
leakage. Be sure to specify the source of the specimen on the requisition before sending
it to the lab.
Routine and HSV Isolation
Throat swabs:
Rub the tonsils and posterior nasal passages.
Throat wash:
Have patient gargle several times with 5-10ml saline.
Collect in sterile container, transfer to transport tube.
Nasopharyngeal swabs:
Use small wire swab, insert gently into nose and down into
pharyngeal area.
Nasopharyngeal wash:
Using 5-10ml saline in syringe with plastic tubing attached
or bulb syringe, expel and withdraw several times.
Transfer to transfer tube.
Sputum/Bronchial wash/
Tracheal Aspirate:
Collect in sterile container and transfer approx. 2ml to
transport tube.
Eye:
Rub conjunctiva and any visible exudate.
Lesion or open vesicle:
Rub lesion or absorb vesicle fluid with swab.
Rectal swabs:
Insert swab 2-3cm into anal canal and rotate. An adequate
(nonrotavirus testing)
sample should have visible fecal material attached to swab.
Transfer to transport tube.
Tissue:
Place tissue in transport tube.
For autopsy specimens, separate sets of sterile instruments
should be used to prevent cross contamination. Sections of
each tissue should be placed in separate labeled transport
tubes.
NO FIXATIVE SHOULD BE USED.
Non-Routine Virus Isolation
These types of specimens do not require viral transport media. Send as instructed with
requisition.
Urine:
Collect 10-20ml sterile screw cap jar.
Feces:
Collect in sterile container aprrox. 5grams in screw cap jar
or container with tight lid.
CSF/Pericardial
or other sterile body fluid:
Collect at least 2ml in sterile tube.
Direct Detection
Rsv EIA:
Nasopharyngeal wash or swab are specimens of choice and
may be sent in viral transport medium.
Rotavirus EIA:
Feces in the specimen of choice. 1 gram or 1ml of stool is
required.
Note:
ALL SPECIMENS SHOULD BE PROCESSED IMMEDIATELY. IF
THER IS A DELAY IN TRANSPORT OR SPECIMEN AFTER NORMAL
LABORATORY HOURS, STORE AT 4
o
C.
CHEERS QAPP 3
Appendix 11: UIC Laboratory: Stool Viral
Culture
C: CPE produced by viruses in monolayer cell
cultures
Table 1.
Description of CPE produced by viruses in monolayer cell cultures
Virus
Characteristic CPE
Devolp
ment
a
(days)
Progression
Comments
Adenovirus
Enlarged, rounded cells in tighly
associated grapelike clusters. Some
isolates may produce lattice-type
arrangement of rounded cells.
4 - 7
Moderate
CPE is less characteristic in diploid
fibroblasts and may initially resemble
that of CMV.
Enterovirus
Rounded highly refractile cells in
loose clusters or dispersed
throughout monolayer
2 - 5
Moderate to
rapid,
depending
on virus
CPE is similar for most enteroviruses
(coxsackie A and B, echoviruses, polio,
and enteroviruses 68 to 71).
Herpes
viruses CMV
Plump, rounded cells in elongated
foci parallel to long axis of host cells
5 - 10
Slow
Some isolates may not progress
beyond a few patches of CPE, while
high-titered urine specimens may yield
extensive CPE within 24 h.
Herpes
simplex
Clusters of rounded, ballooned cells
with or without syncytia. Early CPE
is focal but progresses throughout
monolayer.
1 - 3
Moderate to
rapid
CPE may develop more slowly and be
less characteristic in human bibroblasts.
Simian B virus produces similar CPE in
simian cells, including primary rhesus
kidney.
VZV
Foci of enlarged, rounded, refractile
cells with or without syncytia.
Cytoplasmic strands between
infected cells and granularity may be
prominent as CPE progresses.
4 - 7
Slow to
moderate
Orthomyxovir
uses
Influenza A
and B
Variable. No CPE may be produced
or may include granular and
vacuolated appearance or
nonspecific degeneration. Rounded
refractile cells are more frequently
associated with type B.
3 - 5
Moderate
HAd is independent of presence or
degree of CPE
Orthopoxviru
s Vaccinia
Syncytia and clusters of rounded,
enlarged, refractile cells with
cytoplasmic strands bridging foci of
CPE
1 - 3
Moderate to
rapid
Infected cells hemadsorb chicken
RBCs.
Paramyxoviru
ses
Parainfluenza
, mumps,
Newcastle
disease
Variable, with increased rounding,
granularity, and progressive
degeneration. Syncytia are
associated with mumps and
parainfluenza type 2 and 3 viruses.
3 - 7
Moderate
HAd is independent of presence or
degree of CPE
Measles
(rubeola)
Syncytia appear as large
multnucleated refractile areas.
Nuclei may encircle granular mass of
giant cell. Extensive vacuolization
may also be present.
7 - 14
Slow to
moderate
Infected cells may hemadsorb simian
RBCs.
Respiratory
syncytial
Syncytia appear as large
multinucleated refractile areas.
3 - 5
Moderate
CPE in human bibroblasts is less
characteristic and may resemble
nonspecific cellular degneration.
Reoviruses
Noncharacteristic granular
appearance with progressive
degeneration and detaching of
monolayer
7 - 10
Slow to
moderate
CPE may be difficult to distinguish from
nonspecific degeneration of monolayer.
Rhinoviruses
Enteroviruslike
5 - 7
Moderate
CHEERS QAPP 3
Appendix 12: UIH Laboratory Protocol:
Cryptosporidium/Giardia
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
MERIDIAN CRYPTOSPORIDIUM/GIARDIA DIRECT
IMMUNOFLUORESCENT DETECTION
INTRODUCTION:
Protozoans of the genus Cryptosporidium are encountered worldwide and produce a self
limiting gastroenteritis in immunocompetent individuals. The primary symptoms include
explosive, watery diarrhea, accompanied by vomiting, abdominal cramping and low
grade fever lasting two to fourteen days. In immunocompromised patients, symptoms are
more severe and persistent and may result in mortality particularly in patients with
Acquired Immune Deficiency Syndrome. Attempts to find effective chemotherapeutic
agents have been unsuccessful thus far.
Giardia lamblia is the etiologic agent of giardiasis, an important worldwide intestinal
disease. Infections in the Unites States were initially reported from hikers, campers and
fisherman who drank stream water. The incidence of giardiasis has steadily increased
over the last two decades and has become an urban parasite. There has been an increase
in the prevalence of giardiasis children in day care centers, institutionalized individuals
and the homosexual population. In symptomatic cases there may be irritation of the
mucosal lining, dehydration, epigastric pain, flatulence, diarrhea with increased fat and
mucus and anorexia lasting from several weeks to several months.
An association between Cryptosporidium and Giardia infection has not been
demonstrated. However, numerous incidents of coinfection with both organisms has
been documented. The immunofluorescent technique is simple to perform and has been
demonstrated to have a higher sensitivity than traditional staining procedures.
PRINCIPLE:
The Meridian Cryptosporidium/Giardia test utilizes the principle of direct
immunofluorescence. The Detection Reagent contains a mixture of FITC labeled
monoclonal antibodies directed against cell wall antigens of Cryptosporidium
oocysts and
Giardia
cysts. A prepared fecal specimen is treated with the Detection Reagent a
counterstain. The monoclonal antibodies attach to Cryptosporidium
or Giardia antigens
present in the specimen. The slides are rinsed to remove unbound antibodies. A
coverslip is affixed with mounting medium and the slides are examined for apple green
color and characteristic morphology of Cryptosporidium oocysts and Giardia cysts using
a fluorescent microscope. Background material in the specimen is counterstained dull
yellow to red.
SPECIMEN REQUIREMENTS:
Formalinized preserved stool that has been concentrated by the formalin – ethyl acetate
method.
Page 1 of 5
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
MATERIALS:
Detection Reagent:
FITC labeled anti- Cryptosporidium and anti-Giardia
monoclonal antibodies in a buffered solution containing a
protein stabilizer and 0.1% sodium azide
Counterstain:
Eriochrome Black solution
20X Wash Buffer:
Concentrated wash buffer with a preservative
Positive Control-
Formalinized stool preparation of Cryptosporidium oocysts
and Giardia cysts
Mounting Medium:
Buffered glycerol containing formalin, an anitquencher and
0.1% sodium azide
Transfer Loops
Treated Slides
Distilled or deionized water
Wash bottle
Humidity chamber
Microscope coverslips 22 x 50 mm
Applicator sticks
EQUIPMENT:
Fluorescent microscope
PROCEDURE:
A.
Specimen Preparation
1.
To increase the probability of detection in stools with low numbers of
cysts or oocysts, the specimen should be concentrated by the formalin –
ethyl acetate procedure prior to processing. (Refer to procedure manual)
2.
The removal of fecal lipids by ethyl acetate may be particularly beneficial
if the fecal specimen is mucoid. Delipidation will facilitate the adherence
of fecal material to the treated microscope slide.
3.
Allow the slide to air dry completely at room temperature (usually
requires 15-30 minutes).
4.
Place one drop of Detection Reagent in each well.
Page 2 of 5
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
5.
Place one drop of Counterstain in each well.
6.
Mix the reagents with an applicator stick and spread over the entire well.
Do not scratch the treated surface of the slide.
7.
Incubate the slide in a humidified chamber for 30 minutes at room
temperature.
8.
Use a wash bottle to rinse the slide with a gentle stream of 1X Wash
Buffer until excess
Detection Reagent and Counterstain is removed.
DO
NOT SUBMERGE THE SLIDE DURING RINSING.
Avoid
disturbing the specimen or causing cross contamination of the specimens.
9.
Remove excess buffer by tapping the long edge of the slide on a clean
paper towel.
DO NOT ALLOW THE SLIDE TO DRY.
10.
Add one drop of Mounting Medium to each well and apply a coverslip.
11.
Scan each well thoroughly using 100 – 200X magnification. The presence
of Cryptosporidium
oocysts should be confirmed at higher magnification.
INTERPRETATION:
A.
Control Reactions
1.
Cryptosporidium oocysts are round to slightly oval in shape, 2 – 6 microns
in diameter. The oocyst wall will stain bright apple green. A suture line
may also be visible.
2.
Giradia
cysts are oval shaped organisms 8 -12 microns long. The cyst
wall will stain bright apple green.
3.
Background material should counterstain dull yellow to red.
B.
Test Reactions
1.
Cryptosporidium positive test result: Any stool specimen exhibiting one
or more oocysts with an apple green color and characteristic morphology
should be considered positive for the presence of Cryptosporiduim
sp…
2.
Giardia positive test result: Any stool specimen exhibiting one or more
cysts with an apple green color and characteristic morphology should be
considered positive for the presence of Giardia.
Page 3 of 5
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
3.
Negative test result: A stool specimen with no apple green fluorescence
should be considered negative for the presence of Cryptosporiduim
oocysts and Giardia cysts. Background fluorescence debris which does
not exhibit vibrant apple green color or characteristic morphology should
also be considered negative.
NOTES:
1.
Protect the Detection Reagent and Counterstain from exposure to light.
2.
Patient specimens may contain HIV or other infectious agents and should be
handled by properly trained personnel and disposed of as potential biohazards.
Follow universal precautions and utilized appropriate barrier protection.
3.
Specimens preserved in PVA or MF/MIF are not suitable for use with this assay.
Formalin and SAF preserved specimens may be used.
4.
If stool material is not seen upon scanning the slide wells, loss of sample may
have occurred. This is usually due to an overly vigorous wash procedure or
insufficient specimen drying time.
QUALITY CONTROL:
1.
A Positive and negative control should be evaluated each time patient specimens
are tested.
2.
At the time of each use, the kit components should be visually examined for
obvious signs of contamination, freezing or leakage.
3.
The results of each quality control check must be recorded on the appropriate log.
Do Not Report Patient Results if QC Fails.
REFERENCES
Fayer, R., and B. L. Ungar, 1986. Cryptosporidium spp. and Cryptosporidiosis.
Microbiol. Rev. 50:458-483.
MERIFLUOR Crptosporidium/Giardia Direct Immunofluorescent Detection
Procedure, Package Insert, 1991, Meridain Diagnostics, Inc. Cincinnati, OH
45244.
Clinical Microbiology Procedures Handbook
, American Society for
Microbiology, Washington, D. C., 1992.
Page 4 of 5
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Garcia, L.S., D.A. Bruckner,
Diagnostic Medical Parasitology 4
th
edition
,
American Society for Microbiology, Washington, D. C., 2001.
Page 5 of 5
CHEERS QAPP 3
Appendix 13: UIH Laboratory Protocol:
Rotavirus
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 6
MERIDIAN
IMMUNO-CARD STAT ROTAVIRUS
EIA
INTENDED USE:
The
ImmunoCard STAT!
Rotavirus
immunoassay is a rapid
in vitro
qualitative procedure for
the detection of rotavirus antigen in human stool. The test can be used to aid in the diagnosis of
rotavirus associated gastroenteritis.
PRINCIPLE:
Rotavirus is a major cause of acute gastroenteritis, especially in children 6 to 24 months in age.
In addition, rotavirus infections can produce severe illness as well as asymptomatic infection in
adults. The incubation period of rotavirus infection is usually one to three days, followed by
gastroenteritis with an average duration of five to eight days. Virus titers in stool reach a
maximum shortly after the onset of illness, then decline.
Due to inadequacies in existing culture methods, human rotavirus infection is not routinely
isolated from rotavirus-containing specimens. For many years, electron microscopy has been the
standard method for rotavirus detection. However, newly introduced enzyme immunoassays and
latex agglutination assays with increased sensitivities and specificities are now the methods of
choice. The
ImmunoCard STAT!
Rotavirus
test offers a simple, rapid method for detecting
rotavirus antigen in patient stool.
The
Meridian
ImmunoCard Stat!
Rotavirus
assay detects the presence of rotavirus antigen in
stool. Patient specimen is diluted in Sample Diluent and then added to the sample port of the
device. The sample mobilizes gold particles coated with monoclonal antibody to rotavirus and
migrates along the membrane through the
Test
(polyclonal anti-rotavirus antibody) and
Control
zones. After ten minutes, the
Test
and
Control
zones are observed for the presence of
red/purple line in the
Test
zone. No red/purple line in the
Test
zone indicates a negative result.
The
Control
line serves as a procedural control to assure that the sample has migrated the
appropriate distance along the membrane.
SPECIMEN:
For the best results, specimens should be collected after onset of symptoms. Several authors
have reported declining numbers of rotavirus particles after day eight or nine, with peak counts
occurring on days three through five. Samples collected after day eight or nine may be less
reactive than those collected earlier in the course of the disease. Specimens containing high
levels of blood may fail to flow in the
ImmunoCard STAT!
Rotavirus
device, resulting in an
invalid test result. Testing of an additional specimen is recommended under such circumstances.
Stool specimens must be collected into a clean, dry container free of detergent residue. One
gram of stool (about the size of a pea) or one mL of liquid stool is required for test.
Swabs are
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 6
not acceptable
. Any request for Rotavirus collected on a swab is to be canceled and called to
the floor or clinic.
Handling Conditions:
The specimen should be tested as soon as possible, but may be
stored up to 72 hours at 2 – 8ºC prior to testing. If the test cannot be performed within this
time frame, the specimen should be frozen at -20ºC or lower.
EQUIPMENT AND MATERIALS:
Materials Provided:
ImmunoCard STAT! Rotavirus
device
Positive Control-Inactivated rotavirus (SA-11) in a buffer containing 0.02%
thimerosal
Sample Diluent-Buffer containing 0.1% sodium azide as a preservative
Transfer pipets
Materials Not Provided:
12x75 mm test tubes
Applicator sticks
Timer
Preparation:
Allow kit components to reach 21 – 25ºC prior to use.
Gently mix liquid reagents prior to use.
PRECAUTIONS:
1. All reagents are for
in vitro
diagnostic use only.
2. All reagent concentration, incubation times and temperatures (21 – 25ºC) have been
optimized for sensitivity and specificity. Best results are obtained by adhering to these
specifications. Once the assay has been started, complete all subsequent steps without
interruption.
3. Patient specimens and used
ImmunoCard STAT!
Rotavirus
devices may contain
infectious agents and should be handled at Biosafety Level 2 as recommended in the
CDC/NIH manual “Biosafety in Microbiology and Biomedical laboratories” 1988.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 3 of 6
4. Positive Control reagent contains inactivated rotavirus. However, it should be handled
as potential biohazard.
5. All reagents should be gently mixed and at 21 – 25ºC before use.
6. Do not interchange reagents from different kit lot numbers, or use expired reagents.
7. Hold Positive Control vial vertically to insure proper drop size and delivery. Do not
allow the tips of the vial or pipet to touch the sample port.
8. Use only one transfer pipet per control or specimen. Discard after use.
9. Stool must be mixed thoroughly (regardless of consistency) to insure a representative
sample prior to pipetting. Do not use stools that have dried out.
10. Dilution of stool as described in the
PROCEDURE
section is important. Over-
inoculation of stool into the Sample Diluent may restrict movement within the
ImmunoCard STAT!
Rotavirus
device so as to produce an invalid result.
QUALITY CONTROL:
The Positive and Negative Controls should be assayed along with patients and performed each
day test is performed. Patient tests are invalid if controls do not perform to specifications.
1. Add three drops of Positive control directly to
Sample
Port of appropriate device (do
not dilute Positive Control).
2. Using a transfer pipet, add 150 μL Sample Diluent (Negative Control) directly to
Sample
port of appropriate device.
Interpretation of Control Results:
1. The Positive Control should yield a visually detectable red/purple
Test
and
Control
lines.
2. The Negative control should yield a visually detectable red/purple
Control
line. No
Test
line should be present.
STORAGE REQUIREMENTS:
All components must be at 21 – 25ºC prior to use.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 6
PROCEDURE - STEPWISE:
1. Sample dilution
a. Add 350 μL Sample Diluent to one 12x75 mm test tube for each specimen to be
tested.
b. Mix stool thoroughly, regardless of consistency.
c. Liquid or semi-solid stool: Using a transfer pipet, draw stool to the 25 μL
calibration point (first mark from tip of pipet). Dispense the stool into the Sample
Diluent in appropriate 12.75 mm tube. Using the same pipet, gently withdraw and
expel the stool suspension several times, then vortex ten seconds. Leave transfer
pipet in tube for further use. Note: Do not pipet more than 25 μL of stool. Over-
inoculation with stool may produce invalid results. Proceed to Step 2 within 30
minutes.
d. Solid stool: using a wooden applicator stick, transfer a 2 mm diameter portion of
stool into the Sample Diluent in the appropriate 12.75 mm tube. Emulsify the
stool thoroughly using the applicator stick, then vortex ten seconds. Place transfer
pipet in the tube. Proceed to Step 2 within 30 minutes.
2. Remove appropriate number of
ImmunoCard STAT!
Rotavirus
devices from their
pouches. Label appropriately. Use one device per control or sample.
3. Vortex each diluted specimen for ten seconds. Using the original specimen transfer pipet,
draw diluted sample to the 150 μL calibration point (second mark from the tip of the
pipet) and add to
Sample
port.
4. Incubate ten minutes at 21 – 25ºC. Note: During the ten minute incubation, diluted
specimen must move past the
Control
zone.
5. Visually read
Control
and
Test
zones for the presence of absence of a red/purple line at
the end of the incubation period.
INTERPRETATION OF RESULTS:
Positive Test Result:
Visually detectable red/purple
Test
and
Control
lines. A positive result
indicates the presence of rotavirus antigen.
Negative Test Result:
Visually detectable red/purple
Control
line. No red/purple
Test
line
present. A negative result indicates that rotavirus antigen is absent or below the level of
detection.
Invalid Test Result:
No visually detectable red/purple
Control
line, with or without a visually
detectable red/purple
Test
line. An invalid test result may be due to a Reagent/Device problem,
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 6
a procedural error, or over-inoculation of stool into Sample Diluent during specimen dilution.
Re-dilute stool and repeat test. Stools containing high levels of blood may fail to flow properly,
resulting in an invalid result. Testing with an additional specimen is recommended. On
occasion, a stool may have very high levels of rotavirus antigen and will yield a visible
Test
line
and no visible
Control
line. In such cases, the specimen may be diluted two-fold or greater,
beyond original 1:15 dilution (ex. 25 μL stool + 750 μL Sample Diluent) and retested.
REPORTING RESULTS:
Positive Test Result:
All positive test results must be called to the floor or clinic. Under the
function MEH enter the code PROTA with the name, date, and time of the person notified of the
result.
Negative Test Result:
Under the function MEH enter the code NROTA.
Invalid Test Result:
Under the function MEH enter the code IND and free text the comment
“please recollect sample”.
LIMITATIONS OF THE PROCEDURE:
1. The
ImmunoCard STAT!
Rotavirus
test does not define the presence of rotavirus
associated gastroenteritis, but only demonstrates the presence of the antigen in stool. As
with all
in vitro
diagnostic procedures, test results should be interpreted by a physician in
conjunction with other clinical information.
2. Limit of detection in stool specimens is 1.8 – 3.7x10
6
rotavirus particles per test volume.
3. The use of meconium stools in this assay is not recommended as their performance
characteristics have not been evaluated.
4. A positive result does not preclude the presence of other infectious organisms.
REFERENCES:
Meridian
ImmunoCard Stat!
Rotavirus
package insert 12/01. Meridian Diagnostics, Inc.,
Cincinnati, OH.
Winn, W. et al., Koneman’s Color Atlas of Diagnostic Microbiology, 6
th
Ed., Philadelphia, PA
Lippincott, 2006.
CHEERS QAPP 3
Appendix 14: UIH Laboratory Protocol:
Eye Cultures
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 8
EYE CULTURES
PRINCIPLE:
Inflammatory eye conditions may be due to a variety of diseases, and microorganisms play a major
role in both acute and chronic eye diseases. The detection of infectious agents depends on the
knowledge of the site of infection and the severity of the process, because a variety of organisms
cause infections of the eye. Unlike the procedures with other specimen types, it may be important
for the physician to inoculate culture media at the bedside rather than transport the specimen to the
laboratory for processing.
This procedure describes the clinical syndromes associated with bacterial infections of the
eye, the organisms associated with these syndromes, and the procedure for isolation of these
infections.
MICROORGANISMS ENCOUNTERED IN EYE INFECTIONS
A.
Conjunctivitis
1.
Description of syndrome
Conjunctivitis is an acute or chronic inflammation of the conjunctiva, the mucous
membrane covering of the anterior surface (sclera) of the eye. The symptoms
include reddening of the surface, tearing, and a purulent discharge. The source of
the involved bacterial organism is usually direct inoculation of exogenous
organisms from formites, and the environment, etc, but hematogenous spread from
another focus can occur.
2.
Common causes of infection
a.
Haemophilus influenzae
b.
Staphylococcus aureus
c.
Streptococcus pneumoniae
d.
Neisseria gonorrhoeae
3.
Causes of infections in immunocompromised patients
a.
Members of the family
Enterobacteriaceae
b.
Pseudomonas aeruginosa
B.
Bacterial keratitis
1.
Description of the syndrome
Keratitis is defined as an inflammation of the cornea. It may present with a wide
range of symptoms extending from a superficial infection of the corneal epithelium
to deep stromal ulceration that may lead to perforation and/or loss of the eye. It is a
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 8
serious condition requiring prompt and meticulous investigation. Predisposing
factors for corneal ulceration include prior eye disease, contact lens wear, and use
of topical corticosteriods. Other individuals at risk include alcoholics, burn patient
and other immunocompromised patient.
Symptoms of keratitis include redness of the eye, inflammation of the conjunctiva,
increased pain, decreased vision, and photophobia. Patients feel a foreign-body
sensation in the eye that results in tearing and exudate formation.
2.
Common causes of infection
a.
Pseudomonas aeruginosa
(often progresses rapidly and may result in
corneal perforation in a few hours)
b.
Streptococcus pneumoniae
c.
Moraxella sp.
d.
Alpha hemolytic streptococci
e.
Staphylococcus aureus
f.
Coagulase negative staphylococci
3.
Less common bacterial causes of infection
a.
Enterobacteriaceae
b.
Neisseria gonorrhoeae
c.
Neisseria meningitidis
d.
Haemophilus influenzae
e.
Rapidly growing mycobacteria
f.
Actinomyces sp.
g.
Propionibacterium acnes
h.
Clostridium perfringens
4.
Contact lens associated causes of infection
a.
Bacillus sp.
b.
Serratia sp.
C.
Bacterial endophthalmitis
1.
Description of the syndrome
Endophthalmitis is the most serious and sight-threatening infection of the eye. It is
an inflammation of the ocular cavities and intraocular tissue resulting from trauma
to the eye, including surgery, injury, or corneal suppuration and perforation
following keratitis. Endogenous endophthalmitis as a result of bacterial sepsis or
from a contagious site, ie.,
cellulitis, may also occur. The infection following
surgery will often manifest within 72 hours of surgery, presenting with decreased
vision, pain, lid edema, conjunctival hyperemia, sever iridocyclitis, and hypopyon.
Chronic endophthalmitis may follow.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 3 of 8
2.
Common causes of infection
a.
Postsurgical endophthalmitis
1)
Staphylococcus aureus
2)
Coagulase negative staphylococcus
3)
Streptococcus pneumoniae
4)
Streptococcus sp.
5)
Pseudomonas aeruginosa
6)
Other gram-negative bacilli
b.
Postcataract surgery (chronic, occurring months to years after surgery)
1)
Propionibacterium acnes
c.
Posttraumatic endophthalmitis
1)
Bacillus cereus
2)
Bacillus licheniformis
3)
Bacillus subtilis
4)
Clostridium sp.
d.
Endogenous endophthalmitis
1)
Staphylococcus aureus
2)
Streptococcus pneumoniae
3)
Haemophilus influenzae
4)
Neisseria meningitidis
5)
Bacillus sp.
6)
Mycobacterium sp.
(rapid growers)
D.
Preseptal cellulitis
1.
Description of the syndrome
Preseptal cellulitis is an inflammation of the periorbital tissue resulting from
traumatic injury, laceration, or a puncture wound. It may also result from an
extension of impetigo or erysipelas. Symptoms are a warm erythematous eyelid
with conjunctival edema. The condition needs to be differentiated from orbital
cellulitis, a more-systemic, severe infection of the orbit itself.
2.
Common causes of infection
a.
Staphylococcus aureus
b.
Streptococcus pyogenes
c.
other streptococci
d.
Haemophilus influenzae
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 8
3.
Traumatic injury with foreign body contamination
a.
Clostridium sp.
may be involved alone or mixed with aerobes
4.
Uncommon causes of infection
a.
Pseudomonas sp.
b.
Other gram-negative bacilli
E.
Orbital cellulitis
1.
Description of the syndrome
Orbital cellulitis is an infection of the orbital tissue resulting from trauma, surgery,
or an extension of paranasal infection or panophthalmitis. It is a serious, systemic
infection and may cause blindness, septic thrombosis of the cavernous sinus, or
intracranial infections. Symptoms include fever, leukocytosis, lid edema, and
limitation of ocular motility.
2.
Common causes of infection
a.
Postsurgical infection
1.
Staphylococcus aureus
b.
Trauma
1.
Mixed anaerobic and aerobic infections may occur.
c.
Extension from panophthalmitis
1.
Staphylococcus aureus
2.
Streptococcus pneumoniae
3.
Pseudomonas aeruginosa
d.
Extension of a paranasal infection
1.
Haemophilus influenzae
(especially in children less than 5 years old)
2.
Staphylococcus aureus
3.
Streptococcus pyogenes
4.
Streptococcus pneumoniae
5.
Gram-negative bacilli
F.
Miscellaneous eye infections
1.
Dacryoadenitis
a.
Description of the syndrome
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 8
Dacryoadenitis is an infection of the lacrimal glands. Symptoms include
pain and tenderness in the upper lid.
b.
Common causes of infection
1.
Staphylococcus aureus
2.
Streptococcus pyogenes
3.
Streptococcus pneumoniae
2.
Dacryocystitis
a.
Description of the syndrome
Dacryocystitis is an infection of the lacrimal sac that usually follow
obstruction of the nasolacrimal duct. Symptoms include pain, swelling,
redness, and tenderness of the lacrimal gland. A fistula may form.
b.
Common cause of infection
1.
Streptococcus pneumoniae
2.
Staphylococcus aureus
3.
Streptococcus pyogenes
4.
Haemophilus influenzae
3.
Canaliculitis
a.
Description of the syndrome
Canaliculitis is an inflammation of the canaliculus, the passage that
connects the punctum to the lacrimal sac. Symptoms include swelling, pain,
and tenderness at the corner of the eye. The infection may be accompanied
by unilateral conjunctivitis and hyperemia of the eyelid.
b.
Common causes of infection
1.
Actinomyces israelii
2.
Propionibacterium propionicus
3.
Moraxella sp.
4.
Diphtheroids
5.
Alpha hemolytic streptococci
SPECIMEN:
Acceptable Specimens:
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 6 of 8
1.
Sterile tubes: Fluids can be transported in sterile vacutainer tubes without
preservatives
2.
Swabs
3.
Cornea or lens
4.
Specimen received on plates and broth
Unacceptable Specimens:
1.
Mislabelled and unlabelled specimens
2.
Dried swabs, or swabs with ampules not crushed
3.
Specimens received >24 hours after collection
4.
Specimens received in citrate or EDTA tubes
Specimens Types:
A) Fluid Specimens
•
Vitreous fluid
•
Anterior chamber
B) Specimens sent on swabs
•
Conjunctiva
•
Eye lid
•
Corneal rim
•
Lens
•
Cornea
C) Specimens sent on plates and broth:
•
Corneal scraping
•
Vitreous fluid
•
Anterior Chamber
Handling Conditions:
All specimens must be sent to the laboratory as soon as they are collected.
Once the specimen is in the laboratory, it must be processed promptly. If there is a delay in
the processing, eye specimens must be place in the CO2 incubator.
The type of container the specimen came in must be entered into the computer under
SREQ for each test ordered.
EQUIPMENT AND MATERIALS:
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 7 of 8
Equipment:
35
o
C CO
2
Incubator
Laminar Flow Hood
35
o
C ambient air incubator
Materials:
Chocolate Agar Plate (CHOC)
Blood Agar Plate (BAP)
Eugonic Broth
Glass microscope slides (for requested Gram Stains)
Sterile pipette
Sterile forceps
QUALITY CONTROL:
Quality control on all commercially prepared culture media adheres to CLSI (NCCLS) M22-A3
standards. Refer to Protocol for QC on Media procedure.
PROCEDURE - STEPWISE:
A.
Gram Stains
1.
Gram stains are performed upon request only.
2.
Reporting of Gram Stain results: refer to Gram Stain/Direct Exam Procedure
B.
Culture Examination and Interpretation
1.
Initial examination of plates is made 18-24 hours after specimen is processed.
Preliminary reports are updated daily until report is final.
2.
No growth plates are incubated for 72 hours before final report is sent out. No
growth Eugonic broths are incubated for 72 before final report and held for 10 days
before being discarded.
3.
On cultures with growth, Chocolate plate must be incubated for 72 hours (for
Neisseria gonorrhoeae
) before being discarded. Subculture positive Eugonic
broths which have no growth on original plates or no plates were received.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 8 of 8
4.
Report the quantity and organism identity for each morphological type observed on
culture media. Conjunctive and eye lid cultures which grow few to moderate
numbers of multiple
organisms including Coagulase negative staphylococci,
diphtheroids, alpha Strep, Gram negative rods,
Bacillus sp.
, etc. do not identify,
report the relative quantity and type of organisms only, i.e. "4 colonies Staph." Hold
plates on bench a few days in case physician questions report.
5.
Any organisms found in corneal ulcer, vitreous fluid, other sterile site is considered
significant and should be worked up with sensitivities. If these cultures are mixed
with multiple organisms bring up at rounds.
C.
Antimicrobial Susceptibility Testing
1.
For general policy, follow Antimicrobial Susceptibility Testing Protocol.
2.
If
Haemophilus influenzae
is isolated, beta lactamase testing is done.
REPORTING RESULTS:
1.
Report the quantity and organism identity for each morphological type observed
2.
The appropriate physician is informed by phone about possible
Neisseria gonorrhoeae
,
Pseudomonas aeruginosa
, any growth in vitreous fluid cultures.
3.
Eye bank should be notified on any growth found in eye bank cultures.
NOTE
:
The date and name of the person the report is given to must be recorded in the
CULTURE
field of the Mysis report.
REFERENCES:
Isenberg, Henry D. 1992.
Clinical Microbiology Procedures Handbook
. American Society for
Microbiology, Washington, D.C.
Winn, W. et al. 2006
Koneman’s Color Atlas and Textbook of Diagnostic Microbiology
, 6th ed.
Lippincott-Raven, Philadelphia, PA.
CHEERS QAPP 3
Appendix 15: UIH Laboratory Protocol:
Wound Culures
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 5
WOUND CULTURES
PRINCIPLE:
The accumulation of pus, either within an abscess or exuding from a sinus tract or from a
mucocutaneous surface, is one of the indicators of local sepsis. Varying degrees of redness, pain,
and swelling may also be present. Exogenous wound infections may also be present. Exogenous
wound infections include those associated with traumatic injury wounds or decubitus ulcers,
animal or human bites, burns, or foreign bodies in the skin or mucous membranes.
Endogenous wounds and abscesses may be associated with appendicitis, cholecystitis, cellulitis,
dental infections, osteomyelitis, empyema, septic arthritis, sinusitis, or many other internal
infections. Many of these infections are nosocomial, secondary to invasive procedures, surgical
manipulations, or placement of prostheses. Others originate from hematogenous spread from other
primary sites of infection or by direct extension of bacterial contaminants from ruptured viscera,
particularly the large intestine.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 5
SPECIMEN:
Acceptable Specimens:
1. Specimens received on aerobic swabs, gel swabs or anaerobic transport media.
Swabs specimens are the least desirable for culture, as the quantity of the sample
may not be sufficient to ensure adequate recovery of the infectious agent.
2. Stool or rectal swabs sent to rule out VRE.
3. Any source that is not a fluid (the source dictates the order). For example,
•
Pus (from any site)
•
Fluid (from any site)
•
Abscess drainage
•
Abscess (from any site)
•
Abdominal wound
•
Leg wound
Unacceptable Specimens:
1. Mislabeled or unlabeled specimens.
2. Specimens received >24 hours after collection.
3. Specimens received without appropriate source documentation.
4. Specimens received on dried swabs, or swabs with ampules not crushed.
5. Specimens received on expired BBL plus swabs/anaerobic transport media.
Type:
Surface wounds are more often than not colonized with environmental bacteria, and
swab samples often do not reflect the true cause of the infectious process.
Aspiration of fluid or pus from the depths of pustular or vesicular wounds and
abscesses with a sterile needle and syringe is the most desirable method for
collecting material for culture. Ideally, a biopsy specimen of the leading edge of the
wound is the best specimen for culture. The site from which the culture is to be
obtained should first be decontaminated with surgical soap and 70% ethyl or
isopropyl alcohol.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 3 of 5
Handling Conditions:
All specimens must be sent to the laboratory as soon as they are collected.
Once the specimen is in the microbiology laboratory, it must be processed promptly.
If there is a delay in specimen processing, fluid specimens must be placed in the
incubator and swab specimens must be left at room temperature.
EQUIPMENT AND MATERIALS:
Equipment:
35°C CO
2
incubator
Laminar flow hood
Heating block
Materials:
Chocolate agar plate (CHOC)
Blood agar plate (BAP)
MacConkey agar plate (MAC)
Colisitin-Naladixic acid agar plate (CNA)*
Modified Thayer-Martin agar plate (MTM)**
Sterile loops
Glass Microscope slide
Gram stain slip***
* CNA agar is the only media used if the source of the specimen is rectal swab or
stool and the special request is R/O VRE (vancomycin resistant enterococci). This
order is usually from the Stem Cell floor (8WST). No gram stain should be prepared
for this type of order.
** MTM agar should only be used if the source of the specimen is in a genital region
(penile, labial, perineum, rectal, groin, etc.).
*** Gram stain slip should be prepared for those wound cultures accompanied by a
GRAM STAIN ONLY order. Please note the site of the wound under source on the
Gram stain slip. Gram stains should be read before the end of the shift, unless it is
ordered STAT then it should be read within 1 hour.
QUALITY CONTROL:
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 5
Quality control on all commercially prepared culture media adheres to CLSI (formerly NCCLS)
M22-A2
Standards. Refer to Protocol for QC on Media procedure.
PROCEDURE - STEPWISE:
For the fluid specimen:
1. Label specimen, plates, and a glass slide with patient's name and accession number (use the
SunQuest labels).
*NOTE: All of the following steps must be performed inside a laminar flow hood.
2. Mix specimen very well before inoculating plates. Aspirate approximately 1 ml of specimen
using a sterile transfer pipette. Inoculate media by dropping 1 to 2 drops of the specimen
onto each plate.
3. Place one drop of the specimen on a glass slide for a Gram stain (Do not touch slide with the
pipette). If no Gram stain was ordered, label the slide WDCT and place slide on heating
block to dry. If a Gram stain was ordered, label a Gram stain slip with a GRAM STAIN
ONLY label and place slide on heating block to dry.
4. Using a sterile loop, streak each plate to obtain well isolated colonies. Make sure to use a
different loop for each plate.
5. Make a few small stab marks with the loop into the surface of the BAP for demonstration of
subsurface hemolysis.
6. Incubate plates in the CO
2
incubator.
For the swab specimen:
1. If the specimen is received on a double swab or Port-a-Cul transport system, roll one swab
over a small area at the edge of each plate.
2. Take the second swab and roll it over a small area on the glass slide for Gram stain, and
discard swab. If no Gram stain was ordered, label the slide WDCT and place slide on heating
block to dry. If a Gram stain was ordered, label a Gram stain slip with a GRAM STAIN
ONLY label and place slide on heating block to dry.
3. Using a sterile loop, streak each plate to obtain well isolated colonies. Make sure to use a
different loop for each plate.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 5
4. Make a few small stab marks with the loop into the surface of the BAP for demonstration of
subsurface hemolysis.
5. Incubate plates in the CO
2
incubator.
If the specimen is received in an anaerobic transport system and both an aerobic and
anaerobic culture is ordered it will be necessary to use both the swabs carefully. Use one
swab to setup plates for the cultures and the second swab to setup the two Gram stains
necessary for both cultures (refer to Anaerobic Specimen Processing procedure for further
instructions on processing the anaerobic cultures).
CHEERS QAPP 3
Appendix 16: IDPH Laboratory Protocol:
Shigatoxin
CHEERS QAPP 3
Appendix 17: IDPH Laboratory Protocol:
Norovirus
CHEERS QAPP 3
Appendix 18: UIH Laboratory Description
UNIVERSITY OF ILLINOIS MEDICAL CENTER
CLINICAL MICROBIOLOGY LABORATORY
The Clinical Microbiology Laboratory is a full-service laboratory offering
diagnostic bacteriology, mycology, parasitology, virology, and mycobacteriology. The
laboratory receives specimens from in-patients at the University of Illinois Hospital and
the University’s out-patient clinics, as well as from several outreach sites throughout
Illinois and the United States. The Microbiology Laboratory is composed of several
sections including Aerobic and Anaerobic Bacteriology, Mycology, Parasitology,
Mycobacteriology.
The Aerobic Bacteriology Section isolates and identifies clinically significant
microorganisms from clinical specimens and performs antimicrobial susceptibility testing
on these bacterial pathogens. These functions are performed with the Vitek-2 automated
instrument. Additional reference identification and susceptibility testing methods for
other, more fastidious bacterial agents are also available. Blood culturesare performed
using the cultures using the BactiAlert system, which provides continuous monitoring of
blood cultures for the entire 7-day incubation period. The Aerobic Bacteriology
Laboratory performs amplified probe tests for detection of
Neisseria gonorrhoeae
and
Chlamydia trachomatis
in urogenital specimens and also has the capability to perform
“real-time” PCR on nares swab specimens and other specimen types for rapid
detection/identification of methicillin-resistant strains of
Staphylococcus aureus (MRSA).
This lab section also p
erforms isolation and characterization of clinically significant
anaerobic bacteria. For these purposes, the laboratory is equipped with a glove box, a
gas-liguid chromatograph, and other methods to provide accurate identification of
anaerobes.
The Mycology Section of the Laboratory performs identification and anti-fungal
susceptibility testing on clinically significant yeast isolates and provides identification of
pathogenic moulds recovered from clinical specimens, including dermatophytes, moulds
causing wound and systemic infections, and systemic mycotic agents such as
Histoplamsa capsulatum and Blastomyces dermatiditis
.
The Parasitology Section provides services for the diagnosis of various parasitic
infections and has a great deal of expertise in providing diagnostic parasitology services
to several other local hospitals and clinics. Specimens submitted for parasitology include
stool specimens for the detection of pathogenic amoebae, and flagellates, and for
detection/identification of the ova belonging to various nematode (roundworms), cestode
(tapeworms), and trematode (flukes) species. Blood specimens are also submitted for the
diagnosis and species identification of malarial parasites.
The Virology/STD Section provides laboratory services to aid in the diagnosis of
viral infections. Culture methods are available for several viral agents, and enzyme
immunoassay tests are used for detection of several non-cultivable viral agents such as
rotavirus. The Virology Section also performs “real-time” molecular detection assays for
influenza A and B viruses, and, in cooperation with Molecular Pathology, offers
multiplex molecular detection of several other respiratory viruses. This laboratory section
also performs HIV-1 antibody enzyme immunoassays, syphilis serology, and cultures for
Trichomonas vaginalis
.
The Mycobacteriology Section receives specimens for the isolation and
identification of acid-fast organisms including
Mycobacterium tuberculosis
,
Mycobacterium avium
complex, and other important mycobacterial pathogens. The
laboratory utilizes state-of-the-art methods to detect growth and to confirm the identities
of mycobacterial isolates, including the use of chemiluminescent ribosomal RNA probes
for species identification. This laboratory section also performs the new FDA-approved
Quantiferon TB-Golod blood test for diagnosis of active and latent tuberculosis.
The staff of the Clinical Microbiology Laboratory includes over 22 FTE’s.
Technical staff participate in teaching Medical Students, Pathology Residents, and
Infectious Diseases Fellows. Continuing education is eagerly encouraged and promoted
through weekly/monthly teleconferences, seminars, and ongoing lectures. Staff are also
encouraged to avail themselves of the many other educational opportunities that exist on
the University campus for further education and academic advancement.
The Clinical Microbiology Laboratory is certified by the College of American
Pathologists (CAP). The Laboratory subscribes to the CAP Proficiency Testing Program
and currently receives the following Proficiency Surveys from CAP (dates in parentheses
indicate the lab receipt date of surverys from CAP for the year 2007):
Proficiency Test Area
Surveys Received
Receipt Dates
(if known)
Clinical Microscopy
Survey CMM-A
03/19/07
Survey CMM-B
08/06/07
Blood Parasite Identification
Survey BP-A
01/29/07
Survey BP-B
05/21/07
Survey BP-C
09/24/07
Bacteriology
Survey D-A
Survey D-B
Survey D-C
Gram Stains
Survey D5-A
02/26/07
Survey D5-B
07/09/07
Survey D5-C
10/22/07
Mycobacteriology
Survey E-A
Survey E-B
Mycology
Survey F-A
Survey F-B
Survey F-C
Syphilis Serology
Survey G-A
Survey G-B
Survey G-C
Chlamydia IF Antigen Detection
Survey HC1-A
Survey HC1-B
Survey HC1-C
Chlamydia trachomatis/Neisseria gonorrhoeae
by Nucleic Acid Amplification
Survey HC6-A
Survey HC6-B
Survey HC-6-C
Helicobacter pylori Antigen Detection
Survey HPS-A
04/04/07
Survey HPS-B
09/19/07
Bioterrorism Laboratory Preparedness
Survey LPS-A
Survey LPS-B
Parasitology
Survey P-A
Survey P-B
Rapid Anti-HIV-1 Antibody
Survey RHIVW-A
05/02/07
Survey RHIVW-B
09/19/07
Infectious Mononucleosis
Survey S-A
03/29/07
Survey S-B
07/12/07
Survey S-C
11/15/07
Viral Markers
Survey VM-A
Survey VM-B
Survey VM-C
Virology Culture
Survey VR1-A
Survey VR1-B
Survey VR1-C
Virology-Antigen by Immunofluorescence
Survey VR2-A
Survey VR2-B
Survey VR2-C
Virology-Antigen Detection by eia
Survey VR4-A
Survey VR4-B
Survey VR4-C
Director of Clinical Microbiology:
William M. Janda, Ph.D., D(ABMM)
Phone:
312-996-56o8
Laboratory Manager:
Linda Bruno, M.A., MT(ASCP)
Phone:
312-996-3635
Bacteriology/Blood Culture Section Supervisor:
Kathy Ristow, M.S., MT(ASCP)SM
Phone:
312-996-3175
Virology/STD Section Supervisor:
Jo-Ann Curry, B.A., MT(HEW)
Phone: 312-996-5377
Mycology Supervisor:
TBA
0%
20%
40%
60%
Anatomic Pathology
Bacteriology
Hematology
Molecular Pathology & Genetics
Mycobacteriology
Mycology
Parasitology
Routine Chemistry
Syphilis Serology
TDM/Endo
Toxicology
Virology
SUBSPECIALTY
Percent corrected of total graded challenges tested
4th QTR 2006
100.0%
100.0%
86.5%
97.3%
100.0%
100.0%
90.9%
99.9%
100.0%
3rd QTR 2006
100.0%
99.0%
86.8%
98.5%
100.0%
100.0%
87.6%
99.5%
100.0%
2nd QTR 2006
100.0%
97.3%
99.3%
97.2%
100.0%
100.0%
90.9%
99.5%
100.0%
Anatomic
Pathology
Bacteriology Hematology
Molecular
Pathology &
Genetics
Mycobacteriol
ogy
Mycology
Parasitology
Routine
Chemistry
Syphilis
Serology
U
NIVERSITY OF
I
LLINOIS
M
EDICAL
C
ENTER AT
C
PATHOLOGY LABORATORIES
SUBSPECIALTY PROFICIENCY TESTING OVERVIEW
2ND QTR - 4TH QTR, 2006
CHEERS QAPP 3
Appendix 19: UIH Laboratory CLIA
Certification
CHEERS QAPP 3
Appendix 20: IDPH Laboratory CLIA
Certification
CENTERS FOR MEDICARE
&
MEDICATD SERVICES
CLINICAL LABORATORY IMPROVEMENT AMENDMENTS
CER TIFICA TE OF COMPUNCE
I.'
LABORATORY NAME AND ADDRESS
CLLA ID NUMBER
1
11
&byl
14D0691828
ILLINOIS DEPT OF PUBLIC HEALTH LABS
21 21
W TAYLOR STREET
EFFECTrVE DATE
CHICAGO, IL 60612
02/09/2007
LABORATORY DIRECTOR
EXPIRATION DATE
DAVID
F CARPENTER PHD
02/08/2009
This
date
shall
be valid until
the
expiration
date
abave,
but
is
subject to temcation, suspension, Limitation, or other sanctions
for
violation of the
Act
or the replations pmmnlgated thereunder.
Judith
A. Yost, Director
Division of Laboratory Services
Survey and Certification Group
Center for Medicaid and State Operations
If you currently hold a Certificate of Compliance or Certificate of Accreditation, below is a list of the laboratory
specialtieslsubspecialties
you are certified to perform and their effective date:
LAB CERTIFICATION (CODE)
LAB CERTIFICATION (CODE)
EFFECTIVE DATE
BACTERIOLOGY (1 10)
MYCOBACTERIOLOGY (1 15)
MYCOLOGY (1 20)
PARASITOLOGY (130)
VIROLOGY (140)
SYPHILIS SEROLOGY (210)
GENERAL IMMUNOLOGY (220)
ROUTINE CHEMISTRY (310)
ENDOCRINOLOGY (330)
TOXICOLOGY (340)
FOR MORE INFORMATION ABOUT CLLA, VISIT OUR WEBSITE AT WWW.CMS.HHS.GOV1CLJ.A
OR CONTACT YOUR LOCAL STATE AGENCY. PLEASE SEE THE REVERSE FOR
YOUR STATE
AGENCY'S ADDRESS AND PHONE NUMBER.
PLEASE CONTACT YOUR STATE AGENCY FOR ANY CHANGES TO YOUR CURRENT CERTIFICATE.
CHEERS QAPP 3
Appendix 21: IDPH Laboratory Clinical
Quality Manual
DCA001-06-1105
Page 1 of 65
IDENTIFICATION INFORMATION
Document Title:
Illinois Department of Public Health, Division of
Laboratories (DOL), Clinical Quality Manual, DCA001-06-
1105
Effective Date:
12-08-2005
Organizational Title:
Illinois Department of Public Health, Division of Laboratories
Addresses:
1155 S. Oakland St., P.O. Box 2797
2121 W. Taylor
825 N. Rutledge
Carbondale, IL 62902
Chicago, IL 60612
Springfield, IL 62794
Phone: 618-457-5131
Phone: 213-793-4760
217-782-6562
Responsible Officials:
Bernard Johnson, MS, Chief, Division of Laboratories
Karen Meier, Manager, Carbondale Laboratory
George Dizikes, Ph.D., Manager, Chicago Laboratory
David L. Maserang, Ph. D., CLIA Director, Division of Laboratories
Division of Laboratory Quality Administrator:
Maria Kaminski
Scope of this Document:
This Quality Manual has been developed to address the data
quality needs of the Illinois Department of Public Health, Division of Laboratories, in the clinical
area. This Document reflects the overall QA program framework and the management systems
to ensure that clinical data generated by the Division of Laboratories is of acceptable quality to
meet the needs of users and decision-makers. It also describes the delegation of quality
assurance responsibilities within the DOL. This document meets the requirements for a Quality
Manual for the following laboratory certification program:
-
Medicare, Medicaid and CLIA Programs;
Laboratory Requirements Relating to Quality
Systems and Certain Personnel Qualifications;
Final Rule, January 24, 2003
DCA001-06-1105
Page 2 of 65
APPROVALS*:
Maria Kaminski
11/07/2005
Author
Date
Karen Meier
11/28/2005
Carbondale Laboratory Manager
Date
George Dizikes, Ph.D.
11/14/2005
Chicago Laboratory Manager
Date
David L Maserang, Ph.D.
12/01/2005
CLIA Laboratory Director
Date
Maria Kaminski
11/30/2005
Division QA Administrator
Date
Bernard T. Johnson
12/08/2005
Chief, Division of Laboratories
Date
* Original signatures – LIS file.
DCA001-06-1105
Page 3 of 65
Table of Contents
Section
Number
Title
1.0
Quality Assurance Policy
1.1
Scope of the DOL Clinical Quality Manual
2.0
Organization and Responsibilities
2.1
Carbondale Laboratory Organization
2.2
Chicago Laboratory Organization
2.3
Springfield Laboratory Organization
2.4
Laboratory Key Personnel
2.5
Training Procedures
3.0
Laboratory Physical Facilities
3.1
Carbondale Laboratory Physical Facility
3.2
Chicago Laboratory Physical Facility
3.3
Springfield Laboratory Physical Facility
3.4
Laboratory Improvement Section
4.0
General Laboratory System
4.1
Ethics
4.2
Confidentiality
4.3
Specimen Identification and Integrity
4.4
Customer Complaints and Communication
4.5
Personnel Competency Assessment
4.6
Evaluation of Proficiency Testing Performance
5.0
Quality Assurance Objectives
5.1
Test Variability/Reproducibility
5.2
Accuracy
5.3
Precision
6.0
Preanalytical System
6.1
Specimen Management Protocol
6.2
Specimen Collection
6.3
Established Procedures
6.4
New Work or Procedures
6.5
Specimen Receipt and Logging
6.6
Test Requisition Form
6.7
Specimen Submission, Handling, and Referral
6.8
Purchase and Handling of Consumables and Services
7.0
Analytical System
7.1
Standard Operation Procedurs
DCA001-06-1105
Page 4 of 65
Table of Contents
Section
Number
Title
7.2
Test Systems, Equipment, Instruments, Reagents, Materials and Supplies
7.3
Establishment and Verification of Performance Specification
7.4
Maintenance and Function Checks
7.5
Calibration and Calibration Verification
7.6
Control Procedures
7.7
Comparison of Test Results
7.8
Corrective Actions
7.9
Test Records
8.0
Post Analytical System
8.1
Data Review Procedure
8.2
Final Test Reports
8.3
Records
8.4
Corrective Action Report
8.5
Specimen Retention
9.0
Documentation Management
9.1
CLIA Documents
9.2
Routine QA Operating Documents and Analytical Records
9.3
Administrative Records
9.4
Record Storage Procedure
9.5
Data Security
10.0
System Audits
10.1
Scope and Frequency of Audits
10.2
Types of Audits
10.3
Managerial Review Procedure
11.0
Definitions
12.0
References
13.0
Appendices
Appendix 1
Directive
Appendix 2
Administrative Operating Procedures
Appendix 3
Personnel Training Record
Appendix 4
Calibration/Verification of Laboratory Support Equipment
Appendix 5
Comparison of Test Results
Appendix 6
CLIA Personnel File Documentation
Appendix 7
Quarterly Managerial Review Forms
Appendix 8
Semi-Annual Managerial Review Forms
Appendix 9
Quarterly Quality Assurance Report
DCA001-06-1105
Page 5 of 65
1.
QUALITY ASSURANCE POLICY
The mission of the Illinois Department of Public Health (IDPH) is to promote the health of the
people of Illinois through the prevention and control of disease and injury. The IDPH Division
of Laboratories participates in the fulfillment of this mission by providing data of exceptional
quality. Figure 1-1 shows the placement of the Division of Laboratories in IDPH. The high
level of data quality is maintained by an established quality assurance program that provides a
mechanism to evaluate and monitor work quality, identify and correct non-conformities, validate
accuracy, precision, sensitivity and timeliness of analytical results, and provide personnel
training.
Adherence to the quality assurance program requirements is the responsibility of all laboratory
personnel. This responsibility is stated in Directive 01-01,
Quality Assurance/Quality Control
,
dated October 1, 2002, and signed by the Chief of the Division of Laboratories. In addition,
employees participate in the Division’s continuous quality improvement efforts. Through the
process of continuous quality improvement, the services of the Division are constantly being
examined and improved.
1.1
SCOPE OF THE DOL CLINICAL QUALITY MANUAL
This Quality Manual summarizes the policies and administrative procedures associated with the
Illinois Department of Public Health, Division of Laboratories Clinical Laboratories and
establishes a structured quality system for all phases of the total process; that is, preanalytic,
analytic, and postanalytic, as well as general laboratory systems. All policies and procedures
have been structured in accordance with the Clinical Laboratory Improvement Act (CLIA). This
manual has been prepared in accordance with the documents listed in Section 12. Further details
of the Division’s administrative procedures are contained in directives listed in Appendix 1 and
standard operating procedures listed in Appendix 2.
All laboratory activities performed by the Illinois Department of Public Health, Division of
Laboratories, Clinical Laboratories, are performed in accordance with this document. The
laboratories provide services and assistance to referring physicians, public health care providers,
federal and local law enforcement agencies, other approved providers, and State of Illinois
agencies.
The IDPH Clinical Laboratories analyze clinical specimens in the specialties and sub-specialties
of Microbiology, Serology, Virology, Molecular Diagnostics, Chemistry, and metabolic/genetic
diseases. The laboratories also perform animal rabies testing.
Occasionally, the Division will find it necessary to allow a departure from approved procedures
due to unusual circumstances or specimens. The procedure for this departure is contained in
SOP DXX011 (draft),
Exceptions Permitting Departures from Documented Policies or
Procedures.
The procedure in this SOP provides the only authorized means by which departures
from required procedures and policies can occur.
DCA001-06-1105
Page 6 of 65
Figure 1-1
DIRECTOR
Office of
Epidemiology and
Health System
Development
Office of
Finance and
Administration
Office of Health
Care Regulations
Office of
Women’s Health
Office of Health
Promotion
Office of Health Protection
Division of Environmental Health
Division of Infectious Disease
Plumbing Program
Division of
Food, Drug, and
Dairies
Division of
Laboratories
DCA001-06-1105
Page 7 of 65
2.
ORGANIZATION AND RESPONSIBILITIES
The Division of Laboratories is a division of the Illinois Department of Public Health and is thus
legally identifiable as a State of Illinois agency. For the purpose of clinical analyses, the
Division of Laboratories is divided into three separate organizational units, all under the
supervision of the Chief, Division of Laboratories. They are the Carbondale Laboratory, the
Chicago Laboratory, and the Springfield Laboratory. The Division of Laboratories, Laboratory
Improvement Section is responsible for the overall management of the laboratory quality system
and its implementation. Figure 2-1 is a divisional organizational chart as it relates to clinical
testing. The Laboratory Improvement Section maintains a record of employee’s name, initials,
and signature for all individuals who are responsible for signing or initialing any laboratory
record.
2.1
CARBONDALE LABORATORY ORGANIZATION
The Carbondale Laboratory is headed by the Carbondale Laboratory Manager and the Clinical
Supervisor. The laboratory is organized and operates in such a way that it meets the
requirements of all appropriate standards. The laboratory specifies and documents the
responsibility, authority, and interrelationships of all personnel who manage, perform or verify
work affecting the quality of testing.
2.2
CHICAGO LABORATORY ORGANIZATION
The Chicago Laboratory is headed by the Chicago Laboratory Manager. The Chicago Laboratory
consists of four sections: Microbiology, Virus-Serology, Metabolic/Genetic Diseases and
Molecular Diagnostics, all under the supervision of the Laboratory Manager. These sections are
headed by the technical supervisors (section chiefs). The laboratory is organized and operates in
such a way that it meets the requirements of all appropriate standards. The laboratory specifies
and documents the responsibility, authority, and interrelationships of all personnel who manage,
perform or verify work affecting the quality of testing.
2.3
SPRINGFIELD LABORATORY ORGANIZATION
The Springfield Laboratory consists of two sections: Diagnostic and Molecular, all under the
supervision of the Laboratory Manager. Both sections are headed by a supervisor. The
laboratory is organized and operates in such a way that it meets the requirements of all
appropriate standards. The laboratory specifies and documents the responsibility, authority, and
interrelationships of all personnel who manage, perform or verify work affecting the quality of
testing.
2.4
LABORATORY KEY PERSONNEL
2.4.1
MANAGEMENT
Laboratory management is responsible for defining the minimum level of qualification,
experience, and basic laboratory skills necessary for all positions within the laboratory.
DCA001-06-1105
Page 8 of 65
Laboratory managerial staff and their specific responsibilities are listed here. Each managerial
staff member is provided with the authority and resources needed to discharge his/her duties.
2.4.1.1
CHIEF, DIVISION OF LABORATORIES
The Chief, Division of Laboratories, is responsible for the coordination of analytical services
between the three laboratories. He/she is responsible for ensuring that standardized laboratory
systems are used at each laboratory so that the data generated is similar in format, content, and
quality; providing budgetary oversight of laboratory operations to verify that required financial
controls and accounting procedures are in place; formulating long-term goals in facilities,
staffing, equipment, and analytical capabilities. Specific responsibilities include the following:
-
Delegating authority and responsibilities to the laboratory managers/directors to
implement their duties;
-
Being accessible to the laboratory to provide consultation either on site or by phone or
pager;
-
Developing, establishing and maintaining written laboratory policies.
2.4.1.2
CLIA LABORATORY DIRECTOR
The laboratory director is responsible for the overall operation and administration of the
laboratory, including the employment of personnel who are competent to perform test
procedures, record and report test results promptly, accurately and proficiently, and for assuring
compliance with the applicable regulations. The laboratory director, if qualified, may perform
the duties of the technical supervisor, clinical consultant, general supervisor, and testing
personnel, or delegate these responsibilities to personnel meeting the qualifications. If the
laboratory director reapportions performance of his or her responsibilities, he or she remains
responsible for ensuring that all duties are properly performed. The laboratory director must be
accessible to the laboratory to provide onsite, telephone or electronic consultation as needed.
The laboratory director must:
-
Ensure that testing systems developed and used for each of the tests performed in the
laboratory provide quality laboratory services for all aspect of test performance, which
includes the preanalytic, analytic, and postanalytic phases of testing;
-
Ensure that the physical plant and environmental conditions of the laboratory are
appropriate for the testing performed and provide a safe environment in which employees
are protected from physical, chemical, and biological hazards;
-
Ensure that test methodologies selected have the capability of providing the quality of
results required for patient care;
DCA001-06-1105
Page 9 of 65
-
Verifies that procedures used are adequate to determine the accuracy, precision, and other
pertinent performance characteristics of the method and that laboratory personnel are
performing the test methods as required for accurate and reliable results;
-
Ensure that the laboratory is enrolled in an HHS-approved proficiency testing program
for the testing performed and that the proficiency testing samples are tested as routine
specimens; the results are returned within the timeframes established by the proficiency
testing program; all proficiency testing reports received are reviewed by appropriate staff
to evaluate the laboratory’s performance and to identify any problems that require
corrective action; and an approved corrective action plan is followed when any
proficiency testing result is found to be unacceptable or unsatisfactory as outlined in the
proficiency testing SOP, DAA017;
-
Ensure that the quality control and quality assessment programs are established and
maintained to assure the quality of laboratory services provided and to identify failures in
quality as they occur;
-
Ensure the establishment and maintenance of acceptable levels of analytical performance
for each test system;
-
Ensure that all necessary remedial actions are taken and documented whenever
significant deviations from the laboratory’s established performance characteristics are
identified, and that patient test results are reported only when the system is functioning
properly;
-
Ensure that reports of test results include pertinent information required for
interpretation;
-
Ensure that consultation is available to the laboratory’s clients on matters relating to the
quality of the test results reported and their interpretation concerning specific patient
conditions;
-
Ensure that a general supervisor provides on-site supervision of non-waived test
performance by qualified testing personnel;
-
Employ a sufficient number of laboratory personnel with the appropriate education and
either experience or training to provide appropriate consultation, properly supervise and
accurately perform tests and report test results;
-
Ensure that prior to testing patients’ specimens, all personnel have the appropriate
education and experience, receive the appropriate training for the type and complexity of
the services offered, and have demonstrated that they can perform all testing operations
reliably to provide and report accurate results;
-
Ensure that policies and procedures are established for monitoring individuals who
conduct preanalytic, analytic, and postanalytic phases of testing to assure that they are
DCA001-06-1105
Page 10 of 65
competent and maintain their competency to process specimens, perform test procedures
and report test results promptly and proficiently, and whenever necessary, identify needs
for remedial training or continuing education to improve skills;
-
Ensure that an approved procedure manual is available to all personnel responsible for
any aspect of the testing process; and
-
Specify, in writing, the responsibilities and duties of each consultant and each supervisor,
as well as each person engaged in the performance of the preanalytic, analytic, and
postanalytic phases of testing, that identifies which examinations and procedures each
individual is authorized to perform, whether supervision is required for specimen
processing, test performance or result reporting and whether supervisory or director
review is required prior to reporting patient test results.
2.4.1.3
CLINICAL CONSULTANT, DIVISION OF LABORATORIES
The Clinical Consultant, Division of Laboratories is responsible for providing consultation.
Specific responsibilities include the following:
-
Be available to clients and to the laboratories via phone or e-mail;
-
Provide consultation regarding the appropriateness of the testing ordered and
interpretation of test results;
-
Assist the laboratory’s clients in ensuring that appropriate tests are ordered to meet
clinical expectations;
-
Ensure that reports of test results include pertinent information required for specific
patient test interpretation;
-
Ensure that consultation is available and communicated to the laboratory’s clients on
matters related to the quality of the test results reported and their interpretation
concerning specific patient conditions.
2.4.1.4
DIVISION OF LABORATORIES MANAGERS / SECTION CHIEFS –
TECHNICAL SUPERVISORS
The technical supervisor is responsible for the technical and scientific oversight of the
laboratory. The technical supervisors must be available to the laboratory to provide on-site,
telephone or electronic consultation. The technical supervisor’s responsibilities include:
-
Approve test methodology that is appropriate for the clinical use of the test results
according to the latest version/revision of DAA005,
Standard Operating Procedure for
Technical and Standard Operations Implementation and/or Change
;
DCA001-06-1105
Page 11 of 65
-
Verify the test procedures performed and establishment of the laboratory’s test
performance characteristics, including the precision and accuracy of each test and test
system and document such activity as described in SOP DAA005,
Standard Operating
Procedure for Technical and Standard Operations Implementation and/or Change
;
-
Ensure that the laboratory is enrolled in an HHS-approved proficiency testing program
for the testing performed and that the proficiency testing samples are tested as routine
specimens; the results are returned within the timeframes established by the proficiency
testing program; all proficiency testing reports received are reviewed by appropriate staff
to evaluate the laboratory’s performance and to identify any problems that require
corrective action; and an approved corrective action plan is followed when any
proficiency testing result is found to be unacceptable or unsatisfactory as outlined in the
latest version/revision of SOP, DAA017,
Standard Operating Procedure for Handling,
Analysis, and Reporting of Proficiency Testing Samples
;
-
Establish a quality control program appropriate for the testing performed and the
parameters for acceptable levels of analytical performance and ensure that these levels
are maintained throughout the entire testing process from the initial receipt of the
specimen, through sample analysis and reporting of test results;
-
Ensure that all necessary corrective actions are taken and documented whenever
significant deviations from the laboratory’s established performance characteristics are
identified, and that patient test results are reported only when the system is functioning
properly as outlined in SOP 015,
Standard Operating Procedure for Quality
Improvement Actions
;
-
Ensure that patient test results are not reported until all corrective actions have been taken
and the test system is functioning properly;
-
Evaluate the competency of all testing personnel and assuring that the staff maintain their
competency to perform test procedures and report test results properly, accurately, and
proficiently. See Appendix 6.
-
Conduct quality improvement studies that include the following: identify and monitor
important quality indicators; define and select thresholds in data generated; analyze data
and recommend corrective action to the Director; and, conduct follow-up assessments.
-
Perform Management System Reviews - See Appendices 7 and 8.
The technical supervisor is familiar with the calibration of tests and procedures, the objective of
the calibration or test, and the assessment of results. The technical supervisor reports to the
Laboratory Manager/Laboratory Director and may act as a deputy in the absence of the
Laboratory Manager/Director. The technical supervisor must have a minimum of a bachelor’s
degree in a natural or physical science course work and a minimum of four years laboratory
experience with six months experience in the designated area of responsibility. The technical
supervisor inform the Laboratory Improvement Section of changes in technical supervision.
DCA001-06-1105
Page 12 of 65
2.4.1.5
DIVISION OF LABORATORIES SUPERVISOR – GENERAL SUPERVISOR
The general supervisor is responsible for day-to-day supervision or oversight of the laboratory
operation and personnel performing testing and reporting test results.
-
The general supervisor must be accessible to testing personnel at all times testing is
performed to provide on-site, telephone or electronic consultation to resolve technical
problems in accordance with policies and procedures established either by the laboratory
director or technical supervisor;
-
Is responsible for providing day-to-day supervision of non-waived test performance by
qualified testing personnel;
-
Except as listed below, must be on site to provide direct supervision when non waived
testing is performed by qualified personnel;
*
Exception: For individuals qualified, who were performing non waived testing on
or before January 19, 1993, the requirements provide that all non waived testing
performed by the individual in the absence of a general supervisor is reviewed
within 24 hours by a qualified general supervisor;
-
Is responsible for monitoring test analyses and specimen examinations to ensure that
acceptable levels of analytical performance are maintained;
-
The director or technical supervisor may delegate to the general supervisor the
responsibility for:
* Assuring that all remedial actions are taken whenever test systems deviate from the
laboratory’s established performance specification following the procedure in the
latest version/revision of SOP DAA015,
Standard Operating Procedure for Quality
Improvement Action
.
The general supervisor is familiar with the calibration of test methods and procedures, the
objective of the calibration or test and the assessment of results. The supervisor reports to the
section chief – technical supervisor and may act as a deputy in the absence of the Section Chief.
The supervisor must have a minimum of a bachelor’s degree in a natural or physical science or
medical technology with course work relative to the area of supervision, and one-year experience
in the analysis of clinical specimens in the area of supervision.
2.4.2 LABORATORY IMPROVEMENT SECTION
The Laboratory Improvement Section is responsible for the overall management of the
laboratory quality system and its implementation. For the purpose of clinical testing, the section
consists of the Laboratory Quality Specialists (LQS) who report directly to the Laboratory
Quality Supervisor in the Chicago Laboratory and the Laboratory Quality Specialists (LQS) who
DCA001-06-1105
Page 13 of 65
report directly to the Laboratory Quality Administrator in the Springfield Laboratory. The
Laboratory Improvement Section in Springfield also works with the Carbondale Laboratory.
2.4.1.2 LABORATORY QUALITY ADMINISTRATOR
The Laboratory Quality Administrator is responsible for the overall management of the Division
of Laboratories’ quality operations. The Laboratory Quality Administrator has direct access to
technical supervisors, Laboratory Director(s), and the Chief, Division of Laboratories. The
Laboratory Quality Administrator also functions as a quality manager as defined in ISO 15189.
In addition, the Laboratory Quality Administrator does/has the following:
-
Conducts internal audits of the entire technical operation annually, notifies laboratory
management of deficiencies in the quality system, and monitors corrective action;
-
Has documented training and/or experience in QA procedures and is knowledgeable in
the quality systems defined by CLIA;
-
Has a general knowledge of the analytical procedures;
-
Notifies the regulatory agencies of changes in technical supervision and directorship;
-
Prepares and presents administrative laboratory training, such as general safety, ethics,
general security, and regulation updates;
-
Oversees the Division’s quality documentation.
2.4.2.2 LABORATORY QUALITY SUPERVISOR
The Laboratory Quality Supervisor is responsible for the overall management of the Chicago
Laboratory’s quality operations and assists the Laboratory Quality Administrator with the
resolution of regulatory compliance issues. For CLIA compliance issues, the Laboratory Quality
Supervisor reports directly to the Laboratory Quality Administrator and has direct access to the
technical supervisors and division management.
The Laboratory Quality Supervisor functions as a quality manager as defined in ISO 15189. The
Laboratory Quality Supervisor in the Chicago Laboratory performs those functions listed under
the Laboratory Quality Administrator, Section 2.4.1.2.
2.4.2.3 CLINICAL LABORATORY QUALITY SPECIALIST (LQS)
The Clinical LQS is responsible for assisting the Laboratory Quality Administrator and
Laboratory Quality Supervisor in coordinating the quality system, its implementation, and
certification programs for clinical laboratories. The clinical LQS reports directly to the
Laboratory Quality Administrator or Laboratory Quality Supervisor, has direct access to division
management and the technical supervisors, and can discuss with them any questions or concerns
about the laboratory quality system.
DCA001-06-1105
Page 14 of 65
2.4.3 SAFETY COMMITTEE/OFFICER
The laboratory has a designated safety committee/officer. The safety committee/officer assists
laboratory supervisors in the implementation of the safety program. The safety
committee/officer provides, and/or makes available, safety training for all employees, reviews
the laboratory safety manual and safety standard operating procedures, performs safety
inspections of the facility, and reviews maintenance documentation for laboratory safety
equipment to determine compliance with safety regulations. Areas of noncompliance are
reported to the supervisor, and Director and to the Chief, Division of Laboratories. See the
laboratory Safety Manual for further details.
2.4.4 DIVISION OF LABORATORIES TESTING PERSONNEL
Testing personnel are responsible for sample analysis and identification of corrective actions.
The testing personnel report directly to the designated general supervisor. All testing personnel
are responsible for complying with all quality assurance requirements that pertain to their
organizational/technical function. The testing personnel are responsible for specimen
processing, test performance and reporting test results. Each individual performs only those tests
that are authorized by the laboratory director and require a degree of skill commensurate with the
individual’s education, training or experience, and technical abilities. Each testing personnel
must:
-
Follow the laboratory procedures for specimen handling and processing, test analyses,
reporting and maintaining records of patient test results;
-
Maintain records that demonstrate that proficiency testing samples are tested in the same
manner as patient specimens;
-
Adhere to the laboratory’s quality control policies, document all quality control activities,
instrument and procedural calibrations and maintenance performed;
-
Follow the laboratory’s established policies and procedures whenever test systems are not
within the laboratory’s established acceptable levels of performance;
-
Be capable of identifying problems that may adversely affect test performance or
reporting of test results and either must correct the problems or immediately notify the
general supervisor, technical supervisor, or director;
-
Document all corrective actions taken when test systems deviate from the laboratory’s
established performance specifications.
2.5
TRAINING PROCEDURES
Laboratory personnel are internally trained in health and safety, security, QA procedures, and the
laboratory management system. Laboratory personnel are also required to attend regular health
and safety and laboratory QA procedures refresher courses. New testing staff is trained for a
sufficient number of days by experienced testing personnel prior to performing any laboratory
DCA001-06-1105
Page 15 of 65
work on clinical specimens. The length of training is determined by the technical supervisor.
This training will include basic laboratory skills, as appropriate. Content and dates of training
that each laboratory staff member receives are documented by the immediate supervisor in the
personnel training records. Employee review of the Quality Manual and other administrative
procedures are documented through the use of signed read receipts. This read receipt verifies
that each employee has read, understood, and is using the latest version of the laboratory’s SOPs
that relate to his/her job responsibilities.
2.5.1 GENERAL TRAINING
The employee reviews the current versions of the appropriate method SOPs. This review is
documented by a signed and dated read receipt. Training is provided and documented in the
following areas for specimen preparation if applicable:
-
Glassware cleaning;
-
Specimen logging;
-
Analytical balance;
-
Use of pipets and other liquid handling systems;
-
Quality Control Procedures;
-
Reagent preparation techniques;
-
Testing procedures (SOP).
The form for training documentation is contained in Appendix 3. The documentation of training
is maintained in the employee CLIA personnel file. When a regulatory agency requires
additional training certification such as required by the Select Agent Rule, the detailed process
will be outlined in the corresponding procedure. Records must be maintained accordingly.
2.5.2 CLINICAL SPECIMEN ANALYSES
Upon assignment to testing, the testing personnel will be trained and assessed to verify
capabilities; the personnel assessment will be conducted upon completion of training (initial),
after six months, and yearly, thereafter. Testing personnel are also required to participate in a
blind sample testing program at least yearly. See Appendix 6.
DCA001-06-1105
Page 16 of 65
Figure 2-1
FIGURE 2.1 DIVISION OF LABORATORIES, CLINICAL ORGANIZATIONAL CHART
David Maserang, Ph. D.,
CLIA Director
Maria Kaminski,
Division of Laboratories
Laboratory Quality
Administrator
Karen Meier,
Carbondale
Laboratory Manager
George Dizikes,Ph.D.,
Chicago
Laboratory Manager
Clinical
LQS
Technical/General Supervisor
Technical/General Supervisor
Technical Supervisor
Testing Personnel
Testing Personnel
Clinical Consultant
Testing Personnel
General Supervisor
Chicago Quality
Supervisor
Bernard T. Johnson,
Chief, Division of
Laboratories
Vacant, Springfield
Laboratory Manager
DCA001-06-1105
Page 17 of 65
3
LABORATORY PHYSICAL FACILITIES
3.1
CARBONDALE LABORATORY PHYSICAL FACILITY
3.1.1 ACCOMMODATION AND ENVIRONMENT
The Carbondale Laboratory is located at only one site as listed in the beginning of this manual. The
Carbondale Laboratory is maintained so that the test areas, energy sources, lighting, heating, and
ventilation are adequate to facilitate proper performance of testing. The environment of the laboratory
is such that it does not invalidate the results or adversely affect the required accuracy of measurements.
In instances where monitoring or control of any environmental condition is specified in the test
procedure or by regulation, the laboratory will meet and document adherence to the specification. The
facility is owned by the Illinois Department of Public Health. Problems with environmental conditions
are brought to the attention of the laboratory manager who is then responsible for adjustments and
maintenance. A pest and rodent control program is available. The floor plan of the Carbondale
Laboratory can be found in the
Carbondale Laboratory Emergency Procedure Manual.
3.1.2 WORK AREAS
The laboratory maintains effective separation between neighboring areas when the activities are
incompatible. The contamination of specimens, equipment, instruments, reagents, materials and
supplies is minimized. Molecular amplification procedures that are not contained in closed systems
have a uni-directional workflow including separate areas for specimen preparation, amplification, and
product detection, and, as applicable, reagent preparation. Good housekeeping is practiced in the
laboratory to ensure that contamination does not adversely affect data quality. Enough work space is
available to ensure an unencumbered work area.
3.2
CHICAGO LABORATORY PHYSICAL FACILITY
3.2.1 ACCOMMODATION AND ENVIRONMENT
The Chicago Laboratory is located at only one site as listed in the beginning of this manual. The
Chicago Laboratory is maintained so that the test areas, energy sources, lighting, heating, and
ventilation are adequate to facilitate proper performance of testing. The environment of the laboratory
is such that it does not invalidate the results or adversely affect the required accuracy of measurements.
In instances where monitoring or control of any environmental condition is specified in the test
procedure or by regulation, the laboratory will meet and document adherence to the specification.
Environmental conditions are monitored by the building owner, State of Illinois, Central Management
Services. Problems with environmental conditions are brought to the attention of the building owner
through the building manager who is then responsible for adjustments and maintenance. A pest and
rodent control program is available. The floor plan for the Chicago laboratory can be found in the
Chicago Laboratory Emergency Procedure Manual
.
3.2.2
WORK AREAS
The laboratory maintains effective separation between neighboring areas when the activities are
incompatible. The contamination of specimens, equipment, instruments, reagents, materials and
supplies is minimized. Molecular amplification procedures that are not contained in closed systems
have a uni-directional workflow including separate areas for specimen preparation, amplification, and
DCA001-06-1105
Page 18 of 65
product detection, and, as applicable, reagent preparation. Good housekeeping is practiced in the
laboratory to ensure that contamination does not adversely affect data quality. Enough work space is
available to ensure an unencumbered work area.
3.3
SPRINGFIELD LABORATORY PHYSICAL FACILITY
3.3.1
ACCOMMODATION AND ENVIRONMENT
The Springfield Laboratory is located at only one site as listed in the beginning of this manual. The
Springfield Laboratory is maintained so that the test areas, energy sources, lighting, heating, and
ventilation are adequate to facilitate proper performance of testing. The environment of the laboratory
is such that it does not invalidate the results or adversely affect the required accuracy of measurements.
In instances where monitoring or control of any environmental condition is specified in the test
procedure or by regulation, the laboratory will meet and document adherence to the specification.
Environmental conditions are monitored by the building owner, Southern Illinois University School of
Medicine. Problems with environmental conditions are brought to the attention of the building owner
through the building manager who is then responsible for adjustments and maintenance. A pest and
rodent control program is available. The floor plan for the Springfield laboratory can be found in the
Springfield Laboratory Emergency Procedure Manual
.
3.3.2
WORK AREAS
The laboratory maintains effective separation between neighboring areas when the activities are
incompatible. The contamination of specimens, equipment, instruments, reagents, materials and
supplies is minimized. Molecular amplification procedures that are not contained in closed systems
have a uni-directional workflow including separate areas for specimen preparation, amplification, and
product detection, and, as applicable, reagent preparation. Good housekeeping is practiced in the
laboratory to ensure that contamination does not adversely affect data quality. Enough work space is
available to ensure an unencumbered work area.
DCA001-06-1105
Page 19 of 65
4.0
GENERAL LABORATORY SYSTEM
4.1
ETHICS
In committing to a standard of excellence, the Illinois Department of Public Health Laboratories
expects high ethical standards from all personnel. The management of the IDPH and the Division of
Laboratories has stressed the importance of high ethical standards to the employees through a number
of documents distributed to all employees. These documents include the following:
Directive 88-03, “Personnel Conduct”;
Directive 97-01, “Professional Conduct”;
SOP DAA003, “Standard Operating Procedure for Data Integrity”;
Through the distribution of the above documents, employees agree to abide by these directives and
procedures. Any data reporting errors are to be reported to the appropriate laboratory supervisor in a
timely manner. The supervisor will then take appropriate action.
4.2
CONFIDENTIALITY
All information about clients/patients, specimens, results, and proprietary rights must be considered
confidential. Disclosure of any such information is restricted. Only authorized personnel will be
allowed access to such information. All records, including those pertaining to calibration and test
equipment, certificates and reports are safetly stored, held secure and reported in confidence to the
client. The confidentiality of information is communicated to all employees through the distribution of
Directive 01-04, “Confidentiality of Test Results”. Refer to Section 9.4 concerning storage
requirements.
4.2.2 REPORTS BY FAX
The same limitations apply to Faxed reports as to those by mail. In addition, the following statement
must appear on the fax cover page:
“CONFIDENTIAL REPORTS”
This message is intended for the use of the individual or entity to which it has been addressed and may
contain information that is Privileged, Confidential, and exempt from Disclosure under Applicable
Law. If the reader of this message is not the intended recipient, or the employee or agent responsible
for delivering the message to the intended recipient, you are hereby notified that any dissemination,
distribution or copying of this document is strictly prohibited. If you have received this message in
error, please notify us by telephone to arrange for returning the faxed document to us.
4.2.3 REPORTS BY PHONE
When requests are received to report results by telephone, the person reporting the data must document
the verbal transmission of results either on the work sheet/test requisition form or in a telephone
logbook. The telephone call to report results must either originate from the laboratory to a number
verified to belong to the appropriate agency or be from an individual clearly identifiable by voice to
the person reporting data. Any verbally transmitted data is to be followed up by sending a written
report at the earliest convenience unless a report has been sent or is in transit.
DCA001-06-1105
Page 20 of 65
4.3
SPECIMEN IDENTIFICATION AND INTEGRITY
The Division of Laboratories offers to the submitters a manual of services either as hard copy or an
electronic file. The manual of services includes instructions for the labeling and handling (storage,
shipment) of specimens to maintain the specimen integrity. Upon receipt of the specimen by the
Division of Laboratories, the specimen will enter a control system as described in sections 6, 7, and 8.
4.4
CUSTOMER COMPLAINTS AND COMMUNICATIONS
All customer complaints concerning laboratory test data, whether internal or external to the division,
are handled according to the procedure contained in SOP DAA016,
Service Improvement Actions
.
The
completed resolution of complaints form is retained by both, the laboratory section involved and the
Laboratory Improvement Section and becomes part of the laboratory records.
4.5
PERSONNEL COMPETENCY ASSESSMENT
Evaluation and documentation of the performance of individuals responsible for testing must be done
at six months during the first year the individual tests patient specimens. Thereafter, evaluations must
be performed at least annually unless test methodology or instrumentation changes. If changes to
methodology or instrumentation do occur, the individual’s performance must be reevaluated to include
the use of the new test methodology or instrumentation prior to reporting patient test results.
The procedures for evaluation of the competency of the staff are the responsibility of the technical
supervisors and must include, but are not limited to the following:
-
Direct observation of routine patient test performance, including patient preparation, if
applicable, specimen handling, processing and testing;
-
Monitoring the recording and reporting of test results;
-
Review of intermediate test results or worksheets, quality control records, proficiency testing
results, and preventive maintenance records;
-
Direct observation of performance of instrument maintenance and function checks;
-
Assessment of test performance through testing previously analyzed specimens, internal blind
testing samples or external proficiency testing samples;
-
Assessment of problem solving skills.
Each laboratory unit may expand the personnel assessment form to suit individual unit needs.
Supervisor’s remarks must include employee’s testing status, remedial training, and follow-up as
required. See Appendix 6.
DCA001-06-1105
Page 21 of 65
4.6
EVALUATION OF PROFICIENCY TESTING PERFORMANCE
The laboratory will review and evaluate the results obtained on proficiency testing samples as detailed
in the latest version/revision of SOP DAA017,
Standard Operating Procedure for the Handling,
Analysis, and Reporting of Proficiency Testing Samples.
DCA001-06-1105
Page 22 of 65
5.0
QUALITY ASSURANCE OBJECTIVES
The overall Quality Assurance objectives for the Illinois Department of Public Health, Division of
Laboratories are to develop and implement procedures for laboratory analyses, chain-of-custody (when
appropriate), and reporting that will provide results that are of known and acceptable documented
quality. Below is a general discussion of data quality indicators (DQIs) used in the laboratory. See
specific procedure SOPs for more specific information concerning quality control parameters.
5.1
TEST VARIABILITY/REPRODUCIBILITY
For test procedures that specify parameters for reproducibility, duplicate (or other multiple) analyses
are performed and must meet acceptance limits as required by the technical procedure.
5.2
ACCURACY
Accuracy is the degree of agreement between an observed value and an accepted reference value. It is
the degree of agreement between a specimen’s target (positive or negative) and the actual measured
value. Laboratory accuracy is assessed with the use of positive and negative controls at the time of
media preparation and during analysis. Negative controls are analyzed to demonstrate that equipment,
containers, media and reagents are not contaminated because of improper handling or preparation,
inadequate sterilization, or environmental exposure. Positive controls demonstrate that the medium
can support the growth of the target organism(s), and the medium produces the specified or expected
reaction to target organism(s). Positive controls also are used to determine that the analytical system is
functioning correctly for example: antibiotic testing.
5.3
PRECISION
Precision is the degree to which a set of observations or measurements of the same property, obtained
under similar conditions, conform to themselves. Precision is usually expressed as standard deviation,
variance or range, in either absolute or relative terms.
DCA001-06-1105
Page 23 of 65
6.0
PREANALYTIC SYSTEM
6.1
SPECIMEN MANAGEMENT PROTOCOL
With the exception of Metabolic/Genetic Diseases, Clinical testing is not directly available to
individuals but must be requested through approved Local Health Department, Regional Offices of the
Illinois Department of Public Health, or other public agencies. Requests for special or non-routine
analyses must be coordinated through the laboratory in conjunction with a public agency. The
Division of Laboratories routinely analyzes blood and body fluids specimens for Microbiology,
Serology, Virology, Molecular Diagnostics, Chemistry, and Metabolic/Genetic Diseases. The
laboratories also perform animal rabies testing.
6.2
SPECIMEN COLLECTION
Collection of specimens is performed by the appropriate local health department, clinical laboratory,
hospital laboratory, IDPH program personnel or other authorized health care provider/individual. The
Division of Laboratories personnel do not collect specimens. Testing for public health clinics is
coordinated through the Division of Infectious Diseases. The Division of Infectious Diseases will pre-
approve facilities for laboratory testing. The request for testing for private laboratories can be made by
an individual through the local health department or IDPH regional office. For a detailed list of
procedures and specimen collection instructions, refer to the current manual of services.
6.3
ESTABLISHED PROCEDURES
Microbiology Analyses:
The microbiology specimens are accepted from pre-approved submitters.
Microbiology testing includes bacteriology, enterics, parasitology, mycobacteriology, and mycology.
Serology Analyses:
Serology specimens are accepted only from pre-approved submitters. Serology
testing includes testing for Syphilis, HIV, Encephalitic panel, and Hepatitis.
Virology Analyses:
Virology analyses include viral isolation for enterovirus, respiratory virus and
herpes simplex; viral serology for Measles, Rubella, IgM, IgG, and West Nile Virus.
Molecular Diagnostics:
Molecular diagnostics include testing for Chlamydia and Gonorrhea,
viral load for HIV-1,
Mycobacterium tuberculosis
, Pertussis, molecular strain typing, Norwalk-like
virus, Leptosporosis, Enterohaemorrhagic E. coli (EHEC), and Rabies
Chemistry:
Chemistry testing includes blood lead.
Metabolic/Genetic Diseases :
Metabolic/Genetic Diseases include testing for Congenital
Hypothyriodism, Biotinidase Deficiency, Congenital Adrenal Hyperplasia, Galactosemia, Amino Acid
Disorders, Organic Acid Disorders, Fatty Acid Oxidation Disorders, Sickle Cell and other
Hemoglobinopathies.
Rabies:
Rabies specimens are accepted only from regional, veterinary, animal control, county,
or city sanitarian personnel. The sanitarian contacts the epidemiologist of the IDPH Infectious Disease
Division to receive assistance in determining the type of symptoms experienced by the victims. After
DCA001-06-1105
Page 24 of 65
consulting with the Division of Infectious Disease, the veterinarian or qualified animal control officer
should contact the laboratory with specifics of the time and method for shipment.
6.4
NEW WORK OR PROCEDURES
The laboratories will occasionally receive request for analyses not included in the list of established
procedures or beyond the normal workload. Such requests are first discussed by the Chief, Division of
Laboratories and program to determine the exact nature of the test, the data quality objectives, the
expected sample load, and any regulatory or legal issues. Refer to the following documents:
Standard Operating Procedure Implementation/Change,
Directive 01-02, October 1, 2001
;
Standard Operating Procedure for Proposals for Technical and Standard Operating Procedure
Implementation and/or Change,
DAA005.
New procedures must be reported to the regulatory agency within six months of implementation.
6.5
SPECIMEN RECEIPT AND LOG-IN
Details of the specimen receipt and acceptance policies can be found in the latest version/revision of
the Standard Operating Procedure for specific testing. These standard operating procedures (SOP)
contain information on the following sample handling issues:
Specimen collection kits;
Litigation specimens;
Recording of specimen information;
Specimen preservation, container, storage, and holding times;
Specimen acceptance policy;
Specimen log-in sheets or electronic log-in.
6.6
TEST REQUISITION FORM
Specimens submitted for testing must be accompanied by an Illinois form approved by the Division of
Communications. The laboratory must have a written or electronic request for a patient test from an
authorized person/facility. The laboratory may accept a verbal request for laboratory tests if it solicits
a written electronic authorization within 30 days of the verbal request and maintains the authorization
of documentation of its efforts to obtain authorization. See memo from the Chief, Division of
Laboratories dated April 29, 2003. The memo is available through the Laboratory Improvement
Section.
Test requisition forms must solicit the following information:
Submitter identification (name and address of facility or submitter code);
Patient’s name or unique patient identifier;
Sex and age or date of birth of the patient;
Test(s) to be performed;
Source of specimen, when appropriate;
Date, and if appropriate, time of specimen collection;
Any additional information relevant and necessary for a specific test.
DCA001-06-1105
Page 25 of 65
6.7
SPECIMEN SUBMISSION, HANDLING AND REFERRAL
6.7.1 WRITTEN INSTRUCTIONS
The Division of Laboratories will have available to submitters of specimens either written or electronic
instructions for:
Patient preparation;
Specimen collection;
Specimen labeling, including patient name or unique identification and, when appropriate,
specimen source;
Specimen storage and preservation;
Conditions for specimen transportation;
Specimen processing;
Specimen acceptability and rejection;
Specimen referral.
6.7.2 DOCUMENTATION
The laboratory must document the date and time it receives a specimen. This documentation can be
performed by manually writing the date and time on the test requisition form, using a mechanical or
electronic device to stamp the date and time or by placing the date and time into the database during
the scanning of optical character recognition forms.
6.7.3 SPECIMEN REFERRAL
The laboratory must refer a specimen for testing to a CLIA certified laboratory or a laboratory meeting
equivalent requirements as determined by the regulatory entity.
6.8
PURCHASE AND HANDLING OF CONSUMABLES AND SERVICES
6.8.1 PURCHASE OF REAGENTS AND STANDARDS
The purchasing of reagents and standards is usually performed by the supervisor or designee of each
unit.
6.8.2 INSPECTION/ACCEPTANCE REQUIREMENTS FOR SUPPLIES AND
CONSUMABLES UPON RECEIPT
Labels indicating the following information on receipt and testing are to be used for critical supplies
and consumables:
-
Unique identification name or number (if not clearly shown);
-
Date received;
-
Date opened;
-
Date tested (if applicable);
-
Date to be retested (if applicable);
-
Expiration date (if not clearly shown)
DCA001-06-1105
Page 26 of 65
-
Initials
6.8.3 OUTSIDE SUPPORT SERVICES AND SUPPLIES
Only those outside support services and supplies that are of adequate quality to sustain confidence in
the quality of testing are to be used. Where no assurance of the quality of equipment and supplies is
available, the purchased equipment or supplies are not used until they have been inspected, calibrated
or otherwise verified as complying with any standard specifications relevant to the calibration or tests
concerned. Work performed by outside support services must be verified to be of adequate quality
before any testing dependent on that work is done.
Where applicable, such as in the purchase of specimen collection tubes or specimen collection kits,
supplies will be purchased with a certificate or statement indicating suitability for their intended use
and those certificates/statement retained. Where no indication of a material’s suitability for its
intended use is available, a representative batch of that material is tested by using it to prepare test
blanks and/or standards and the testing records retained.
DCA001-06-1105
Page 27 of 65
7.0
ANALYTIC SYSTEM
7.1
STANDARD OPERATING PROCEDURES (SOPs)
Standard Operating Procedures (SOPS) developed within the Division of Laboratories are reviewed by
the technical staff, management, Laboratory Improvement Section, and other designated personnel.
SOP development and revisions are initiated by management or Laboratory Improvement staff and
approved by the Laboratory Director and the Chief, Division of Laboratories. The DOL SOPs
encompass laboratory activities, and quality control practices. SOP documents will be issued and
maintained on file in either hard copy or electronically by the pertinent laboratory section, and SOP
recipients. The Laboratory Improvement Section maintains on file the historical folders for all SOPs.
The procedures for SOPs are detailed in the following documents and SOPs:
Standard Operating Procedure Implementation/change,
Directive 01-02, October1, 2001;
Standard Operation Procedure for Proposals for Technical and Standard Operations Procedure
Implementation and/or Change,
DAA005;
Standard Operating Procedure for the Initiation, Draft, Approval, and Distribution of Standard
Operating Procedures, DAA021
.
7.1.1 SOP DISTRIBUTION
Copies of SOPs will be distributed to all signatories. Other laboratory personnel will receive copies of
SOPs based on their job functions. A distribution list will be generated and maintained by the
Laboratory Improvement Section for each SOP as determined by the Chief, Division of Laboratories.
7.2.
TEST SYSTEMS, EQUIPMENT, INSTRUMENTS, REAGENTS, MATERIALS AND
SUPPLIES
Laboratory testing must be performed following the manufacturer’s instructions and Standard
Operating Procedures in a manner that provides test results within the laboratory’s stated performance
specification for each test system.
7.2.1 REAGENT, STANDARD AND SPECIMEN STORAGE
All reagents and commercial kits are marked with initials, date received, date opened, and expiration
date. If reagents are prepared, the concentration and content of the solution must also be marked on
the container. Standards are stored in each section’s designated location for standards and reagents
only. Storage of reagents and standards must be consistent with the manufacturer’s instructions, if
provided. Storage of specimens must be consistent with test manufacturer’s instructions, if provided.
Specimens must be stored in conditions that support accurate, reliable test operation and results. These
conditions must be monitored and documented and, if applicable, include the following:
Water quality;
Temperature;
Humidity;
Light exposure;
Protection of equipment and instruments from fluctuation and interruptions of electrical current.
DCA001-06-1105
Page 28 of 65
7.2.2
DOCUMENTATION AND LABELS OF STANDARDS AND REAGENTS
Label and maintain records for all reagents, solution, culture media, control materials, calibration
materials, standards, reference cultures, and other supplies including the following:
Manufacturer/vendor;
The manufacturer’s Certificate of Analysis or purity (if supplied);
Identity and, when significant, titer, strength or concentration;
The date of receipt;
Initials;
Recommended storage conditions;
Preparation and expiration date;
An expiration date after which the material must not be used;
Other pertinent information required for proper use.
Components of reagent kits of different lot numbers must not be interchanged unless otherwise
specified by the manufacturer. Original containers must be labeled with in expiration date unless
labeled by the commercial manufacturer. Records are maintained on reagent and standard preparation.
These records contain the following, where applicable:
Traceability to purchased stocks, or reagents;
Reference to the method of preparation;
Final concentration;
Date of preparation;
Expiration date;
Preparer’s initials.
This information is recorded in a standards/reagent preparation logbook(s), appropriate quality control
form, or electronic record. All containers of prepared reagents and standards bear a unique identifier
and expiration date that links the reagent or standard to the documentation requirements recorded in
the logbook.
Reagents, solutions, culture media, control materials, calibration materials, and other supplies must not
be used after their expiration date has been exceeded, they have deteriorated, or they are found to be of
substandard quality.
7.3 ESTABLISHMENT AND VERIFICATION OF PERFORMANCE SPECIFICATION
Any new method that is being considered for routine testing and is FDA-cleared and approved must be
subject to a verification of performance specifications study including accuracy, precision, and
reportable range. Any FDA-cleared and approved method that has been modified or any test system
that is developed by the laboratory must be subject to an establishment of performance specifications
study including accuracy, precision, analytical sensitivity, analytical specificity to interfering
substances, reportable range, reference intervals, and other performance characteristics. See SOP
DAA005,
Standard Operation Procedure for Proposals for Technical and Standard Operations
Procedure Implementation and/or Change.
7.4
MAINTENANCE AND FUNCTION CHECKS
DCA001-06-1105
Page 29 of 65
7.4.1 ANALYTICAL SUPPORT EQUIPMENT
Analytical support equipment includes the following: balances, incubators, laboratory reagent water
dispensors, refrigerators, freezers, temperature measuring devices, pH meters, conductivity meters and
volumetric dispensing devices. All such support equipment is maintained in proper working order and
the records of all activities including service calls are retained. Support equipment is calibrated or
verified at least annually, using NIST traceable references, when available, over the entire range of use.
The results of the calibration or verification must be within the specifications required of the
application for which the equipment is used or the equipment is removed from service until repaired.
Refer to the corresponding Preventive Maintenance manual.
Prior to use on each day of use, refrigerators, incubators (day of use), and freezers are checked with
NIST traceable references (where possible) in the expected range of use. The acceptability for use or
continued use is according to the needs of the analysis or application for which the equipment is being
used. Mechanical volumetric dispensing devices (except Class A glassware) are checked for accuracy
quarterly. See Appendix 4 for a minimum schedule for the calibration/verification of laboratory
support equipment.
7.4.2 LABORATORY INSTRUMENT MAINTENANCE
All laboratory instruments are maintained in proper working order, and the records of all routine and
non-routine maintenance activities including service calls are retained. A record of maintenance is
maintained for each instrument to record the date and details of each maintenance activity.
Manufacturer’s instructions and manuals for the operation of the instruments are stored in an area
readily accessible to the instrument operator. Function checks are performed as defined by the
manufacturer and with at least the frequency specified by the manufacturer. Function checks must be
within the manufacturer’s established limits before patient testing is conducted. Function checks must
be documented.
7.4.3 LABORATORY INSTRUMENT CALIBRATION
All laboratory instruments are calibrated in a manner consistent with the appropriate reference method
protocols and/or the laboratory’s standard operating procedures. In cases where the laboratory needs
to use equipment outside its permanent control, the laboratory assures that all requirements for the
equipment are met by establishing and maintaining a protocol that ensures performance necessary for
accurate and reliable test results and test results reporting. The laboratory must also define a function
check protocol that ensures equipment, instrument, and test system performance necessary for accurate
and reliable test results and test result reporting. The laboratory must perform and document the
function checks, including background or baseline checks specified in the respective procedure
manual. Function checks must be within the laboratory’s established limits before patient testing is
conducted. The results of instrument calibration must meet acceptance criteria. If the calibration
acceptance criteria are not met, the instrument is removed from service until repaired or a deviation
curve prepared, and all measurements corrected for the deviation. Raw data for all calibrations are
retained, as either electronic or hard copy, so that the conditions of the calibration can be
reconstructed.
The SOP for each analysis performed in the laboratory describes the calibration procedures, their
frequency, acceptance criteria and the conditions that will require re-calibration. When calibration is
performed, sufficient raw data is retained to permit reconstruction of the instrument calibration
DCA001-06-1105
Page 30 of 65
including the following when appropriate: date, test method, instrument test name, analyst’s initials,
concentration and response, calibration or response factor, or unique equation or coefficient used to
reduce instrument responses to concentration.
7.5 CALIBRATION AND CALIBRATION VERIFICATION
Calibration and calibration verification procedures are required to substantiate the continued accuracy
of the test method throughout the laboratory’s reportable range for patient test results. Calibration
verification is the assaying of calibration materials in the same manner as patient specimens to confirm
that calibration has remained stable throughout the laboratory’s reportable range for patient test results.
7.5.1 CALIBRATION PROCEDURE AND FREQUENCY
The laboratory will perform and document calibration procedures following the manufacture’s test
system instructions, using calibration materials provided or specified, and at least the frequency
recommended by the manufacturer. All analytical instruments are calibrated with a standard or series
of standards, or equivalent materials. These standards are either purchased from various vendors in
premixed solutions or prepared directly from neat compounds. The preparation of all standard
solutions is documented in standard preparation logbooks. All stock standards are labeled with date
received, date opened, date prepared (if prepared in the laboratory), and expiration date. The standards
are stored in designated areas and checked for expiration on a regular schedule and prior to use. The
laboratory must also perform and document calibration procedures whenever calibration verification
fails to meet the laboratory’s acceptable limits for calibration verification; at least once every 6
months, and whenever any of the following occur:
-
A complete change of reagents for a procedure is introduced, unless the laboratory can
demonstrate that changing reagent lot numbers does not affect the range used to report patient
test results, and control values are not adversely affected by reagent lot number changes.
-
There is a major preventive maintenance or replacement of critical parts that may influence test
performance.
-
Control materials reflect an unusual trend or shift, or are outside of the laboratory’s acceptable
limits, and other means of assessing and correcting unacceptable control values fail to identify
and correct the problem.
-
The laboratory’s established schedule for verifying the reportable range for patient test results
requires more frequent calibration verification.
Specific calibration requirements for major classes of analytical procedures are described in the
following sections.
7.5.2 STANDARD RECEIPT AND TRACEABILITY
A standard (including reference cultures) is a solution or culture containing the organism or test
analyte of interest with verifiable accuracy that is used to evaluate that constituent in the specimen or
the performance of the test process in the presence of that organism or material. The purity of a
material, or an organism/culture must be verified and the accuracy requirements for its measurement
available from the source through certification and traceability statements that are kept on file in the
DCA001-06-1105
Page 31 of 65
laboratory. All laboratory standards and reference cultures must be traceable to a national standard
such as NIST, ATCC, or other documented source. Where traceability to national standards is not
possible, the laboratory participates in inter-laboratory testing programs to ensure the quality of the
results. Other reagents must have the purity specifications (grades) required by the method. The
safety requirements are checked with the material safety data sheets (MSDS) supplied by the
manufacturer. Information concerning specific grades of materials used in reagent and standard
preparation, appropriate glassware and container for preparation and storage, and labeling and record
keeping for all reagents are detailed in the procedure SOPs.
7.5.3 VERIFICATION
-
Select a minimum of three solutions spanning the reportable range (minimum or 0, mid-point,
and maximum), to be run in replicate (recommend two to three times);
-
Using appropriate, validated software, plot the assigned value (x axis) versus the mean of the
replicates for each level, the observed value (y axis). Draw a line through zero at 45 degree
angle where y=x.
-
Compare the observed values with the assigned in terms of the laboratory defined
tolerance limits for acceptability. These limits may be specific for each analyte, or may vary
with concentration.
-
Visually inspect the data for linearity or use linear regression analysis.
Note:
For calibration verification, CLIA requires at a minimum that observed values fall within the
laboratory's acceptable limits, having no regard for linearity.
If all the points are within the laboratory’s tolerance limits then calibration verification is acceptable. If
calibration verification is unacceptable, then recalibrate and repeat calibration verification. If still
unacceptable, then check reagents, troubleshoot the instrument, seek manufacturer technical assistance,
etc. until verification is acceptable. Document all corrective actions.
7.6
CONTROL PROCEDURES
7.6.1 GENERAL REQUIREMENTS
The data acquired from quality control (QC) procedures are used to evaluate the quality of analytical
data, to determine the need for corrective action in response to identified deficiencies, and to interpret
results after corrective action procedures are implemented. Each SOP includes a QC section that
addresses the QC requirements for the procedure. Internal QC requirements are specific for each
method but are summarized in this section. The frequency, acceptance limits, and corrective action for
QC checks are also described in each SOP. The requirements and procedures for initiating and
implementing corrective action are detailed in the following documents:
Remedial Action, Directive 01-03,
October 1, 2001;
Standard Operating Procedure for Corrective Action,
DAA015.
For quality control purposes the laboratory will measure and assure constant and consistent test
conditions (both instrumental and environmental) where required by the test method such as
DCA001-06-1105
Page 32 of 65
temperature, humidity, light, or specific instrument conditions. Figure 7-1 contains examples of the
minimum frequency of quality control samples. Figure 7-2 contains examples of the test quality
control requirements. Each procedure SOP details the required, minimum quality control specimens,
the minimum frequency, and acceptance criteria.
FIGURE 7-1 QUALITY CONTROL SAMPLE SUMMARY
QC Sample
Frequency
Sterility checks
Each new lot number of media, sample bottles,
membrane filters, etc
Positive control
Each new lot of media, supplies, each day patient
specimens are tested
Negative control
Each new lot of media (where available), media,
supplies; each day patient specimens are tested.
Two control materials, positive and negative
Each day patient specimens are tested, extraction
phase, molecular amplification
Comparison testing
Each new lot of reagents, new lot of media, new
controls
Two control materials of different concentration Each quantitative procedure
Negative and positive controls
Each qualitative procedure
Negative control and control material with
graded or titered reactivity
Each procedure producing graded or titered results
Two control materials including one that is
capable of detecting errors in the extraction
process
Each test system that has an extraction phase
FIGURE 7-2 MINIMUM QUALITY CONTROL REQUIREMENTS SUMMARY
Test
Quality Control Requirement
Frequency
Beta-lactamase
Positive and negative organisms
Each day of use
Gram stain
Positive and negative organisms
Each week of use
Media
Positive and negative organisms
Each batch or each lot
Bact-antiserum
Positive and negative organisms
Upon preparation /open;
every six months thereafter
Antimicrobial Susceptibility Appropriate control organisms
Each day test performed
Acid-fast
Positive and negative organisms
Each day of use
TB susceptibility
Positive and negative organisms
Each week test is performed
Lactphenol Cotton Blue
Positive and negative organisms
Each batch or lot
Fungal susceptibility
Appropriate control organisms
Each day of test
Parasitology
Collection of slides or
photographs
Each day of testing
Permanent stain
Sample control material
Each month of use
Virus Isolation
Cell substrate control, bland
Simultaneously with test
Chemistry
Low and high value controls
Each 8 hours
Staining materials
Positive and negative controls
Each day of use
DCA001-06-1105
Page 33 of 65
7.7 COMPARISON OF TEST RESULTS
Laboratories performing the same test using different methodologies (distinct analytical, chemical,
biochemical, molecular etc. techniques or kits) or instruments, must have a system that, twice a year,
evaluates and defines the relationship between test results using different methodologies or
instruments. Previously tested proficiency test samples or patient specimens may be used. If all
samples used to compare test results are within the laboratory’s established acceptable range, the test
comparison exercise is acceptable. If the test comparison is unacceptable, then calibrate and repeat
calibration verification. If still unacceptable, then check reagents, troubleshoot the instrument, etc.
until comparison of test results is acceptable. The comparison of test results must be documented
using “Comparison of Test Results Report” (Appendix 5).
The laboratory must have a system to identify and assess patient test results that appear inconsistent
with the following relevant criteria, when available: patient age, sex, diagnosis or pertinent clinical
data, and distribution of patient test results relationship with other parameters.
7.8 CORRECTIVE ACTIONS
The laboratory must document all corrective actions taken, including test systems not meeting the
established verification specifications as specified in the procedure; equipment and methodology
perform outside parameters; patient test values are outside reportable range; or when the laboratory
determines that the reference intervals for a test procedure are inappropriate. Corrective action must be
taken any time the results of controls or calibration fail to meet the established criteria. Patient test
results obtained in the unacceptable test run and since the last acceptable test run must be evaluated.
Any time the criteria for proper storage of reagents and specimens is not met, a corrective action must
be implemented and documented. See DAA015,
SOP for Quality Improvement Actions.
7.9 TEST RECORDS
The laboratory must maintain an informational record system that includes:
-
Positive identification of specimens which initiates an audit trail;
-
The test requisition form bearing the date and time the specimen was received. When an
optical character form is used, a date/ time record will be recorded upon scanning in an
electronic file;
-
Worksheets documenting the condition and disposition of specimens that do not meet
acceptable criteria;
-
Dates of all specimen testing;
-
Identity of the personnel who performed the test;
-
Records of testing including, if applicable, instrument printouts or electronic equivalents.
DCA001-06-1105
Page 34 of 65
8.0
POSTANALYTIC SYSTEM
8.1
DATA REVIEW PROCEDURE
Data resulting from the analysis of specimens are calculated according to protocols described in the
laboratory procedure. Computer programs used for data calculation are validated before use. All
information used in the calculations (e.g., raw data) is recorded in order to enable reconstruction of the
final result at a later date. Information on the preparation of the specimen (e.g., weight or volume of
specimen used, dilution factor used) is maintained in order to enable reconstruction of the final result
at a later date. All specimen results are reviewed by the unit supervisor or designee before being
released.
8.2
FINAL TEST REPORTS
The results of each test, or series of tests, are contained in a final laboratory report and include all the
information necessary for the interpretation of the results. Final results may be reported to an
interfaced system or manually transcribed or electronically transmitted by the analyst for all
specimens. Final reports must be sent promptly only to the authorized person, and if applicable, the
individual responsible for using the test results and the laboratory that initially requested the test. Test
results are reported according to the specific test SOP. Each final report sent to the customer includes
information to interpret the results. The report will include some or all of the following information as
required by the accrediting authority:
-
Name, and address of the laboratory where the test was performed;
-
Unique identification number of the report and all pages numbered. The report identification
number and specimen number are equivalent;
-
Outbreak number. Outbreak specimens are collected in groups. The group of specimens is
reported on one form that is identified with the outbreak number, date and time of collection,
and specimen numbers;
-
Name and address of test requesting facility or submitter code number;
-
Test report date;
-
Test performed;
-
Specimen source, when appropriate;
-
Test results and, if applicable, the units of measurement or interpretation, or both;
-
Any information regarding the condition and disposition of specimens that do not meet the
laboratory’s criteria for acceptability.
After issuance of the report, the report must remain unchanged. Any material amendments to a report
after issue are to be made in the form of a further document with the statement “Corrected Report,
serial number” or “Amended report, serial number”. Amendments will meet all relevant requirements
as the original report. In the event that anything is discovered after the issuance of a report that casts
doubt on the validity of that report, the authorized person ordering the test and, if applicable the
individual using the test results is to be notified promptly. Issue a corrected report and maintain
duplicates of the original report, as well as the corrected report.
The laboratory must have available pertinent “reference intervals” or “normal” values. Upon request,
the laboratory will make available a list of methods employed and the performance specifications
established or verified. Pertinent updates on testing information must be provided to clients whenever
changes occur that affect the test results or interpretation of test results.
DCA001-06-1105
Page 35 of 65
When the laboratory cannot report test results within its established time frame, the laboratory must
determine, based on the urgency of the patient test requested, the need to notify the appropriate
individual of the delay.
When the laboratory refers patient specimens for testing, the laboratory must not revise results or
information related to the test interpretation; the referring laboratory may send test results directly to
the authorized person who initiated the test request. In that case, the laboratory must maintain an exact
duplicate of each testing laboratory’s report. The laboratory initiating the test request must be notified
by the referring laboratory of the name and address of each laboratory where the test was performed.
When test results are submitted to clients by telephone, telex, fax, or other electronic or
electromagnetic means, all requirements stated in this section and those in the Section 4.2,
“Confidentiality”, must be followed.
8.3
RECORDS
Accurate records provide the direct evidence, support, and documentation for the necessary technical
interpretations, judgments, and discussions concerning laboratory results. These records, particularly
those that are anticipated to be used as evidentiary data, provide the historical evidence needed for later
reviews and analyses. Records should be legible, identifiable, and retrievable, and protected against
damage, deterioration, or loss. All records referenced in this section are retained for a total of six
years; Metabolic/Genetic Diseases will keep records for 21 years. All records will be made available
to the accrediting authority upon request. All records are maintained on site and thus immediately
available for a period of 2 years.
Electronic or hard copy data is stored securely in each of the laboratories or in the off-site
storage/archive facility. Original laboratory logbooks, analyst logbooks, and laboratory worksheets are
changed at the beginning of the year or as determined by the technical supervisor, replaced with new
ones, and the old logbook stored. Logbooks are also replaced if they show signs of deterioration or as
determined by the technical supervisor.
8.4
CORRECTIVE ACTION REPORTS
The process of corrective action is detailed in each method SOP and in SOP DAA015,
Standard
Operating Quality Improvement Actions.
Corrective action reports and all pertinent information are
retained by the Laboratory Improvement Section.
8.5
SPECIMEN RETENTION
Most specimens and specimen products are discarded shortly after the analysis has been completed and
the sample results are reported. The specimens and specimen products are disposed of by the
laboratory in accordance with federal and state laws and regulations. Figure 8-1 summarizes the
minimum specimen retention schedule. All documents pertaining to specimen storage and tracking are
retained, including shipping receipts, transmittal forms, and internal routing and assignment records.
If the specimen is part of litigation, disposal of the physical specimen occurs only with the concurrence
of the affected legal authority, specimen data user and/or submitter of the specimen. All conditions of
DCA001-06-1105
Page 36 of 65
disposal and all correspondence between all parties concerning the final disposition of the physical
specimen are recorded and retained.
Records indicate the date of disposal, nature of disposal (such as specimen depleted, specimen
disposed in hazardous waste facility, or specimen returned to submitter), and the name of the
individual who performed the task.
DCA001-06-1105
Page 37 of 65
Figure 8-1
IDPH Specimen Retention Schedule
TEST
NEGATIVES
POSITIVES
UNSATS
Ch/GC probe
7 days
10 days (frozen)
10 days
GC culture
3 days
3 days
3 days
Throat culture
1 day
5 days
1 day
Syphilis
10 days
3 months (frozen)
10 days
CDC requests
Indefinite (frozen)
Indefinite (frozen)
Indefinite (frozen)
HIV
10 days
5 years (frozen)
10 days
Rabies
1 year (frozen)
1 year (frozen)
1 day
Parasitology
3 days
3 days
1 day
Clinical specimens (enteric) /
subcultures
10 days after report
1 year
3 days
Blood lead
5 days
5 days
5 days
Referred cultures (enteric)
1 year
1 year
6 months
Reference culture (misc.)
1 month/final
1 month/final
Till report
MTB RFLP Testing (mail-out)
2 years (frozen)
2 years (frozen)
N/A
Bacteriology culture ID / susceptibility
Subculture 1 week
Subculture 1 week
10 days
TB clinical specimens
5 weeks or until final
Specimen & isolates 2
years (frozen)
10 days
TB referred cultures
N/A
Slants 3 months
Isolates 2 years
10 days
Urine culture
24 hours or until final
Specimen & isolate 2
years (frozen)
10 days
Mycology
1 month/final
1 month/final
Until reported
Blood Film
Indefinite
Indefinite
Until reported
HBsAg
10 days
5 years
10 days
Anti-HBs
10 days
5 years
10 days
Anti-HBc
10 days
5 years
10 days
Anti-HBc IgM
10 days
5 years
10 days
Toxoplasmosis
1 month
5 years
10 days
Viral serology
30 days
5 years
10 days
Rubella
Negative &
equivocals
3 months (frozen)
10 days
10 days
Metabolic/Genetic Diseases
Negative/Unsat
Positives
BDL*s and Unsats
Biotinidase Deficiency
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
Congenital Adrenal Hyperplasia
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
Galactosemia
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
Hypothroidism
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
Phenylketonuria
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
Sickle Cell Disease and other
hemoglobinopathies
1 wk refrigerated
2 months @ rm. temp.
Frozen indefinitely
4 months refrigerated
balance of year @ rm. temp.
*Borderline
DCA001-06-1105
Page 38 of 65
9.
DOCUMENTATION MANAGEMENT AND RECORD RETENTION
Managing quality documents is the responsibility of the Laboratory Improvement Section with
assistance from the laboratory supervisors. The process of document management serves to assure that
records are accessible, reviewed and revised on a regular schedule, and protected in storage from
damage and deterioration. The IDPH Division of Laboratories follows the provision of the State
Records Act (5ILCS 160/1) and the rules established by the State Records Commission to implement
this act.
9.1
CLIA DOCUMENTS
Copies of national guidance or requirements document issued by the CLIA’s Quality Assurance
Division and all HHS documents are maintained by the Laboratory Improvement Section in
Springfield. They are distributed by the Laboratory Improvement Section to Division personnel upon
request and when documents are updated or changed.
9.2
ROUTINE QA OPERATING DOCUMENTS AND ANALYTICAL RECORDS
All routine QA documents (logbooks, temperature charts, balance checks, specimen receipt logs, etc.)
and specimen analysis records are used, properly maintained and stored by the laboratories. All
documents and records are maintained on site for a period of three years either as hard copy or
electronic equivalents. At the end of on site storage period, documents are inventoried, boxed up and
sent to the Secretary of State Records Retention Center and are kept for the appropriate time. See
Guideline Record management 91-02 and DAA010,
SOP for Record management.
See section 8 for detailed information on final reports and laboratory records. Allowing for specific
requirements of the analytical process, quality assurance forms established by the Division of
Laboratories may be revised by the technical supervisor in order to more accurately reflect the
documentation required. The revised forms must contain at a minimum the criteria set by the Division.
The revised forms must be reviewed by the Laboratory Improvement staff and be approved by the
Laboratory Director and Chief, Division of Laboratories, prior to implementation. Electronic records
are considered equivalent to hard copy.
9.2.1 QUALITY MANUAL
This Quality Manual was developed by the Division of Laboratories and is reviewed by the Division’s
management and Laboratory Improvement Section. The Division’s Quality Manual is a controlled
document that is distributed to all employees in the clinical and research sections/units in the DOL.
Receipt will be documented with signed read receipts.
9.2.2 DOCUMENT TRACKING
A master list of controlled documents for the Quality Manual, SOPs, and QA policies will be
developed and maintained by the Laboratory Improvement Section. All controlled documents and
subsequent approved revisions will be entered into the system by the LQS. The master list of
controlled documents includes the title, revision number, effective date, and retirement date of all
SOPs. The LQS will monitor the status of all documents to assure timely drafting, review, and
approval or revisions.
DCA001-06-1105
Page 39 of 65
9.2.3 RETIREMENT OF DOCUMENTS
The LQS will ensure that all DOL quality documents are current. Should one of these documents
become outdated, the LQS will initiate a revision of the document. Copies of all quality documents,
both current and retired, will be maintained in the Laboratory Improvement Section in a centrally
located file area under secure conditions for a minimum of six years.
9.2.4 MAINTAINING SENSITIVE DOCUMENT INTEGRITY
The DOL will take special care to preserve the integrity of all sensitive documents such as audit
reports, performance evaluation reports, personnel assessments, and service improvement forms. At
the Springfield and Chicago laboratories, these records are in the Laboratory Improvement Office.
This office is locked when the Laboratory Quality Specialist/Manager is absent. Access to these
records is by permission of the Division Chief, Laboratory Director, and/or the Laboratory
Improvement Section only. In Carbondale, these records are located and kept secure in the Laboratory
Manager’s office.
Analysts have access to those specimen records that are currently being completed. After the records
are completed and submitted to the unit supervisor, they are accessible to analysts only with
permission of the supervisor. Records that have entered permanent storage are accessible only to the
Division Chief, Laboratory Director, Section Chief, Laboratory Improvement Section, or the
designated supervisor. If sensitive (confidential, proprietary, evidentiary) documents are used at a
workstation, due care and document control processes are used in order to maintain integrity of the
data or records. No data or records identified as, or deemed to be confidential, proprietary or
evidentiary documents will be distributed outside the Division of Laboratories workstation where they
are being used without specific written or verbal permission by the appropriate laboratory technical
supervisor. Any issues or questions concerning the internal Division of Laboratories distribution of
such documents must be brought promptly to the attention of the applicable section or unit supervisor.
All records will follow the departmental requirements of the Health Insurance Portability and
Accountability Act of 1996 (HIPPA).
9.2.5 STANDARD OPERATING PROCEDURES
All methods performed in the Division of Laboratories will have a standard operating procedure that
details the procedure and requirements of the method. See SOP DEA021,
The Initiation, Draft,
Approval, Distribution, and Revision of Standard Operating Procedures (SOPs).
9.2.6 EQUIPMENT MAINTENANCE DOCUMENTATION
Documents detailing the receipt and specification of analytical equipment are retained. A history of
the maintenance record of each instrument serves as an indication of the adequacy of maintenance
schedules and parts inventory. The performance of all instrument maintenance, either regular or
emergency, will be recorded in a logbook, electronic record, or log sheet. The information recorded
will include date and initials, the maintenance performed, and the reason for the maintenance.
9.2.7 TEST REQUISITIONS AND AUTHORIZATIONS
DCA001-06-1105
Page 40 of 65
Records of test requisitions and test authorizations must be retained including documentation of the
laboratory’s efforts to obtain authorization to perform the test when a verbal request for test and/or
patient demographics has been made. See memorandum dated April 29, 2003,
Operational Change –
CLIA Request.
A copy of this memorandum can be obtained from the Laboratory Improvement
Section.
9.2.8 SPECIMEN ANALYSIS LOGBOOKS/LOGSHEETS
Specimen analysis logbooks/logsheets/worksheets are used to document the conditions and date of
specimen analysis. The information in these logbooks includes specimen numbers and the date of
analysis, the method used, and any instrument or specimen information that may be relevant.
9.2.9 ORIGINAL DATA
The raw data and calculated results for all specimens are maintained in laboratory notebooks, logs,
bench sheets, files or other sample tracking or data entry forms or electronic equivalents. Instrumental
output is stored in a computer file or hard copy report. These records include the following:
-
Laboratory specimen ID code;
-
Date of analysis;
-
All specimen handling and analysis logbook/logsheets or electronic equivalents;
-
Instrumentation identification and instrument operating condition/parameters (if appropriate);
-
Analysis type and specimen preparation information;
-
All manual, automated, or statistical calculations;
-
All data system reports including instrument printouts or electronic equivalents;
-
Confirmatory analysis data, when required;
-
Review history of specimen data; and,
-
Analyst’s or operator’s initials/signature.
9.2.10 QC DATA
The raw data and calculated results for all QC specimens and standards are maintained in the manner
described in the preceding paragraph. Documentation allows correlation of sample results with
associated QC data. Documentation also includes the source and lot numbers of standards for
traceability. QC specimens include, but are not limited to, sterility checks, positive and negative
controls and multiple level controls.
9.2.11 SYSTEM PERFORMANCE
Systems performance includes specification that the laboratory has established and verified the test
performance specification including verification studies and calibration verification.
9.2.12 FINAL REPORT
A copy of any report issued and any supporting documentation is retained by the laboratory, either in
hard copy or retrievable electronic form for a minimum of 6 years (per HIPPA requirements). Reports
issued by Metabolic/Genetic Diseases must be retained for 21 years.
DCA001-06-1105
Page 41 of 65
9.2.13 CORRESPONDENCE
Correspondence related to specific specimens is retained along with the specimen test requisition form.
Correspondence and/or records of conversations concerning the final disposition of rejected specimens
are maintained.
9.3 ADMINISTRATIVE RECORDS
Each analyst performing testing must be qualified to perform laboratory testing in accordance with the
accrediting agency. The laboratory supervisor assigns the performance of testing to each analyst. The
technical supervisor or his/her designee upon staff assignment to that laboratory initiates CLIA
personnel files. CLIA personnel files include educational background, initial and continuing
demonstrations of capability or personnel assessments, and record of training. CLIA personnel files
are maintained in the Laboratory Improvement Section. See Appendix 6.
9.3.1 CORRECTIVE ACTION REPORTS
The process of corrective action is detailed in each method SOP and in SOP DAA015,
Standard
Operating Quality improvement Actions.
Corrective action reports and all pertinent information are
retained at a minimum by the Laboratory Improvement Section.
9.3.2 AUDIT INFORMATION
Files of audits (internal and external), as well as the responses and corrective actions associated with
them including, but not limited to checklists, correspondence, and follow up are retained at a minimum
by the Laboratory Improvement Section. Internal audits will be conducted according to SOP DAA022,
Standard Operating procedures for Internal Audits.
9.3.3 PERFORMANCE EVALUATION DATA
These records include but are not limited to the following: all proficiency test raw data, results,
corrective action and follow-up (if any). The files are arranged chronologically by survey and retained
by the Laboratory Improvement Section and/or the unit supervisor.
9.3.4 SUPPLIER AND SUPPLIES RECORDS
All documentation from suppliers of services is retained. These documents include, but are not limited
to the following: records of service contracts, copies of certifications, schedules of services provided.
All records associated with goods received are also retained. These records include, but are not limited
to instrument manuals and certifications and certificates of analysis for standards and reagents.
9.4
RECORD STORAGE PROCEDURE
All records, including all information necessary for the historical reconstruction of data, must be stored
for a minimum of 6 years from the date of completion of analysis; 21 years for Metabolic/Genetic
Diseases. For the first two years the records are stored in-house. The data is then stored at the State
Records Center in Springfield, Illinois under the authority of the Secretary of State for the remainder of
the required time. Stored documentation includes all raw analytical data (computer print-outs, etc),
test requisition forms, logbooks, maintenance records, quality control records, controlled documents,
DCA001-06-1105
Page 42 of 65
etc. In the event of the closure of the laboratory or when a test is discontinued, the records will be
transferred to the State Records Center in Springfield, Illinois. The department programs will be
notified in writing of the closure and procedures for obtaining records.
9.5
DATA SECURITY
Analog data from the instruments is converted to digital information by vendor supplied software
installed on the laboratory’s computers. Consistent, correct analyses of the QC specimens indicate that
the data is being received and calculated correctly.
Data from the laboratory’s computers is backed up on a regular basis. A copy of this back up is
maintained by the Division of Information Technology. Records stored only on electronic media are
supported by the hardware and software necessary for their retrieval.
Data is further protected by physical methods. Access to the laboratory is restricted. A series of
passwords are also required for entry into any database. Access is restricted to authorized personnel.
Computer and automated equipment are maintained to ensure proper functioning and provided with the
environmental and operating condition necessary to maintain the integrity of calibration and test data.
DCA001-06-1105
Page 43 of 65
10.0 SYSTEMS AUDITS
10.1 SCOPE AND FREQUENCY OF AUDITS
The Laboratory Improvement Section (LIS) will, on an annual basis, audit all laboratory operations to
verify compliance with the requirements of the quality system: preanalytic, analytic, and postanalytic.
These audits form the basis of quality improvement action requirements and constitute a permanent
record of the conformity of laboratory operations to quality requirements. When audit findings suggest
that further investigation may be necessary, the laboratory will investigate and when deemed
necessary, take immediate corrective action and immediately notify, in writing, any client whose work
may have been affected.
10.2 TYPES OF AUDITS
10.2.1 QUALITY SYSTEM AUDITS
The quality system audits are based on the quality system elements, activities, and requirements
contained in a quality manual. The activities subject to quality system audits are those activities that
have a significant effect on the quality of DOL services. The laboratory must monitor and evaluate the
overall quality of the preanalytic, analytic, and postanalytic system. Audit procedures and scheduling
are detailed in the latest version/revision of SOP DAA022,
Standard Operating Procedure for Internal
Audits
.
10.2.2 GENERAL LABORATORY AUDITS
The general laboratory systems audit must include a review of the effectiveness of improvement
actions taken to correct problems, revisions of policies and procedures necessary to prevent recurrence
of problems, and discussion of general laboratory systems audit reviews with appropriate staff.
10.2.3 PREANALYTIC SYSTEMS AUDITS
The preanalytic systems audit must include a review of the effectiveness of improvement actions taken
to correct problems, revisions of policies and procedures necessary to prevent recurrence of problems,
and discussion of preanalytic systems audit reviews with appropriate staff.
10.2.4 ANALYTIC SYSTEMS AUDITS
The analytic systems audit must include a review of the effectiveness of improvement actions taken to
correct problems, revision of policies and procedures necessary to prevent recurrence of problems, and
discussion of analytic systems audit reviews with appropriate staff.
10.2.4 POSTANALYTIC SYSTEMS AUDIT
The postanalytic systems audit must include a review of the effectiveness of improvement actions
taken to correct problems, revision of policies and procedures necessary to prevent recurrence of
problems, and discussion of postanalytic systems audit reviews with appropriate staff.
DCA001-06-1105
Page 44 of 65
10.2.5 PERFORMANCE AUDITS – PROFICIENCY TESTING SPECIMENS
Performance audits are periodic audits to ensure the quality of results provided to clients by
implementing checks to monitor the quality of the laboratory’s analytical activities. The audits provide
assessment of laboratory performance through the analysis of proficiency testing specimens. The
specimens are to be handled, analyzed, and reported according to the procedure in SOP DAA017,
Standard Operation Procedure for Handling, Analysis, and Reporting of Proficiency Testing Samples
.
The Division of Laboratories, clinical units, participate in the following programs:
-
CAP – College of American Pathology;
-
WSLH – Wisconsin State Laboratory of Hygiene;
-
CDC – Centers for Disease Control and Prevention;
-
AAB – American Association of Bioanalysts.
Other programs may be added as they become available.
10.2.6 EXTERNAL AUDITS
External audits are conducted to verify compliance with rules, regulations, or certification criteria.
External audits are conducted with a high degree of formality upon notification and scheduling with
the auditing agency. The laboratories are surveyed during the renewal period by the US Department of
Health and Human Service, Centers for Medicare and Medicaid Services for Disease Control and
Prevention (CMS) for all clinical analyses.
10.3 MANAGERIAL REVIEW PROCEDURE
10.3.1 QUARTERLY SUPERVISOR’S REVIEW OF LABORATORY TESTING
Each laboratory supervisor must quarterly review its policies and procedures for sample and results
management, quality control, the relationship of sample information to test results, corrective action,
and customer complaints. See Appendix 7 for the form to be used for this review.
10.3.2 SEMI-ANNUAL QUALITY ASSURANCE REVIEW AND REPORT
Each laboratory supervisor must perform a semi-annual review of its QA program including the
outcome of recent internal audits and assessment by external organizations. The supervisor must then
submit a report to the Laboratory Director and the Laboratory Improvement Section using the forms in
Appendix 8. This report summarizes all quality assurance activities for the six-month period.
10.4 DOCUMENTATION
The laboratory must document all preanalytic, analytic, and postanalytic systems audit activities.
DCA001-06-1105
Page 45 of 65
11.0 DEFINITIONS
Accuracy:
the degree of agreement between an observed value and an accepted reference value.
Arithmetic Mean (Mean X):
the number obtained by dividing the sum of a set of quantities by the
number of quantities in the set; average. The mean is an arithmetical measure of central tendency.
The mean is commonly referred to as the “average”. The mean may be used to indicate the accuracy
of a test or analysis.
Audit
: a formal examination of an organization's or individual's accounts or financial situation; an
assessment.
Calibration:
to determine, by measurement or comparison with a standard, the correct value of each
scale reading on a meter, instrument, or other device.
Calibrator or Standard:
any material that meets established identity, labeling and performance
criteria and is used or recommended for use in a calibration process to establish the basis by which
specimen values are determined.
Chain-of-Custody
: a record that documents the possession of the sample from the time of collection
to receipt in the laboratory through the analytical testing process, and storage or retention; This record
generally includes the number and types of containers, the mode of collection, collector, time of
collection, preservation, and requested analysis.
Coefficient of Variation – CV
: a relative measure of variation, sometimes referred to as the Relative
Standard Deviation, RSD; The CV is expressed as percent by the follow formula: (standard deviation
divided by the mean) times 100.
CMS
: US Department of Health and Human Services, Centers for Medicare and Medicaid Services
for Disease Control and Prevention.
Corrective action
: the action taken to eliminate the causes of an existing conformity, defect, or other
undesirable situation in order to prevent recurrence.
Data audit
: a qualitative and quantitative evaluation of the documentation and procedures associated
with environmental measurements to verify that the resulting data are of acceptable quality.
Deficiency:
an unauthorized deviation from acceptable procedure or practices, or a defect in an item
or system.
DOL
: Division of Laboratories
Holding times (maximum allowable holding time):
the maximum times that samples may be held
prior to analysis and still be considered valid or not compromised. Holding time measurement begins
with the time of specimen collection.
ISO:
the International Organization for Standardization; ISO standards are developed by technical
committees comprising experts on loan from the industrial, technical, and business sectors that asked
for the standards and will subsequently put them to use.
DCA001-06-1105
Page 46 of 65
LQS
: Laboratory Quality Specialist
May
: denotes permitted action, but not required action.
Median
: the middle-ranking number in a set of numbers.
Must
: denotes a requirement that must be met.
Negative Control – media:
an analysis consisting of the inoculation of media using an organism that
should not grow in the media or should not respond in a specific way.
Negative Control – analytical testing
: an analysis consisting of a sample that does not contain the
analyte(s) of interest. A negative control is performed in an analytical test to ensure the test is
responding appropriately.
NIOSH
: National Institute of Occupational Safety and Health
NIST
: National Institute of Standards and Technology, US Department of Commerce
PT
: Proficiency Test
Parallel Testing:
the system used to compare lots and/or shipments of diagnostic reagents/kits or
methods. Parallel testing uses identical controls and specimens/samples to determine the consistency
of the test system being evaluated (i.e., the only variable is the reagent/kit/test material being
evaluated). All reagents are parallel tested prior to use for diagnostic purposes or as screening tests.
Performance Audit
: the routine comparison of independently obtained qualitative and quantitative
measurement system data with routinely obtained data in order to evaluate the proficiency of an
analytical system.
Positive Control – media
: an analysis consisting of the inoculation of media using an organism that
should grow in the media or should respond in a specific way.
Positive Control – analytical testing:
a sample containing the analyte(s) of interest. A positive
control is performed in an analytical test to ensure the test is responding appropriately.
Predictive Value
: the expression of the likelihood that a test result reflects the disease or condition
status of an individual; Predictive values may be applied to either positive or negative results.
Predictive value is especially useful when screening for diseases or conditions in a population with a
low prevalence of the disease or condition.
Preventive Maintenance – PM:
upkeep, repairs, or maintenance that prevents or impedes breakdown
or loss of function in equipment, machinery, or complex systems.
Precision
: the degree to which a set of observations or measurements of the same property, obtained
under similar conditions, conform to themselves. Precision is usually expressed as standard deviation,
variance or range, in either absolute or relative terms.
DCA001-06-1105
Page 47 of 65
Preservation
: the act of preserving or controlling the conditions (e.g., environmental, chemical,
temperature, etc.) at the time of sample collection (or later) to maintain the chemical and/or biological
integrity of the sample.
Proficiency Testing
: a means of evaluating a laboratory’s performance under controlled conditions
relative to a given set of criteria through analysis of unknown samples provided by an external source.
Quality Assurance (QA):
an integrated system of activities involving planning, quality control,
quality assessment, reporting and quality improvement to ensure that a product or service meets
defined standards of quality with a stated level of confidence.
Quality Control (QC):
the overall system of technical activities whose purpose is to measure and
control the quality of a product or service so that it meets the needs of the users.
Quality Manager
– ISO 15189 – has delegated responsibility and authority to oversee compliance
with the requirements of the quality management system and reports directly to the level of laboratory
management at which decisions are made on laboratory policy and procedures.
Regression:
a statistical measure of the strength of linear association of X and Y; Complete linear
association equals unity, while no linear association equals zero.
Relative Standard Deviation – RSD:
see Coefficient of Variation – CV
Screening:
the presumptive identification of unrecognized disease or defect by the application of tests,
examinations, or other procedures which can be applied rapidly to sort out apparently well persons
who probably have a disease from those who probably do not.
Should:
denotes a guideline or recommendation
Slope
: the slope is the rate of change of Y for a unit change of X.
Standard Deviation (SD):
a statistical tool used as a measure of dispersion in a distribution; The SD
is the square root of the variance. The standard deviation is a measure of dispersion of results about
the mean value of the frequency distribution of the observation. Standard deviation may be used to
indicate the precision of a test or analysis.
Traceability:
the property of a result or a measurement where by it can be related to appropriate
standards, generally international or national standards, through an unbroken chain of comparison.
Validation:
the process of substantiating specified performance criteria.
Verification
: the confirmation by examination and provision of evidence that specified requirements
have been met.
DCA001-06-1105
Page 48 of 65
12.0 REFERENCES
12.1
Health Care Financing Administration 42 CFR Part 493
Medicare, Medicaid, and CLIA
Programs: Laboratory Requirements Relating to Quality Systems and Certain Personnel
Qualification;
Final Rule January 24, 2003.
12.2
General Requirements for the Competence of Testing and Calibration Laboratories,
ISP/IEC17025, International Standard, 1999.
12.3
Laboratory Procedure Manuals,
National Committee for Clinical Laboratory Standards,
Volume 4, Number 2.
12.4 James P. Rux, Ph. D.,
Handbook of Quality Assurance for the Analytical Chemistry
Laboratory,
Van Nostrand Reinhold Company, N.Y., 1986.
12.5 Stanley L. Inhorn, M.D., Editor,
Quality Assurance Practices for Health Laboratories,
American Public Health Association, Washington, D.C., 1978.
12.6 John Keenan Taylor,
Quality Assurance of Chemical Measurements,
Lewis Publishers.,
Chelsea, MI, 1988.
12.7 Health Care Financing Administration, 42 CFR Part 405, et. al.,
Clinical Laboratory
Improvement Amendments of 1988,
Final Rule, February 28, 1992.
12.8 J. S. Mausner and A. K. Bahn,
Epidemiology: An Introductory Text,
W. B. Saunders Co.,
Philadelphia, 1974.
12.9 P.E. Leaverton,
A Review of Biostatistics,
Little, Brown & Co., Boston, 1978.
12.10 B. L. Therrell, Jr., Editor,
Laboratory Methods for Neonatal Screening,
American Public
Health Association, 1993.
12.11 ISO15189,
Medical Laboratories – Particular Requirements for Quality and Competence,
ISO
15189:2003(E), ISO 2003
DCA001-06-1105
Page 49 of 65
13.0 APPENDICES:
13.1 Appendix 1 – Directives
13.2 Appendix 2 – Standard Operating Procedures
13.3 Appendix 3 – Training Documentation
13.4 Appendix 4 - Calibration/Verification of Laboratory Support Equipment
13.5 Appendix 5 – Comparison of Results
13.6 Appendix 6 – CLIA Personnel File Documentation
13.7 Appendix 7 – Quarterly Managerial Review Forms
13.8 Appendix 8 – Semi-Annual Managerial Review Forms
DCA001-06-1105
Page 50 of 65
Appendix 1
Directives
Directive Number
Title
Effective Date
01-01
Quality Assurance/Quality Control
October 1, 2001
01-02
Standard Operating Procedure
Implementation/Change
October 1, 2001
01-03
Remedial Action
October 1, 2001
88-03
Personal Conduct
October 15, 1988
97-01
Professional Conduct
March 1, 1997
01-04
Confidentiality of Laboratory Test
Information
October 1, 2001
DCA001-06-1105
Page 51 of 65
Appendix 2
Administrative SOPs
SOP No.
SOP Title
Effective Date
DAA003
SOP for Data Integrity
11/05/2001
DAA005
SOP for Technical and Standard Operation
Implementation/Change
11/01/2001
DAA007
Information Technology Procedure Manual
01/03/2005
DAA0010
Standard Operating Procedure for Record
Management
02/10/2004
DAA015
SOP for Quality Improvement Actions
12/12/2003
DAA016
SOP for Service Improvement Actions
12/12/2003
DAA017
Standard Operating Procedure for Handling,
Analysis, and Reporting of Proficiency Testing
Samples
12/12/2003
DAA018
Chemical Hygiene Plan
12/05/2004
DAA021
SOP for Initiation, Draft, Approval, and
Distribution of Standard Operating Procedures
12/12/2003
DAA022
Standard Operating Procedure for Internal Audits
01/07/2004
DAA023
SOP for Risk Assessment
3/28/2005
DAA025
SOP for Fiscal Ordering
8/16/2005
DAA028
SOP for Exposure Control to Blood borne
Pathogens
1/03/2005
DAS001
SOP for Specimen/Sample Log-In and Receipt
01/01/2004
DCS001
SOP for Preventive Maintenance
09/26/2005
DCA001-06-1105
Page 52 of 65
Appendix 3
Illinois Department of Public Health – Division of Laboratories
Personnel Training Record
Employee Name: _____________________
Supervisor: __________________
I understand that by initialing, I acknowledge having been instructed and evaluated on each time
below. Furthermore, I agree to follow the laboratory’s procedures and policies for the items below.
Employee signature: ___________________
Date: ________________________
Items
Date of Evaluation
Employee
Initials
Supervisor
Initials
Glassware cleaning
Specimen log-in
Analytical balance
Use of pipets
Quality Manual
Emergency Response Plan
Safety Manual
Reagent preparation techniques
Testing procedures (SOP number)
A.
B
C.
D.
E.
F.
G.
DCA001-06-1105
Page 53 of 65
Appendix 4
Calibration/Verification of Laboratory Support Equipment
Equipment
Activity
Minimum
Frequency
Acceptance
Incubators
Temperature check
Once/day
Varies
Balances
Check with three weights
Monthly
"5%
Refrigerators
Temperature check
Once/day
Varies
Freezer
Temperature check
Once/daily
#
-15
o
C
Water bath
Temperature check
Day of use
Varies
Pipets, Pipettors
Volume check
Quarterly
"5%
Temperature monitoring
devices
Verification at temperature
of use against a NIST
thermometer
Yearly
"1
o
C
pH meter
Calibration
Day of use
Slope 95-102%
or
"0.1
of
standard pH
DCA001-06-1105
Page 54 of 65
Appendix 5
Illinois Department of Public Health – Division of Laboratories
Comparison of Test Results Report
Specimen
Method/Instrument
Result
Acceptable
Initials/Date
Summary:
Corrective Action:
Follow-up action to be taken to document that the problem has been solved:
Prepared by: _______________________________________
Date: __________________
General/Tech Supervisor: ____________________________
Date: __________________
Reviewed by QA Staff: ______________________________
Date: __________________
Approved by Laboratory Director: _____________________
Date: __________________
DCA001-06-1105
Page 55 of 65
Appendix 6
Illinois Department of Public Health – Division of Laboratories
CLIA Personnel File
Checklist
Employee Name:
Position:
Date Hired
Certificate, license, registry number
Foreign education equivalency
High school diploma, degrees, certificates
Job responsibilities (copy of job description)
Personnel assessment
In-service training
Safety orientation
Hepatitis B
Tuberculosis test
Rabies vaccination
DCA001-06-1105
Page 56 of 65
Appendix 6 – continued
Illinois Department of Public Health – Division of Laboratories
CLIA Personnel File
Laboratory Qualification Appraisal
Employee Name
:
Section assigned:
Hours:
High School
Career School
Position
DIR
CC
TC
TS
GS
TP
Name of College, University, or Professional School
Attach any copies of license, transcript evaluation,
certification or diplomas
Dates
Major
Degree/Cert.
Experience: Facility’s name
Dates
Position
Test: Extension of test procedures
Eval Date/ Emp. Initials
Supervisor Init.
Signature: General/Technical/Supervisor:
Date:
Director’s Signature:
Date:
DCA001-06-1105
Page 57 of 65
Appendix 6 – continued
Illinois Department of Public Health – Division of Laboratories
CLIA Personnel File
Personnel Assessment
Assessment
Date _____________________
Semiannual
Annual
Other
Employee:
Employee SSN
Job title
Test
Performance
Sat.
Un-sat.
Comment
Direct observation
Sat.
Un-sat.
Comment
Monitoring, reporting
& recording of results
Sat.
Un-sat.
Comment
Monitoring, reporting
& recording of results
Sat.
Un-sat.
Comment
Review of worksheets
PM, QC, PT
Sat.
Un-sat.
Comment
Direct observation
of PM activities
Sat.
Un-sat.
Comment
Blind test samples
Sat.
Un-sat.
Comment
Problem solving skills
General supervisor’s remarks
General supervisor’s signature:
Date
Technical supervisor’s remarks (if applicable):
Technical supervisor’s signature:
Date:
DCA001-06-1105
Page
58 of 65
Appendix 7
Illinois Department of Public Health – Division of Laboratories
Supervisor’s Review of Laboratory Testing
Month(s) of _____________________________ Date of Review:________________________________Reviewed by:_________
The laboratory must perform a quarterly review of its policies and procedures for patient test management, quality control, proficiency testing, relationship of
patient information to test results, communications and complaint investigations. To comply with this requirement, review a representative number of specimens
for compliance in the indicated headings. Indicate Sat
, if satisfactory, Unsat, if unsatisfactory. Use the comment/corrective action space to document
unsatisfactory findings and corresponding corrective action.
Test
Specimens
reviewed
Requisitions Reports Worksheets
QC
PM
Calibration
Storage
Corrective
action
PT
%TAT Communications
Requisitions: Review specimen processing for accuracy: Specimen identification, test ordered, correct specimen type, appropriate handling, appropriate storage.
Reports:
Assure that requisition information has been accurately transferred to test report; test ordered was performed and reported to authorized person.
Worksheets: Has all required information including lot #, results from worksheets, instrument printout or electronic transmission been accurately reported.
QC
: Control samples are tested in the dame manner as patients, meet established criteria, corrective action is followed when controls are out of limits.
PM
: Records of all instruments requiring preventive maintenance, including corrective action and proper labeling, assure PM schedule is observed.
Calibration: Records of all instruments requiring calibration to assure that calibration and/or verification is performed at least every 6 months, review for
compliance.
Storage
: Specimens, records, and reagents are stored according to schedule or corresponding expiration date.
Corrective Action
: Evaluate corrective actions for effectiveness and follow up.
PT
: PT samples are treated as patients, if unsat participation, review patients’ specimens, review corrective action and follow-up.
% TAT: Indicate the % of specimens that meet the established TAT for the review period.
Communications
: Review documents that resolve communication problems and complaints, effectiveness of solutions.
Comments: Findings/ Corrective Action:
Follow-up:
DCA001-06-1105
Page
59 of 65
Appendix 8
Quality Assurance Semi-Annual Report
Lab. Section/Unit:
Date:
PT results/Deviances:
PT results/Deviances:
QC Reports:
Personnel Assessment reports:
Lab investigation reports:
Preventive maintenance records:
Safety:
Other Quality Assurance Issues:
Technical supervisor:
Date:
Q.A. Staff :
Date:
Laboratory Director:
Date:
DCA001-06-1105
Page
61 of 65
Appendix 8 - continued
Illinois Department of Public Health – Division of Laboratories
Quality Assessment Semi-Annual Managerial Review
Performed by: _________________________Date: ___________________Test(s): _______________
The technical supervisor or his/her designee must review the level of compliance in his/her area(s) of
responsibility with the Division’s quality assurance policies and procedures semiannually. Indicate Y,
N, or NA in the findings box. Use the comments/corrective action space to documented the proposed
corrective action, where appropriate.
ASSESSMENT REQUIREMENTS
Finding
Comments/ corrective action
FACILITY
Work area arranged to minimize problems in
specimen handling, examination and testing of
specimens, and reporting of test results
Workbench space is sufficient for test performance, is
well lit, and has water, gas, suction, and electrical
outlets as necessary
Instruments, equipment, and computer systems are
placed in locations where their operation is not
affected adversely by physical or chemical factors
such as heat, direct sunlight, vibrations, power
fluctuations, or fumes from acid or alkaline solutions
Equipment tops are not used as workbench space
Stable electrical source is maintained, e.g. outlets, not
extension cords, and meets the power requirements
for each piece of equipment
Follows contamination prevention practices to
minimize contamination of patient specimens,
equipment, instruments, reagents, materials, and
supplies
Performs wipe test of areas where radioactive
material or amplification procedures are used in order
to monitor and prevent contamination
Processing of mycobacteriology cultures is performed
in a manner that prevents contamination of the
environment
Molecular amplification procedures have a
mechanism to detect cross contamination of patient
specimens
Molecular amplification procedures have a uni-
directional workflow – separate areas for reagent
preparation, pre-amplification, and post-amplification
Has equipment and/or instruments capable of
producing results within the stated test performance
specifications
Has test systems, equipment and/or instruments
DCA001-06-1105
Page
62 of 65
ASSESSMENT REQUIREMENTS
Finding
Comments/ corrective action
necessary to perform the laboratory’s volume of
testing within established turnaround times
Data capacity in the laboratory information system is
sufficient for current data entry and reporting
Safety procedures are established, accessible, and
observed
Has clean up spill kits (chemical, biological,
radiological)
Has determine the amount of waste that can be safely
contained and precautions are taken to ensure that
liquid waste does not spill or splash while in travel
status
Maintains records in a secure area
Records are stored according to the established
schedule
Maintains copies of the original test requisition or
electronic equivalents
Retains copies of all reports including original,
preliminary, corrected, and final reports
GENERAL LABORATORY SYSTEMS –
CONFIDENTIALITY
Controls visitor access to the laboratory areas
Has safeguards to ensure confidentiality of patient
information and test reports
GENERAL LABORATORY SYSTEMS –
IDENTIFICATION & INTEGRITY
Ensures positive specimen identification from
specimen receiving to the reporting of results
Maintains optimum integrity of patient’s specimen,
follows instructions for performance of each test
method and analyzes the specimen within the
limitations of the test methodology
GENERAL LABORATORY SYSTEMS –
COMPLAINT INVESTIGATION and
COMMUNICATION
Documents all complaints and problems reported to
the laboratory
Investigates all complaints and implement corrective
action when applicable
GENERAL LABORATORY SYSTEMS –
PERSONNEL ASSESSMENT
Monitors each individual’s competency and identify
remedial training or continuing educational needs
GENERAL LABORATORY SYSTEMS – PT
Reviews and evaluates laboratory’s PT results
Reviews its menu to determine if it tests for any
DCA001-06-1105
Page
63 of 65
ASSESSMENT REQUIREMENTS
Finding
Comments/ corrective action
analyte for which there is no PT
Twice annually verifies the accuracy of the test
without PT
Documents review of PT score and corrective action
taken
PREANALYTIC SYSTEM
Retains all laboratory test requests
Documents attempts to collect written lab orders
following a verbal request
Uniquely identifies patient specimens
Ensures that individuals who entered data including
clerical staff correctly match patient information
Provides written instructions or electronic equivalents
to submitters to ensure that patient preparation
requirements have been followed
Specimens are properly stored
Follows referral laboratory’s instructions, as
appropriate, for transport specimens
Notifies submitter when specimen meets its
unsatisfactory criteria and it is unsuitable for testing
Has a current service manual for each reference
laboratory
Documents the date and time it receives specimens
Maintains copy of CLIA certificate of reference
laboratory
ANALYTIC SYSTEM
Has current and approved SOP’s
SOP includes calibration and calibration verification
procedures
SOP includes quality control procedures and
corrective action
Laboratory information system SOP are available to
operators
Follows manufacturer’s instructions for the use of
equipment, instruments, reagents, materials and
supplies
Assesses water quality (may include pH, silicate
content, particular matter, bacterial and organic
content)
Monitors and documents results for acceptable
temperature range on temperature controlled spaces,
equipment, and instruments
Documents dates for expiration of kits/ reagents and
date opened
Uses reagents, solutions, control materials, within
their expiration date, free of contamination, or other
DCA001-06-1105
Page
64 of 65
ASSESSMENT REQUIREMENTS
Finding
Comments/ corrective action
signs of deterioration
Uses components of reagents kits with the same lot
number unless otherwise approved by the
manufacturer/supplier
Verifies or establishes performance specifications for
any new test/ major change to the procedure (SOP
DAA005)
Verifies or establishes performance specifications for
each instrument when multiple instruments are used
Performs maintenance and function checks as defined
in the preventive maintenance manual
Maintains instrument / equipment maintenance
service records
Follows and documents the necessary function checks
as stated by the laboratory information system for the
computer and devices such as monitors, printers and
modems
For instruments that automatically perform function
checks and flag problems, the laboratory documents
the corrective actions in response to the flagged
problem
Fluorescent light source has not exceeded the
manufacturer’s established optimal timeframe
Autodiluters, microdiluters and/or pipettors are
checked for adequate and consistent delivery
For systems that perform simultaneous fluid delivery
to multi well plates or tubes, the laboratory checks for
uniform deliver of reagents or washing solutions to all
wells or tubes
The laboratory monitors the accuracy and precision of
each phase of the analytical testing process by using
control procedures
Control procedures to the test system includes
checking for reagent contamination or deterioration,
reagent lot variation, reaction temperature fluctuation,
inadequate sampling, improper loss of calibration,
electronic or mechanical failure, power supply
variances
Controls procedures that affect the test environment
include checking for temperature, airflow, light
intensity, humidity
Testing personnel are trained prior to testing on the
test performed (SOP)
Uses control samples, at a minimum, as frequent as
specified by the manufacturer
Each quantitative procedure include 2 controls of
different concentration
DCA001-06-1105
Page
65 of 65
ASSESSMENT REQUIREMENTS
Finding
Comments/ corrective action
Each qualitative procedure includes a negative and
positive control
Each procedure producing graded or titered results
include a negative control and a control with graded
or titered reactivity
Verifies acceptable ranges for control materials
Media are checked for sterility, ability to support
growth
Maintains QC documentation from manufacturer and
documents receipt and condition of each batch, lot, or
shipment
Uses ATCC controls or have established reactivity for
each organism
Investigates QC failures and document all corrective
action taken
POSTANALYTIC SYSTEM
Test reports indicate the location where the test was
performed
Test report dates are not changed
Test report corrected copies leave an audit trail
Test results are released to authorized persons
Laboratory documents the date, time test results, and
person’s name to whom test results were given
verbally to alert of panic values
Laboratory maintains an “exact duplicate “ test report
or electronic equivalent
PERSONNEL
A current CLIA file is maintained for each testing
staff member and supervisor.
Personnel assessments are performed for each testing
staff member for each test performed
Monitors each individual’s competency and identifies
remedial training or continuing educational needs
CHEERS QAPP 3
Appendix 22: UIH Procedures for Archiving
Cultures
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 1 of 11
Procedure: Maintenance of Stock Bacteriology Cultures
Prepared by
Date Adopted
Supersedes Procedure
Kathy Ristow
Review Date
Revision Date
Signature
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 2 of 11
PRINCIPLE:
Long term storage of isolates may be achieved by freezing in a storage medium at -70
o
C.
Isolates may be obtained from commercial sources (i.e. ATCC) or prepared from in-house
isolates. The storage medium used is Remel Pras Milk (skim milk) which is dispensed into 2 ml
cryovials. The cryobox used for freezing the cryovials contains 81 positions. The cryobox is
given the name of the type of organisms to be stored (i.e. ATCC strains) and a sequential
number. Information about the organisms stored in each of the 81 cryobox positions is written on
the cryobox logsheet. There are two type of logsheets used, one for VRE isolates (Appendix A),
and the other is for all other isolates (Appendix B). The data or pedigree of particular isolate is
maintained in a data binder by including the Sunquest worksheet.
SPECIMEN:
Pure culture from overnight growth on an appropriate medium
EQUIPMENT AND MATERIALS:
Equipment:
-70
o
C Freezer
35 – 37
o
C Incubator (ambient or CO
2
as appropriate)
Materials:
Sterile 2ml Cryovial
Plastic Cryobox
Sterile loop
Sterile swab
Sterile applicator stick
Appropriate culture media
PROCEDURE - STEPWISE:
Freezing of Stock Strains
1.
Obtain the binder of the organism type to freeze (i.e. ATCC strains, VRE strains, etc). On
the cryobox logsheet locate a vacant location. Write the name of the organism on the
logsheet. Include the ATCC number and/or Sunquest accession number where appropriate.
Note the location to be used.
2.
Label cryovial with organism name and location in cryobox, i.e.
S. aureus
Box 1 #23.
University o
Pathology L
Clinical Microbiology Laboratory
f Illinois Medical Center at Chicago
aboratories
Page 3 of 11
3.
Using a sterile disposable pipet, dispense approximately 2 ml of Remel Pras milk into
cryovial.
3.
Using a sterile swab or loop, suspend a heavy inoculum into the vial and cap tightly.
4.
Label the Sunquest workup printout with the cryovial box used and location. Insert sheet
into binder in numerical order.
5.
Place vial in proper cryobox location in the -70
o
C freezer located in Room 241.
Retreival of Frozen Stock Strains
1.
Locate required isolate in stock culture binders
2.
Retrieve cryobox from -70
o
C freezer located in Room 241.
3.
Remove cryovial from cryobox. Slightly warm vial so the top portion of skin milk may be
removed using a sterile applicator stick or loop. DO NOT COMPLETELY THAW VIAL.
4.
Subculture to appropriate culture media.
REFERENCES:
Isenberg, Henry D, et al. 2006.
Clinical Microbiology Procedures
Handbook
, 2
nd
ed. American Society for Microbiology, Washington, D.C.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 4 of 11
Appendix A
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 5 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 6 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 7 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 8 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
Page 9 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
f Illinois Medical Center at Chicago
aboratories
Page 10 of 11
Linezolid Results
MIC Results: S, I, or R.
Patient Name
Med. Rec. No.
Acc.
No.
Date
of
culture
Isolate
Date
of
Test
AST method
(KBS or E-
test)
Lot #
MIC or
Zone
size
Inter
(S,I,R)
Tech
Vanc
Amp
Syne
Chlor
Eryth
Rif
Tetra
78.
79.
80.
81.
University o
Pathology L
Clinical Microbiology Laboratory
University of Illinois Medical Center at Chicago
Pathology Laboratories
Clinical Microbiology Laboratory
CRYO BOX NUMBER ______
1
28
55
Appendix B
2
29
56
3
30
57
4
31
58
5
32
59
6
33
60
7
34
61
8
35
62
9
36
63
10
37
64
11
38
65
12
39
66
13
40
67
14
41
68
15
42
69
16
43
70
17
44
71
18
45
72
19
46
73
20
47
74
21
48
75
22
49
76
23
50
77
24
51
78
25
52
79
26
53
80
27
54
81
Page 11 of 11
CHEERS QAPP 3
Appendix 23: IDPH Procedures for
Archiving Cultures for PCR
IDPH Protocol for archiving stool specimens for later extraction of nucleic acids and
viral identification by PCR
As part of the Norovirus identification procedure, stool specimens are prepared for RNA
extraction by adding 0.5 mL of buffer to a small amount of stool and 10 glass beads in a
1.5 mL microfuge tube. After vortexing to disrupt the stool matrix, the suspension is
clarified by centrifugation. One-third of the supernatant is used to prepare RNA, and the
remaining supernatant (over the disrupted stool and glass beads) is frozen at -70
o
C for
long-term storage (archiving).
Note: Bacterial cultures are set up from the raw stool when it is received in the lab, but
raw stool is not frozen/archived. Consequently, in the future, only viral identification
(including other molecular manipulations – e.g., sequencing or cloning) would be
possible from the archived material. We have been able to successfully amplify
Norovirus RNA from supernatants that have been frozen for several years.
CHEERS QAPP 3
Appendix 24: Unusual Occurrence Log
CHEERS
LOG OF SPECIMENS UNSATISFACTORY FOR TESTING
SPECIMEN
NUMBER
PROBLEM
DATE
REPORTED
COMMENTS
FINAL ACTION
DATE
RESOLVED
INITIALS
1
CHEERS: THE CHICAGO HEALTH,
ENVIRONMENTAL EXPOSURE, AND
RECREATION STUDY
STATISTICAL ANALYSES
Project Statistician: Li Liu, PhD
Division of Epidemiology and Biostatistics
University of Illinois at Chicago
School of Public Health
2
1. Power/sample size calculation
1.1. The sample size calculations and data analyses are based upon the two primary
aims of this research study, namely, 1) to determine rates of acute gastrointestinal
and non-gastrointestinal illness attributable to recreation on the CAWs, and 2) to
define the relationship between concentrations of microbes and rates of illness
among individuals with limited recreational water contact. As noted in the
District’s Expert Review Report (2006-038), should an epidemiologic study be
conducted, it would require “sufficient statistical power to detect risks at levels
deemed to be acceptable for regulatory purposes.”
1.1.1. For Aim 1, we need to make sure that the number of participants is big
enough in the study in order to detect with confidence, differences that
may exist between the groups. The subtler the difference between groups
(e.g., in rates of AGI), the larger the requisite sample size (e.g., the
number of participants). Given that the background rate of AGI may be
approximately 75 cases/1,000 people, detecting an excess risk in one
group of 10/1,000 would mean differentiating rates of 75/1,000 in the
“background rate” group, from a rate of 85/1,000 in an exposed group.
Mathematical formulas allow the calculation of the required sample size
for the comparison of rates between groups, and a given level of
confidence desired in the conclusion that differences in rates are not due to
chance alone.(Fleiss 1981) Using this formula, given an anticipated
background rate of 75 cases of AGI/1,000 participants in the unexposed
recreator group, in order to identify with confidence a rate of
97cases/1,000 in the CAWs group (a 22 cases/1,000 increase in rates), a
sample size of 2,644 participants per group is required. Larger samples
sizes would allow the detection of more subtle differences. Detecting an
increase of 10 cases/1,000 exposures would require recruiting more than
40,000 participants, which would not be feasible.
1.1.2. For Aim 2, the calculation of the required number of study participants
would be based on the development of a mathematical relationship
3
between concentrations of microbes in the water and rates of illness. With
data obtained in such a study, it would be possible to model rates of illness
as a function of water quality, allowing the prediction of rates of illness for
a given concentration of microbes in the water. The sample size required
for such a study can be calculated if one specifies 1) an estimated slope of
the concentration-disease rate line, 2) an estimated background rate, and 3)
the desired level of statistical confidence desired (Tosteson et al. 2003).
Therefore, given that a 10-fold increase in microbe concentrations in
freshwater increases the risk of AGI by approximately 60% among
swimmers,(Wade et al. 2003) we will assume, for the purpose of sample
size calculation, that the risk of illness among non-swimming recreators is
one-fourth that of swimmers. In order to detect a 15% increase in risk of
illness for a 10-fold increase in microbe concentrations, assuming a
background rate of 75 cases of AGI/1,000, a sample size of 5,028
participants in the water-recreation groups (CAWs and general use waters)
is required.
1.2. Given the above calculations for Aims 1 and 2, we will seek complete data on
2,644 subjects in each of the three groups or a final sample size of 7,932.
Assuming a projected attrition rate of 15% (those who do not complete follow-
up), a total of 9,330 participants will be recruited in order to obtain a final dataset
for analysis with records of 7,932 individuals.
2. Health outcomes, Non-water related and Water Quality Measures
Potential outcome variables, effect modifiers and non-water related confounders, as well
as water exposure and water-quality related predictors are presented in the tables at the
end of this section.
Health outcomes of interest include Acute GI illness and Non-GI illness such as ear,
eyes, skin, and upper respiratory illness. GI symptoms include abdominal cramps or
stomach ache, diarrhea, nausea, and vomiting. Three syndromic definitions (any two or
more of the symptoms; Highly Credible Gastroinsteinal Illness; or the definition used in
the NEEAR study, as outlined in the QAPP 2: Survey Methods) will be explored. Two GI
4
illness outcome variables will be considered in modeling stage: 0/1 indicator of illness
and an ordinal variable of number of symptom items presented. For non-GI illness, a 0/1
indicator for any non-GI illness, indicators for ear, eyes, skin, and respiratory illness, and
an ordinal variable of number of illness presented will be used in logistic regression
models.
In evaluation of the overall risk, potential non-water related confounders include
participant demographics, recent exposures (animal, food, someone ill) prior to
recreation, pre-existing health conditions (such as chronic gastrointestinal illness, asthma,
diabetes), and post-recreation exposures. In assessing the relationship between water
quality and health outcomes, potential behavioral predictors include type and duration of
activity, get wet at all, food/water during or after activity, sanitary measures, and other
activity-specific exposure measures. Water-quality related predictors include
E. coli
,
enterococcus, and coliphage concentrations, concentrations of coliphage serotypes linked
to human sources; recent combined sewer overflow events and rainfall conditions.
3. Data Analysis
3.1. After data is collected, descriptive statistics will be conducted. This will include:
3.1.1. Descriptive statistics of study participant demographics (age,
gender, race, distance traveled, and socioeconomic characteristics).
Demographic distributions for all participants, as well as
distributions by study group, location site, and year of enrollment
will be examined.
3.1.2. Descriptive statistics of participant pre-existing health conditions
and pre- and post-exposures. Overall distributions as well as
distributions by study group, location site, and year of enrollment
will be examined.
3.1.3. Summary statistics of all potential behavioral predictors, including
type and duration of activity, water exposure measures, sanitary
conditions, and food/water intake during or after activity, etc.
5
3.1.4. Summary statistics of indicator organism concentration (
E. coli,
enterococci, coliphages and coliphage serotpyes) in water, by
location and year.
3.1.5. A correlation matrix of concentrations of indicators and pathogens,
measured in samples collected at the same location, at the same
point in time.
3.1.6. Rates of all illness, including acute GI illness and Non-GI illness
such as ear, eyes, skin, and upper respiratory illness. For GI illness,
distributions of the number of GI symptoms presented (abdominal
cramps or stomach ache, diarrhea, nausea, and vomiting) will be
examined. For Non-GI illness, number of illness presented will
also be examined. All rates and distributions of the ordinal
outcomes will be presented by study group, location, and year.
3.1.7. Descriptive statistics of organisms identified in clinical specimens,
by study group.
3.2. Formal statistical analysis for this data includes:
3.2.1. Crude tests of equality of rates among the three study groups for all
acute GI and non-GI illnesses. For these overall tests, chi-square
test of homogeneity of proportions will be performed for all illness
and symptoms of interest. Chi-square test for comparisons of
proportions has the advantage of allowing for more than two
groups. These tests are the first step in revealing whether the rates
of illness are significantly different for the three study groups
without considerations of other effect modifiers or confounders.
3.2.2. Evaluation of the overall risk associated with the study groups. At
this stage, the water-quality related measures are not included. For
each illness of interest, logistic regression models will be used to
estimate the study group effect on illness rate while adjusting for
non-water related covariates such as participant demographics, pre-
existing health conditions, pre- and post-activity exposures.
6
Interactions between covariates and study groups will also be
examined. Model selections will be performed for all logistic
models. Once a final model is selected for an outcome of interest,
adjusted odds ratios for each group from the logistic regression
model can be obtain by holding covariates constant at reasonable
values. For ordinal outcomes of number of GI symptoms presented
and number of Non-GI illness presented, proportional odds models
in logistic regression will be applied, and common odds ratios will
be reported. In fitting the logistic models for ordinal outcomes, the
proportional odds assumption can be examined by the Score test in
SAS.
3.2.3. Modeling the relationship between water quality and health
outcomes. Since water-quality measures are often approximately
log-normally distributed (El-Shaarawi 1989; El-Shaarawi and
Viveros 1997; Noble et al. 2003), base 10 log (log
10
)
transformation of the count is likely to be done for all organism
densities. Different types of water quality measures will be
explored as predictor of the health outcomes. At participant level,
since the time of entering and exit of water will be recorded, water
qualities measured after (or close to) entering time and before (or
close to) the exit time can be averaged to obtain measures that are
subject specific. At access site level, average of measures specific
to the activity location can be obtained. And overall daily water
quality, such as the average of all measures of the day, and one-
time down-stream measure, can be used as well. In the modeling
stage, water-related covariates including behavioral factors,
activity-specific exposure measures, and water quality indicators
will be added into the logistic regression models. A model with
nested interaction terms between water exposures and water
quality measures will be included. These nested interaction models
effectively assign zero exposure values by the use of indicators for
7
water-related activities and water exposures. For instance, in
modeling the probability of illness
p
, the covariates of interest are:
X
1
= 1 if participant engaged in water-related activities, 0
otherwise; X
2
= 1 if participant got wet during the activity; and X
3
is the water organism density measure. The nested interaction
model is parameterized as follows:
log(
p
)
p
XX
XX
XX
XX
1
0112
2
313
4
2
3
512
−
=+
+
+
+
ββ
+
β
β
β
β
X
3
Model selections will be done, and adjusted odds ratios for unit
increase in indicator organisms will be reported to reflect the effect
of water quality on illness.
8
4.0 Overview of analyses to be performed using final datasets
Prior to reaching conclusions from CHEERS regarding rates of illness, risk factors
for illness, and causes of illness, numerous data analyses will be performed. These
include:
4.1 Water data: quality monitoring
4.1.1 Quality monitoring: indicator microbes
4.1.1.1
E. coli
: splits, blanks, recoveries (multi-level and single-
level spiking)
4.1.1.2 Enterococci: splits, blanks, recoveries (multi-level and
single-level spiking)
4.1.1.3 EPA dilution/plate concentration requirements for E. coli
and Enterococci
4.1.1.4 F+ coliphages: splits, blanks, recoveries (multi-level and
single-level spiking)/method 1602 validation for surface
waters
4.1.1.5 Somatic coliphages: splits, blanks, recoveries (multi-level
and single-level spiking) /method 1602 validation for
surface waters
4.1.2 Quality monitoring: pathogens
4.1.2.1
Giardia
: splits, blanks, recoveries (multi-level and single-
level spiking)
4.1.2.2
Crytposporidium
: splits, blanks, recoveries (multi-level and
single-level spiking)
4.2 Quality monitoring: sampling strategy
4.2.1 Analyses of spatial and temporal predictors of microbe
concentration
4.2.1.1 Location (access point)
4.2.1.2 Cross-section
9
4.2.1.3 Upstream/downstream
4.2.1.4 Date
4.2.1.5 Time within day
4.2.2 Holding time
4.2.3 Sample temperature
4.3 Descriptive measures of water quality
4.3.1 Microbiology
4.3.1.1 Concentrations of indicator microbes by location, overall
and by year
4.3.1.1.1 E. coli
4.3.1.1.2 Enterococci
4.3.1.1.3 F+ coliphages
4.3.1.1.4 Somatic coliphages
4.3.1.2 Concentrations of pathogens by location, overall and by
year:
4.3.1.2.1 Giardia
4.3.1.2.2 Cryptosporidium
4.3.2 Water chemistry, by location, by year
4.3.2.1 pH
4.3.2.2 temperature
4.3.2.3 dissolved oxygen
4.3.2.4 conductivity
4.3.2.5 turbidity
4.4 Environmental observations, by location, by year
4.4.1 wave
4.4.2 wake
4.4.3 wind
4.4.4 wildlife
10
4.5 Meteorology
4.5.1 By month, by year:
4.5.1.1 air temperature
4.5.1.2 precipitation
4.5.2 By time of day, by month: cloud cover
4.6 Analyses of water quality
4.6.1 correlations among indicators, with factor analysis
4.6.1.1 Overall
4.6.1.2 By location
4.6.1.3 By group of locations
4.6.1.4 GUW
4.6.1.4.1 Overall
4.6.1.4.2 Small lakes + lagoons
4.6.1.4.3 Rivers
4.6.1.4.4 Lake Michigan
4.6.1.5 CAWS
4.6.1.5.1 Overall
4.6.1.5.2 Locations above WRPs vs. below
4.6.1.5.3 Locations below WRPs overall
4.6.1.5.4 Locations below WRPs, North vs. South
4.6.2 Correlations among indicators (with factors) and pathogens
(
Giardia
and
Cryptosporidium
)
4.6.2.1 Overall
4.6.2.2 By location
4.6.2.3 By group of locations
4.6.3 GUW
4.6.3.1.1 Overall
4.6.3.1.2 Small lakes + lagoons
4.6.3.1.3 Rivers
4.6.3.1.4 Lake Michigan
11
4.6.3.2 CAWS
4.6.3.2.1 Overall
4.6.3.2.2 Locations above WRPs vs. below
4.6.3.2.3 Locations below WPRs overall
4.6.3.2.4 Locations below WRPs, North vs. South
4.6.4 Correlations among indicators and water chemistry
4.6.4.1 Overall
4.6.4.2 By location
4.6.4.2.1 By group of locations
4.6.5 GUW
4.6.5.1.1 Overall
4.6.5.1.2 Small lakes + lagoons
4.6.5.1.3 Rivers
4.6.5.1.4 Lake Michigan
4.6.5.2 CAWS
4.6.5.2.1 Overall
4.6.5.2.2 Locations above WRPs vs. below
4.6.5.2.3 Locations below WPRs overall
4.6.5.2.4 Locations below WRPs, North vs. South
4.6.6 Correlations among indicators and environmental observations
4.6.6.1 Overall
4.6.6.2 By location
4.6.6.3 By group of locations
4.6.6.4 GUW
4.6.6.4.1 Overall
4.6.6.4.2 Small lakes + lagoons
4.6.6.4.3 Rivers
4.6.6.4.4 Lake Michigan
12
4.6.6.5 CAWS
4.6.6.5.1 Overall
4.6.6.5.2 Locations above WRPs vs. below
4.6.6.5.3 Locations below WPRs overall
4.6.6.5.4 Locations below WRPs, North vs. South
4.6.7 Effect of rainfall, overall and by location
4.6.7.1 Rainfall (≥0.1 inch, present vs absent) for indicators
4.6.7.1.1 on day of sampling
4.6.7.1.2 24 hours prior
4.6.7.1.3 48 hours prior
4.6.7.1.4 72 hours prior
4.6.7.2 Rainfall (≥0.1 inch, present vs absent) for pathogens
4.6.7.2.1 on day of sampling
4.6.7.2.2 24 hours prior
4.6.7.2.3 48 hours prior
4.6.7.2.4 72 hours prior
4.6.8 Effect of rainfall, overall and by location
4.6.8.1 Rainfall (≥0.5 inch, present vs absent) for indicators
4.6.8.1.1 on day of sampling
4.6.8.1.2 24 hours prior
4.6.8.1.3 48 hours prior
4.6.8.1.4 72 hours prior
4.6.8.2 Rainfall (≥0.5 inch, present vs absent) for pathogens
4.6.8.2.1 on day of sampling
4.6.8.2.2 24 hours prior
4.6.8.2.3 48 hours prior
4.6.8.2.4 72 hours prior
13
4.6.8.3 CAWS locations only: effect of CSOs on day of sampling,
24 hours prior, 48 hours prior, 72 hours prior on:
4.6.8.3.1 Indicators
4.6.8.3.2 Pathogens
4.7 Effect of WRP
4.7.1 Concentrations above vs below WRP on same day: both plants,
North Side, Calumet:
4.7.1.1 Indicators:
E. coli,
enterococci, F+ coliphage, somatic
coliphage total and for each serotype; factors
4.7.1.2
Pathogens:
Giardia, Cryptosporidium
4.7.1.3 Water chemistry parameters: pH, temperature, DO,
conductivity, turbidity
4.8 Participants
4.8.1 Total participant recruitment, by date by location by group
4.8.2 Summary of recruitment by location by group by study year
4.8.3 Participants recruitment: without telephone follow-up, by reason
(no interview A, no interview B, disqualified by swimming)
4.8.4 Loss to telephone follow-up: overall, by group
4.8.5 Demographic, location, activity differences for those with vs. those
without telephone follow-up
4.8.6 Demographics of study participants: participants in phone follow-
up total, and by group: age (mean, median, SD; <5, 5-<10,10-<20;
20-<40; 40-<65;>=65), gender, race, ethnicity
4.8.7 Recreational activity, by group, by year
4.9 Non-water-related risk factors for illness (potential confounders)
4.9.1 Underlying GI illness: total and by group
4.9.2 Underlying respiratory illness: total and by group
4.9.3 Contact with someone who is sick
14
4.9.4 Contact with animals
4.9.5 Recent ingestion of raw fish, undercooked meat, hamburger, salad
4.10 Water exposure
4.10.1 Recreational activity by location, by group
4.10.2 Duration of recreational activity by activity by location
4.10.3 Recreational activity by age, gender, by group
4.10.4 Did you get wet at all? overall, by group
4.10.5 Did you get wet all? by activity by group
4.10.6 How wet did your feet/legs get: overall, by group
4.10.7 How wet did your feet/legs get by activity by group
4.10.8 How wet did your hands/arms get: overall, by group
4.10.9 How wet did your hands/arms get: by activity by group
4.10.10 How wet did your torso get: overall, by group
4.10.11 How wet did your torso get: by activity by group
4.10.12 How wet did your face/head get: overall, by group
4.10.13 How wet did your face/head get: by activity by group
4.10.14 How much water did you swallow: overall, by group
4.10.15 How much water did you swallow: by activity by group
4.10.16 Eating, drinking and hand washing during/after recreation
4.10.17 Last water contact prior to enrollment
4.10.18 Water contact since enrollment
4.10.19 Boaters:
4.10.19.1
Did you capsize?
4.10.19.2
Get wet at launch?
4.10.19.3
Launch from pier/dock/shore?
4.10.20 Fishers:
4.10.20.1
Catch fish?
4.10.20.2
Bait use?
4.10.20.3
From shore or boat?
4.10.20.4
From shore: use of hip boots/waders?
15
4.11 Recreation, risk perception
4.11.1 Frequency of water recreation at CAWS, other sites
4.11.2 Distance traveled
4.11.3 Leichert scale risk perception
4.12 Health measures
4.12.1 Frequency of symptoms at baseline: GI, respiratory, skin, eye, and
AGI symptom complex definitions
4.12.2 Rates of onset of symptoms following recreation: Kaplan-Meier
curves and survival rates: overall and by group:
4.12.2.1
for each symptom (GI, respiratory, skin, eye)
4.12.2.2
for symptom complexes (specific definitions of
AGI)
4.13 Clinical Microbiology
4.13.1 Frequency of specimen collection vs. frequency of symptoms
4.13.2 Results of stool cultures
4.13.2.1
overall
4.13.2.2
by group
4.14 Predictors of health events (individual symptoms and symptom complexes)
4.14.1 Demographic variables
4.14.1.1
Age
4.14.1.2
Gender
4.14.1.3
Race/ethnicity
4.14.2 Temporal factors
4.14.2.1
Study year
4.14.2.2
Season
4.14.3 Non-water related risk factors
4.14.3.1
Dietary factors
4.14.3.2
Ill contacts
4.14.3.3
Animal contact
16
4.14.4 General water exposure factors
4.14.4.1
Study group
4.14.4.2
Recreational activity
4.14.4.3
Duration of recreational activity
4.14.4.4
Self-reported water exposure variables during
recreation
4.14.4.5
Water recreation prior to enrollment
4.14.4.6
Water recreation subsequent to enrollment
4.14.4.7
Eat/drink during recreation
4.14.5 Specific exposure factors (CAWS and GUW groups only)
4.14.5.1
Did you get wet at all?
4.14.5.2
How wet did your feet/legs get?
4.14.5.3
How wet did your hands/arms get?
4.14.5.4
How wet did your torso get?
4.14.5.5
How wet did your face/head get?
4.14.5.6
How much water did you swallow?
4.14.5.7
Boaters: Capsize? Wet at launch? Launch from
pier/dock/shore?
4.14.5.8
Fishers: Catch fish? Type of bait? Fish from shore or
boat? Use hip boot/waders?
4.14.6 Microbial measures of water quality
4.14.6.1
Indicator concentrations, principal component factors
at launch:
4.14.6.1.1 At time point closest to launch/start
4.14.6.1.2 At time point closest to return/end
4.14.6.1.3 Average concentration during period of water
recreation
4.14.6.2
Pathogen concentrations at launch:
4.14.6.2.1 At time point closest to launch/start
4.14.6.2.2 At time point closest to return/end
17
4.14.6.2.3 Average concentration during period of water
recreation
4.14.6.3 CAWS locations: Indicator concentrations, principal
component factors at sites above vs. below WRP
4.14.6.3.1 At time point closest to launch/start
4.14.6.3.2 At time point closest to return/end
4.14.6.3.3 Average concentration during period of water
recreation
4.14.6.4 CAWS locations: pathogen concentrations, principal
component factors at sites above vs.below WRP
4.14.6.4.1 At time point closest to launch/start
4.14.6.4.2 At time point closest to return/end
4.14.6.4.3 Average concentration during period of water
recreation
4.14.6.5 CAWS locations: indicator and pathogen
concentrations at non-WRP locations upstream of
recreation
4.14.7 Non-microbial measures of water quality
4.14.7.1
Water chemistry
4.14.7.2
Environmental observations
4.14.7.3
Rain and CSO
4.14.8 Exploration of propensity scores
4.14.9 Modeled integrated microbial exposure
4.14.10 Spatial-temporal model of integrated exposure
4.14.11 Final model selection using predictors identified in steps 4.14.1-
4.14.10.
18
References
Fleiss J. 1981. Statistical Methods for Rates and Proportions. 2 ed. NY: John Wiley &
Sons. Chapter 3.
Tosteson TD, Buzas JS, Demidenko E, Karagas M. 2003. Power and sample size
calculations for generalized regression models with covariate measurement error. Stat
Med 22(7): 1069-1082.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati
on&list_uids=12652554
Wade TJ, Pai N, Eisenberg JN, Colford JM, Jr. 2003. Do U.S. Environmental Protection
Agency water quality guidelines for recreational waters prevent gastrointestinal illness?
A systematic review and meta-analysis. Environ Health Perspect 111(8): 1102-
1109.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=
Citation&list_uids=12826481
.
El-Shaarawi A. 1989. Inferences about the mean from censored water data. Water
Resources Res 24(4): 685-690
El-Shaarawi A, Viveros R. 1997. Inferences about the mean in log regression with
environmental applications. Envirometrics 8: 569-582
Noble RT, Weisberg SB, Leecaster MK, McGee CD, Ritter K, Walker KO, et al. 2003.
Comparison of beach bacterial water quality indicator measurement methods. Environ
Monit Assess 81(1-3): 301-312.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati
on&list_uids=12620023