| | - BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
- (Service List-Via First Class Mail)
- R07-8
- (Rulemaking-Land)
- NOTICE
- DATE: February 14,2007
- THIS FILING IS SUBMITTED ON RECYCLED PAPER
- BEFORE THE ILLINOIS POLLUTlON CONTROL BOARD
- R07-8
- (Rulemaking-Land)
- MOTlON FOR ACCEPTANCE
- Christian J. Liebman for the above-captioned matter.
- Respect fully submitted,
- Assistant Counsel Division of Legal Counsel
- THIS FILING IS SUBMITTED ON RECYCLED PAPER
- BEFORE THE ILLINOIS POLLUTlON CONTROL BOARD
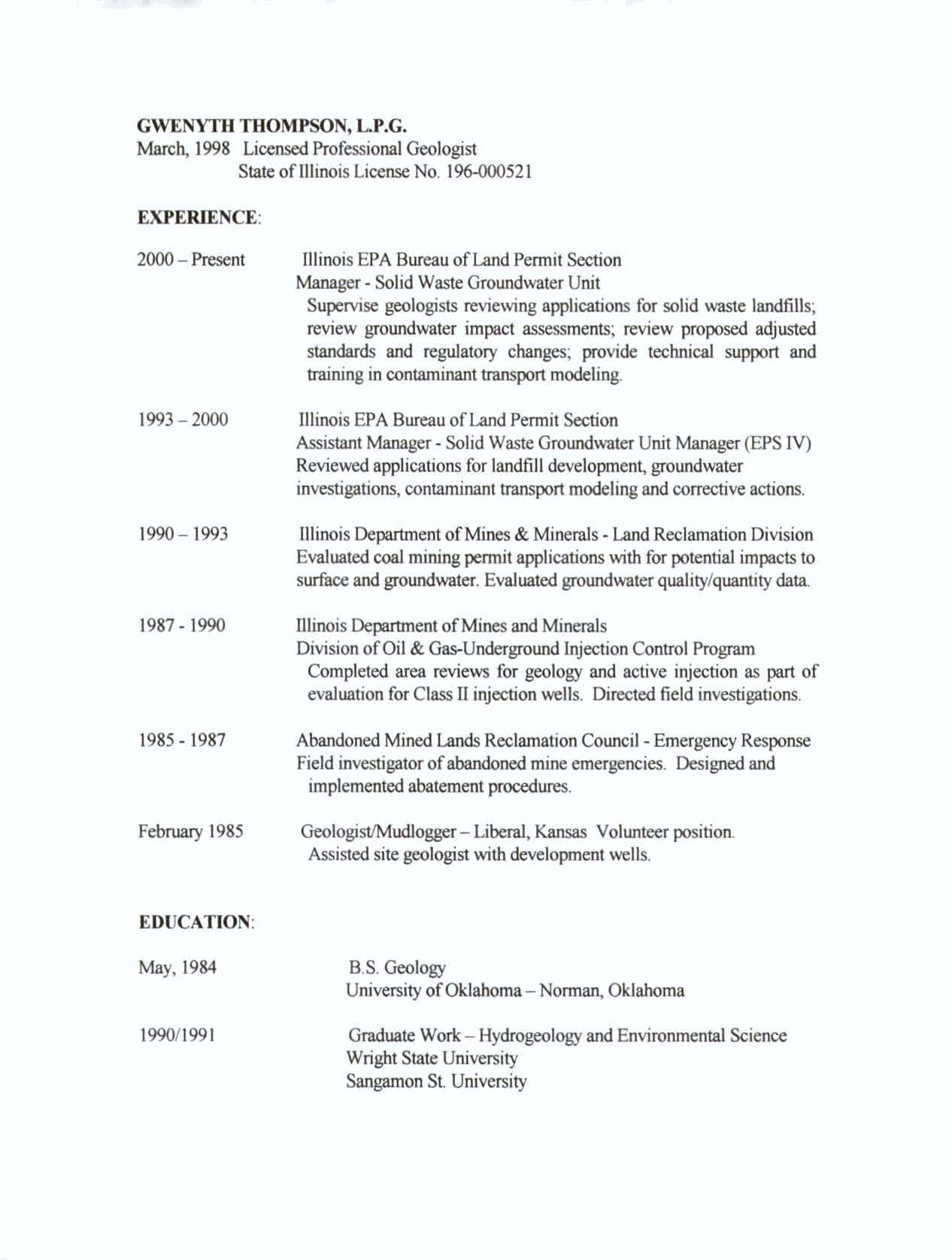
- TESTIMONY OF GWENYTH THOMPSON
- Environmental Protection Agency ("Agency").
- for attending these hearings and for their interest, questions, and input.
- hearing.
- applicability of 302 to groundwaterer
- 302.
- The standards come from the AGQS.
- parmeters must meet is background, as discussed below.
- Maximum Allowable Predicted Concentrations MAPCs").
- Agency took action.
- This concludes my testimony.
- THIS FILING IS SUBMITTED ON RECYCLED PAPER
- EXPERIENCE:
- EDUCATION:
- May, 1984
- Sincerely,
- REC" JED
- 2171524-3300
- Existing Program:
- Page 2
- Supporting Information and Discussion:
- Bervllium (total)
- Cop~er (total)
- Nickel (total)
- Siher (total)
- Ammonia (dissolved)
- Arsenic (dissolved)
- Cadmium (dissolved)
- Chloride (dissoIved)
- Chromium (dissolved)
- Cyanide (total)
- Mercury (dissolved)
- Nitrate (dissolved)
- Page 8
- Zinc (dissolved)
- Sincerely,
- References
- ATTACHMENT I
- Parameter Storet
- Acetone AcroIein Acrylanitri le Alachlor
- AIdicarb Aldrin
- Antimony
- Arsenic
- BOD (ma) Boron
- Bromoform vribromomethane) Bromomethane (Methyl Bromide) n-Butylbenzene
- sec-Butylbemne tert-Butyl benzene Cadmium
- p-C hlorotoluene Chromium
- Parameter Sioret
- Cobalt Copper p-CresoI Cyanide (mg/L) Palapon
- Dichlororaethane (Methylene Chloride) Dieldrin
- Endrin
- Isophomne Isopropyl benzene p-Isopropyltoluene Lead
- Lindane Magnesium (mgfL) Manganese
- Mercury
- Methoxyclor Naphthalene Nickel
- Nitrate-Nimgen (m@) Oil(Hexane-Soluble or Equivalent) (m&)
- Parameter Storet
- Parathion Pentachlam phenol pH
- Silver
- Tetrachloroethylene m@)
- Vanadium
- Vinyl Chloride Vinyl Acetate Xylenes
- Parameter
- cis-1 $2-Dichloroethylene trans- 1 ,ZDicMoroethylene 1,2-Dichloroehe
- ATTACHMENT 11
- Parameter
- Parameter
- Methyl ethyl ketone Methyl iodide; Iodamethane 4-Methyl-2-pentanone Naphthalene
- Phenols
- n-Prop y benzene Styrene
- 1,1,1,2-Tetrachloroethane 1,1,2,2-Tetrachloloethanel Tetrachloroethylene
- BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
- PROPOSED AMENDMENTS TO SOLD WASTE DISPOSAL LANDFILL RULES
- TESTIMONY OF CHRISTIAN J, LIEBMAN
- the first hearing on this rulemaking regarding unpermitted, on-site landfills.
- variability. These changes were initially suggested by the Agency.
- according to the literature, be found in leachate.
- codify tbe approach we have been using.
- Appendix C [Refmd to as Prsposed Amendments 4,5,6 and 9 in the filing].
- Ja. SPATIAL VARtABILITY
- may not be detected at all due to dilution by leachate from other areas.
- fewer leachate monitoring points are needed due to site specific circumstances.
- IV, FREQUENCY OF LEACHATE SAMPLING
- points would be sampled.
- amendments, two such landfills would do the same amount of leachate sampling.
- [Referred to as Proposed Amendments 4 and 8 in the filing].
- V. WERMITTED, ONSITE LANDFILLS
- each year.
- VI. CONCLUSION
- consideration of these changes,
- THIS FILING IS SUBMITTED ON RECYCLED PAPER
- CHRISTIAN J. LIEBMAN
- Springfield, Illinois 62974-9276
- EDUCATION
- WORK EXPERIENCE
- PROFESSIONAL LICENSES
- ATTACHMENT 1: LIST OF UNPERMITTED, ON-SITE LANDFILLS REGULATED UNDER PART 815
- BOL Site No. Contact Name Facility Name
- Renissance Restoration
- Gerry Allen Gerry Allen Solid Waste Site
- Star Industries Inc. Dixon Marquette Cement Co.
- 1 0825 Lake of Egypt Rd. Marion 4416 Prairie Hill Rd. South Beloit
- 500 S. Park Ave. South Beloit
- STATE OF ILLINOIS
- COUNTY OF SANGAMON
- PROOF OF SERVICE
- Dorothy Gunn, Clerk
- 2007, with sufficient postage affixed as indicated above.
- Notary hblic
- THIS FILING SUBMITTED ON RECYCLED PAPER
|

BEFORE
THE
ILLINOIS POLLUTION CONTROL BOARD
IN
THE
MATTER
OF:
PROPOSED
AMENDMENTS
TO
SOLID WASTE LANDFILL RULES
(35
Ill.
Ah.
Code 810
and
81
1)
Dorothy
Gum, Clerk
Illinois Pollution Control Board
James
R.
Thompson
Center
100
W.
Randolph, Suite 1 1-500
Chicago,
Illinois 60601
(VLA
COOL)
Matt
Dunn
Environmental Bureau Chief
Office of the
Attorney
General
James R. Thompson Center
100
W.
Randolph, 1
2"
Floor
Chicago, Illinois
60601
(Via First Class Mail)
(Service
List-Via First
Class
Mail)
R07-8
(Rulemaking-Land)
NOTICE
Bill
Richardson, General Counsel
Illinois Dept. of Natural Resources
One Natural
Resources
Way
Springfield, Illinois
62702-2
271
(Via First Class Mail)
Timothy
J.
Fox
Ill. Pollution Control Board
James
R.
Thompson Center
100 W.
Randolph,
Suite 1 1-500
Chicago, Iflinois
60601
(Via First Class Mail)
PLEASE TAKE
NOTICE
that
I
have
today
filed
with
the Office of the Clerk
of
the
Illinois Pollution Control
Board
the Illinois Environmental Protection Agency's Pre- filed
Testimony of Gwenyth Thompson and Christian J. Liebman,
a
copy of each of which is
herewith served upon
you.
ILLINOIS ENVIRONMENTAL
PROTECTION
AGENCY
Assistant
Chunse.1
v
Division of Legal Counsel
DATE: February
14,2007
Electronic Filing, Received, Clerk's Office, February 14, 2007
102 1
North Grand Avenue kt
.
P.O. Box
19276
Springfield, Illinois
62794-9276
(2
17)782-5544
THIS FILING IS SUBMITTED
ON
RECYCLED PAPER
Electronic Filing, Received, Clerk's Office, February 14, 2007
BEFORE
THE ILLINOIS POLLUTlON CONTROL BOARD
IN
THE MATTER
OF:
PROPOSED AMENDMENTS
TO
SOLID WASTE
LANDFILL
RULES,
(35
111.
Adm. Code 810
and
811)
R07-8
(Rulemaking-Land)
MOTlON FOR ACCEPTANCE
NOW COMES the Illinois Environmental Protection
~~enc~
("filids
EPA") and, purmant
to
35
IH.
Adm. Code 101
.Subpart
C
and
35
Ill.
Adm. Code 102.424,
moves
the
Lllinois PolIution
Control Board rBoard')
to
accept the attached written testimony of Gwenyth Thompson and
Christian J. Liebman for the above-captioned matter.
Respect fully submitted,
ILLINOIS ENVIRONMENTAL
PROTECTION AGENCY
A
'
~imberl
f A.
Geving
V
Assistant
Counsel
Division of Legal
Counsel
DATE:
~ebruary
14,2007
102 1
North Grand
Ave.
East
P.O.
Box
19276
Springkld, IlIinois 62794-9276
(2
17)782-5544
THIS FILING IS SUBMITTED
ON RECYCLED
PAPER
Electronic Filing, Received, Clerk's Office, February 14, 2007
BEFORE
THE
ILLINOIS POLLUTlON CONTROL BOARD
IN
THE MATTER
OF:
PROPOSED AMENDMENTS
TO
SOLD WASTE
LANDFILL
RULES,
(35
XII.
Adm. Code 8 10
and 8 1
1)
TESTIMONY OF GWENYTH THOMPSON
My name
is
Gwenyth Thompson.
I
am
currently
the manager of the Groundwater
Assistance Unit to Solid Waste in the Permit Section of the Bureau
of
Land at the Illinois
Environmental Protection
Agency ("Agency").
I would like to
thank
everyone involved in this rulemaking effort. I would particularly
like to thank the National Solid
Wastes
Management Association
representatives
for their
cooperation with the
Agency
in
addressing our concerns regarding changes
to
the
current
regulations, including incorporation of Agency input into the proposed changes.
In
addition,
I
would like
to
thank
the individual citizens, representing themselves and special interest gmups
for attending these hearings and for their interest, questions, and input.
The
purpose of
my
tdmony is
to
address issues
raised at the
first hearing in
this
matter.
My
testimony is structured
to
track
the
amendments
as
addressed
in
the
transcript from the first
hearing.
I. Amendment
10,
p. 63, line
9,
I would first like to point out that
this
regulation,
35
111.
Adm. Code
81 1.315(e)(l)(GNi), incorporates the
35
Ill.
Ah.
Code 620
("620"')
list of
Electronic Filing, Received, Clerk's Office, February 14, 2007
parameters, not the
standards
associated with those parameters.
Let
me stress that
we
do not
use
the standards (values)
from
the
620
regulations,
j
usl
a
we
did not
use
b
&mda&
hm
3
5
111.
Adm.
Code
302 ("3Q2"') in
the pa,
nor
wiU
we use
them
in
the
future.
hdfilb subject
to
35
Ill.
Adm. Code
8
1 1
have their
own
standards, which will be discussed below in detail
(see
discussion of Applicable
Groundwater
Quality
Standards
("AGQS')). The
620
reference in these
proposed amendments is solely intended to use the 620 list of parameters. It should also be
noted that
the same
rulemaking that promulgated the
620
standards,
R1989-0
14,
struck the
applicability
of
302
to
groundwaterer
To
address
Ms.
Andria's question directly,
I
compared
the
constituents
hm
Part
302,
public
water
supply
standatds to those
of
the
Part
620,
Class I potable
groundwater standards.
By my
cow here
are
11
more
inorganic parmeters and standards
in
620
than
there
are
in
302.
In addition, there are
40
more organic parameters apd standards in
620
than
there
are in
302.
Themfore,
by
virtue
of having more parameters,
620
is
more comprehensive for these rulw
than
302.
At ME;.
Andria's
quest,
I compared the standards for
the 22
parameters that inhabit both
the
302
and
620
lists. For
1
3 of
the
parameters,
the
620 standards were the
same
or had lower
values (meaning that
620
values were
as
conservative or more protective). The exceptions were
Barium, Chromium,
Iron,
SeIenium, Sulfate, Total Dissolved Solids
(TDS),
Heptachlor,
Heptachlor Epoxide, and
Sylvex,
which had slightly higher standards. For those parameters, I
reiterate
my
previous testimony that,
to
the
best
of my
knowledge, the standards promulgated for
35
Ill.
Adm. Code 620,
Class
I,
have been developed specifically
to
protect human hedth and the
environment in potable water supplies. However,
to
reiterate,
these values are moot for
this
Electronic Filing, Received, Clerk's Office, February 14, 2007
regulation because
we
don't
use
the
standards
in 620;
rather,
we
use
620 far its list of parameters.
The
standards
come from the
AGQS.
There
are
only
5
constituents listed in the 302
standards
that are not found in
620:
Aldrin,
Dieldrin, DDT, Oil, and Paratlion.
Of
these,
Oil
exists
on
the
proposed detection monitoring
list, The
others
are pesticides,
and
pesticides are
a11
included in the
assessment
monitoring list.
The
standard
that these
parmeters must meet is background,
as
discussed below.
2.
Amendment
10,
p.
65,
lines
7
through
23.
In
refdg
to
35
Ill.
Adm.
Code 620
standards,
Mr. R210
asked which groundwater quality
standards
apply
to
landfills. For
35
IIL
Adm.
Code
8 1 1
landfills,
the
groundwater standard is the
AGQS,
which is
defmed as
ambient
background
as
cbnnined
by
statistical analysis of existing
groundwater
quality. Tbis is the
groundwater standard for
8
1 1
landfills
at
the
edge
of the zone of attenuation or compliance
boundary
(100
feet
hm
the
edge
of the
waste
or the property
bundary,
if closer).
In
other
P.
words, if natmd background contains lower concentrations
than
allowed
by
any standard,
then
the facility
must meet the lower concentrations; the regulations do
not
allow
exceedences
up
to a
given standard However, within the zone of attenuation
(the
area between
the waste
and
the
1
00-foot
compliance
boundary),
Class
IV
groundwater
applies,
as
stated
in
35
11.
Adm.
Code
620.240(a),
which acknowledges
35
Ill.
Adm. Code
8
1 1
.
The Class
IV
designation allows for
Maximum Allowable Predicted Concentrations MAPCs").
As
found in
3
5
ILI.
Ah.
Code
8
1 1.3 1 7
and
8
1 1
-3
1
SIC),
a
landfill operator
is
required
to
develop MAPCs using
a
contaminant transport model
as
an
early warning mechanism, MAPCs
apply
at wells
located midway between
the waste
boundary and compliance
boundary.
If
an
MAPC
is
exceeded
50
feet
fbm
the
waste
bunday, then the AGQS may potentially
be
Electronic Filing, Received, Clerk's Office, February 14, 2007
exceeded at
the cmpliance boundary. Therefore,
an
exc&ce
of an MAPC would initiate
assessment
in
order
to
prevent imptic@
at the
compliance
boundary. Outside the landfill
zone
of
attenuation, the applicable groundwater standard is
the
standard
as defined
by
3
5
Ill. Adm.
Code
3. Amendment
t
9,
pp.
80-83.
The
amendment deletes total: metal monitoring from the
detection monitoring program, though retains
them
in assessment monitoring.
A
number of
the
total metals
are
quired
for
d~tection
monitoring
by
40
CFR
258, Appendix
I
(the federal
quiremmb
for mupicipal =lid waste landfills),
However, the
federal
rules
allow
the
State
to
alter the required list upon making
a
damstration
to
USEPA,
in writing, and received their
coocwrence
that
tbe
proposed alternab
list was
an
adequate substitution. Copies of
the
comqmndence
accompany
this
testimony.
4.
Amendment
1
9,
p.
84.
Ms.
Andria offers the concern
that
tbe
mas
m
tested
". .
.after
the groundwater
co~ltamhalicln
h
wcurred." Qur pmaw
at
new
landfills
quires
background development for an extensive list of parameters, which
can
be found
itl
Attachment
I
at the end of the document called LPC-PA19. It can be found
at
the Agency's website:
http:/lwww
.epa.state.
i1,usllmd~re~uIatary-v~mwd~ermi
t
s-andtmann~ernent/foms/pl
9-
instructions.df.
The
parameter list contains most parameters required during assessment.
Functionally, background has
already
been developed for most parameters
on
the assessment
monitoring list, wbich are available for comparison, should assessment monitoring be required in
the
futuEe.
In
addition, regulation
35 111.
Adm.
Code
8
1 1.3
19@)(5)(C)
requires
that background
be
developed for
any
parameter that is detected in
groundwater
during assessment monitoring.
In
the
circumstance
where background has not been developed, this regulation
requires
that it be
Electronic Filing, Received, Clerk's Office, February 14, 2007
developed. Background must be established
at
locations unaffected
by
the landfill.
5.
Amendment
26,
p.
125.
The
Agency
committed
to
provide
the exact
link
on
the
Agency website
where
all active
(under
review) and inactive,
(acted
upon)
applications are
described. That web address
is: http:/lefladataepas~te.il.usI1mdlso~idwt.
This
link allows
-
I
rfi
a
user
to
search for
both
active and inactive applications
by
several variables
(e.g.,
facility name,
site number, city, county).
The information
includes the following: the
date
the application is
received, the
date
that the Agency is required
to
take final action,
a
brief summary of
the
application's
purpose, names
of
Agency reviewers, and, for inactive applications,
dates
that the
Agency
took action.
6,
Amendment
45,
p.
1
64.
I would like
to
clarify
my
previous
mponse
to Ms.
Liu.
Aitchison's
adjustment,
as
we11 as
Cohen's,
are
general
1
y
used
when non-detects are between
15%
and
so%;
the
data sets
must be normally distributed
.
This concludes
my
testimony.
THIS FILING IS SUBMITTED
ON
RECYCLED PAPER
Electronic Filing, Received, Clerk's Office, February 14, 2007
GWEm TBOMPSON, W.G.
Mmh,
1998
Licmed
hf~onal
Geologrst
Stak
of
lllinois
License
No.
1%-00052 1
EXPERIENCE:
February
1985
EDUCATION:
May,
1984
Illinois
EPA
Bureau of
Land
Permit Won
Maqpx
-
Solid Waste
Grouadwater
Unit
Supervise
geologists
miming
applW01ls
for solid
waste
lmd6Uq
review groundwater impact
wessmmb;
review
propid
adjusted
stadds
and
regulatmy chmp; provide
technical
support
and
tminingin~~~~
Illinois
EPA
Bureau of Land Permit
Section
Assistarrt
Manager
-
Solid Waste
Gmdwta
Unit
Manager (EPS
IV)
Reviewed
applications for
hdfi1I
development,
groundwas
investi~ons,
contaminant&msprtm&ling
andco&actioasoas
Illhis Department
of
Minw
&
Minerals
-
Land Reclamalion
Division
Eduated
Goal
mill&
pmit
appli~otls
with
fa
potdid
impacts
to
surface
and
gmudwhx.
Evaluated
gmwhter
qualityIqwhty
data.
kis
mmt
of
Mines
and
Minerrrls
Division
of
Oil &
~UnchgroImd lnjectian Control
Prograra
Completed area
reviews
for geology
and
active
injection
as part
of
evaluation for Class
II
injection wells.
Dkbd
field investi@ons.
Abandoned
Mined
Lands
Rechation
Council
-
Emergency Resp~lse
Field
bvd@or
of
hwhed
mine mqemies. Designed
atad
impl-w-.
Geo1~udl~-~~
Kansas
Vol~~tioa
Assid
site
geologist
with
development
wHs.
B.S.
Geology
University of OkIahoma
-
Norman, Oklahoma
&&&
Work-H~log~aadE~~dd
Scim
wigfit
state
UMty
s8ngUnan st
University
Electronic Filing, Received, Clerk's Office, February 14, 2007
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY
REGIONS
5
77
WEST JACKSON BOULEVARD
CHICAGO, IL 60604.3590
REPLY
TO THE ATTENTION
OF
DW-81
October
1 1,2006
Mr. Stephen
Nightingale,
PE
Permit
Section
Manager
Illinois Environmental Protection Agency
1021
N.
Grand
Avenue
East
P.O. Box
19276
Springfield, Illinois
62794-9276
Mr,
Nightingale:
Your
letter, dated September 18,2006, requesting 40
CFR
Part
258
Appendix
I
Total
Metals Proposed DeletionReplacement, has been reviewed.
Your
letter provides detailed
informa?mn
on
how
he
proposed changes
to the
Illinois Environmental
Protection
Agency
detection monitoring program will provide
a
reliable indication
of
inorganic
releases
born
MSWLF units to groundwater, taking into account the factors outlined
40
CFR
25 8+54(a)(Z)(i-iv). The information you submi
ttd is considered sufficient
justification
for
implementing the proposed detection
monitoring program.
As always
the
XEPA approach
of communicating
with
Region
5
staff
on
proposed
program
changes
in
advance
of the formal submittal is greatly appreciated.
Sincerely,
Donna
Twickler
Environment
a1
Engineer
REC" JED
~
O
C
.J 2006
~
Rocyehdhcyatmbh
Wed
mth VapatrbM Od Based Inks on 1OOx R-
Paw
(50% Pos!eanwmar)
Electronic Filing, Received, Clerk's Office, February 14, 2007
1
1 021 NORTH
GRAND
AVENUE
EAST, P.O. BOX
1
9276, SPRINGFIELO, ILLINOIS
62794-9276
- !
2
1 7)
782-3397
2171524-3300
September
18,
2006
CerWed
Mail
7002 2030 0001 1879
4442
Ms.
Dom
Twickler
Environmental Engineer
USEFA
Region
5,
D
W-U
77
West Jackson Blvd.
Chicago, Illinois 60604
Re:
40
CFR
Part
258 Appendix 1 Total Metals Proposed DeletiodReplactment
The
Illinois Environmental Protection
Agency @PA)
requests a determination from the US EPA
whether proposed detection monitoring program changes are considered consistent with 40
CFR
Subtitle
D. The
proposal eliminates
certain
unfiltered
metal
parameters from the Illinois
annual
detection monitoring constituent list. These metals are contained on'the
40
CFR Part
258
Appendix I list. The specific proposal and associated rationale is detailed below.
Existing Program:
The existing detection-monitoring program in
Hlinois
includes quarterly monitoring of the
G1
list
of
parametm and annual monitoring of the G2 list of parameters (see Attachment
I).
The
G
1
list
is monitored quarterly for filtered metals including arsenic,
cadmium,
iron,
lead,
manganese, and
mercury.
The
G2
list is a comprehensive list of inorganic and organic parameters: it includes
organics
from
40
CFR
258
Appendix
1,40
CFR
141.40, Illinois Administrative Code 620,
organics found
at
solid
waste
facilities
from publications, and unfiltered
inorganics
antimony,
arsenic, barium, beryllium, cadmium, chromium,
cobalt,
copper, iron, lead, manganese,
mercury,
nickel, selenium, silver, thallium, vanadium, and
zinc. After the collection of a minimum
of
5
years of
data a
facility
may request a reduction fiom quarterly to semi-annual sampling in
detection monitoring wells.
Proposed New Program:
It is proposed that
an
alternative
list
of inorganics be
substituted for the
40
CFR
Part 2
5
8
Appendix I total
metals
based on criteria stipulated
in
40
CFR Part
258.54(a)(2),
The proposed
list
of
indicator constituents (new
G
1
list)
to
be
analyzed
quarterly includes
total
Cyanide and the
following
filtered parameters: Ammonia-Nitrogen,
Arsenic,
Boron, Cadmium,
Chloride,
Chromium, Lead,
Magnesium,
Mercury, Nitrate, Sulfate, Total Dissolved Solids and Zinc. In
addition,
any
facility accepting
more
than
50% by
volume
non-municipal
waste
must
also
monitor for additional parameters based on leachate and
waste
content.
As with the existing
program, under the
new
program, a facility
may
petition for a reduction from quarterly to
semi-
annual
monitoring after
5
years of data have been collected.
Roc--4302
NorthhinSbC#.R~,Il61103-(815)987-7760
DE~PL~INES-~S~I
W.HarrimSt, kPlaim,lL60016-18.17)29/-4000
ELGIN
-
595
South
Sbk, Elg~n, 11 601
23
-
(847)
608-31
31
Pfonl~
-
5415 N Univenlty
St,
Peorra, 11 61614
-
(309) 693.963
BWU
w
LMlD
-
R~A-
7620
N. Unimriw St., Pm~a,
It
61 61 4
-
(303)
693-5462
*
CMWAICN
-
21
25
%uth
F~rst Sw Clumpa~gn. IL 61820
-
01
f)
2785800
SPIIHGFIUO
-
4500
S.
Sixth
Street
Rd., Springfield,
1i
62706
-
(21 7)
786-6692
4 COLLIWIUE
-
2009 Ma11 Ulmt, Coll~nsv~lle,
I1
61234
-
(6181 346-5120
M~mm-2309 W.
Main
St, Suite 116. Marron,
It
62959
-
(61 8)
993-71W
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page 2
In addition, revisions are proposed for
the
G2 list.
The
new list would include
an
expanded
list
of 40 CFR Part
258
Appendix
I
volatile organics and
be
sampled more frequently. All detection
mooitoring
wells
will
be sampled semi-annually for all organic parameters
fiom
40 CFR Part
258
Appendix I,
as
well
as A0
CFR Part
14 1.40
organics
(see
Attachment
II).
Leachate continues to
be
monitored far the parameters in Attachment 1. Proposed
revis
jam
,
.
include sampling monitoring points
on
rn
al@rnatieg
schedule.
The
facility
would
monitor
the
Attacbent
1
list
on
a
semi-annual
basis
with
each sampling point monitored at least biennially
Supporting Information and Discussion:
This
section presents
an ovemiew
af
the total
heavy meld constituents
as
a
compound elass
in
terms of their utility as detection monitoring parameters
aad
presents
an
alkrnative list.
The
alternative
list is proposed in
accordance
with 40
CFR Part
25
8.54(4)(2),
the Director of
an
approved
State
may establish
an
alternative list of inorganic indicator parameters for a MSWLF
unit,
in
lieu
of
some
or
all of
the
heavy metals listed under Appendix I, if
tbe
alternative
pameters provide a reliable indication of inorganic
releases
horn
the MSWLF unit
to the
ground
water.
In
determining alternative parameters, the Dircctor shall consider the following
factors:
(i)
Tfie
types, quantities,
and
concentrations of constituents in wastes managed
at the
MSWLF unit;
(ii)
The mobility, stability, and persistence of
waste
constituents
or their reaction products in
the
zmsaturahd zone beneath
he
MsWLF
unit;
[iii)
The
detectability of
indicator
pmeters,
waste
constituents,
and reaction products
in
the
ground
water;
and
(iv)
The concentration
or
values and coefficients of variation of monitoring parameters or
constituents
in
the ground water bapkgaund.
The
proposed changes
to the
detection monitoring program are
baxd
upon examinatiop of
literature pertaining to the frequency
of
detected
compounds
in
leachate,
studies and information
pertaining to mobility, stability and persistence, and
the
contrast of concentrations
hetween
leachate and ambient groundwater. The program does not propose to delete all
40
CFR
Part
25
8
Appendix I insrgmics,
as five
(arsenic,
cadmitun,
chromium. lead
and
zinc)
will
be
rchd
9s
quarterly indicators.
Potential indicator constituents for landfills have
been
studied
as to
their relative mobility,
stability
and
persistence in
the
subsurface ~nvironment According
ta
published research
the
heavy melds
as
a
compound
class
are among
the
least mobile of detection monitoring parameters
when compared
to
othet inorganic parameters
and
VOCs (Christensen,
et
al.,
1
994).
In
leachate
characterization
studies
conducted, it has been shown
that
the
detection frequency of the trace
metals monitored range from
45%
(RUST E&I,
1995) to 67%
(US
EPA,
1998).
The
WMI
study
performed
by
RUST E&I was based
on
10
landfills located in Illinois, Louisiana, New Yark
and
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page 3
Pennsylvania.
MSW
leachate data
was
studied for the purpose of identifying which constituents
ate
prevalent
in
leachate
and
for determining which would provide an indication of a relax.
The study included the trace metals summarized below,
as
well
as
inorganic
constituents and
organic
compounds
(not
shown).
Concentration ranges and detection frequencies
of
the trace metals in MSW leachate.
WMI
Study
(1
989-
i
992)
,
Literatwe Data
(2)
Trace
Metal
Minimum
.
Maximum
Detection
Minimum
Maximum
Dttection
(u&)
(u&l
Frequency
I%)
(u@)
Frequency
(%I
Antimony
<I
9.3
-
8
1,5
47,OQO
,A --,- -
30
{I)
-
RUSTEM,
1995.
LcachatcChmckrizalion
Study,
(2)
"Draft
B-
Dccument.
Summy
of Dafd
on
Murkipd
Solid W-
hdfi
Chwkhik",
USEPA July
1988). Summay of83
Smirsry
Sitcs. Inclwh
data
fmn
Wicomin S~dy
(19845,NUS
Sbdy
(L9%7J,
Sobalta
Study (19861,
'Rdc
Auoc.
Studks (1985),
Tetar
AM
SiU* (1986). IDd Wmt~
M-tnt
Sbdy
(1987).
Christensen,
et
al.
(1994) state
that
"heavy metals do
not
constitute
a
groundwater poilution
problem
at
landfills because landfi I1 leachates
usually
contain
only modest heavy-metal
concentnitions
.
.
.and..
.
are
subject
to
strong attenuation by sorption
and
precipitation." Though
the
same
work cautions against the possible effect
of
long-term leachate changes toward
increased
mobility of heavy metals,
a
recent paper
by Kjeldsen,
et
al., (2002) concludes that
the
"postulated
enhanced
release of
accumdated heavy
metals" would not
take
place in
the
long
tan
according
to
model analyses.
The mobility of metals is controlled by physical
factors
reiated
to the
geologic matrix
(e.g.,
rock
type, mineralogy, grain size) and hydrochemical factors
related
to
the subsurface environment
(e .g.,
pH, Eh, complexing ligands, competing ions). The physiochemical properties of the metals
and
the
subsurface conditions
govern
the partitioning, transport, and fate
of
each of
the
mehis.
Metals
in
the god
may exist
as:
free ions, insoluble precipitates, metal ligand complexts,
adsorbed species, species held
on
to
by
ion exchange, and species that differ in oxidation
states.
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page
4
Due ta
the
positive charge of metal ion species> adsorption of metals onto negatively charged
clay minerals or organic matrer
is
an
important limiting process
with
respect
to
metals
mobility.
Ilissolved parameters
exist
truly in
solution
(such
as
groundwater).
Dissolved parameters cannot
be removed fiam
a
liquid
without
a
phase
change
(such as distillation, precipitation, adsorption,
or extraction) (MacKenzie et
al,
1
998).
Suspended (or total) solids are large enough
to
either
settle out
of solution or
be
removed by filtration (MacKenzie
at
al,
1998).
The mobility of
inorganic parameters
is
therefore betrer reflected by monitoring
dissolved
parmeters
as
compared
to
total
metals, wbh
have
been rendered immobile due to physw
or
chemical
processes. This
is
especially
tnre in
fine-grained
environments.
Many detection-monitoring programs
at facilities
in
Illinois
are
developed in siltyl~layey
subsurface materials, which often yield twbid samples. Alteration
of
sampling
pmcedures (we
of low
flow
sampling)
to
decrease turbidity
b
yielded varied results
of
success.
Turbidity
creates a large bias in
the
resultant
data,
which results
in
wholly unreliable
data
for
detection
monitoring purposes (Gibbons, R.
D.
and Sara, M.,
1
995).
Our experience
in
administering
the
current regulatory program in Illinois underscores this unreliabiiity. Turbidity issues contribute
to
an
unacceptable false positive rate
that
necessitates complex
md
continual
statistical treatment
of
the
data
to
maintain acceptable statistical power.
Specifically,
the
following
parameters proposal
for
removal
from
the Illinois detectian-
monitoring
program. Justification is provided
as
required by
40
CFR Part
25
8(a)(2).
Antimosv
(total)
Detection
frequency
of antimony in MSW landfill Ieachate is less than
30%.
Additionally,
the
maximum
concentration cited
in
the
leachate study
was
detected
below the Iliinois Class
n
groundwater standard
(24
ug/l).
Barium
is
commonly
detected
in
groundwater fram
the
presence of
b~um
containing
mineral
deposits. Barium is detected in the majority of leachate samples collected but afkn not
at
concentrations
above
ambient groundwater concentrations.
The
major attenuation mechanisms
for
barium
are adsorption,
exchange, and
precipitation. Barium
also
can be released from natural
soils under reducing conditions or
in
presence of sulfate and
may
give false positive results.
Additionally, the
maximum
concentration provided in the ltachate
study
was
detected
below the
Wbis Clm
I groundwater
standard (2000
ugh).
Bervllium (total)
Beryllium
is
rarely
detected in the groundwater or
MS
W
leachates.
The
major attenuation
mechanisms are precipitation and exchange. Additionally,
both
maximum
concentrations
detected in the leachate and literature studies were below the
Illinois
Class TI groundwater
standard (500 ugll).
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page
5
Cobalt (totab
Detection frequency of cobalt leachate samples is less
than
42%.
It
is thought that cobalt
can
coprecipitate or be
absorbed
by
manganese
and iron oxides (Fetter, 1999). In addition,
both
maximum
concentrations
detected
in
the leachate and literature studies
were
below the Illinois
Class
I
groundwater standard
(1
000
ud),
Cop~er (total)
Sources
of
copper in the environment include
natural
deposits, industrial deposits, wood
preserving, and plumbing. Copper was detected in most
MSW
leachate samples monitored.
Copper is attenuated
in
the
soil through exchange
and
adsorption
mechanisms. Adsorption of
copper occurs at a greater
extent
than for most other metals. The major attenuation mechanisms
for copper are adsorption, exchange, and precipitation.
In
addition, bath
maximum
concentrations detected in the leachate and literature studies
were
below the Illinois
Class
I
groundwater standard (650
ufl).
Nickel (total)
Nickel is detected in the majority of MSW leachate samples, but is generally not found in
groundwater because it occurs mainly as insoluble hydroxides or sulfides. The
major attenuation
mechanisms
axe
adsorption
and
precipitation. Additionally, the
maximum
concentration
detected
in the leachate study
was
detected below the Illinois Class
II
groundwater standard (2000 ug/l).
Selenium
(total)
The detection frequency of selenium in
MSW
leachate
is
less
than
44%. It is naturally occurring
as mineral deposits
in
one of four oxidation
states. The
major attenuation mechanisms are
adsorption and exchange but
this
varies with
the
selenium species, controlled
by
pH, redox, and
soil composition. Additionally, the
maximum
concentration
detected in
the leachate study was
detected below the Illinois Class I groundwater
standard
(50 ug/l).
Siher (total)
The detection frequency of silver in MSW leachate is less than
47%.
Silver is generally not
found
in
groundwater
as
it
will
form highly insoluble precipitates and is strongly adsorbed
by
clay, making it relatively immobile in soil.
In
addition,
both
maximum concentrations
in
the
leachate and literature studies
were
detected
at
or below
the
Illinois Class
I
groundwater standard
(SO
udl).
Thallium (total)
The detection fkquency of thallium in MSW leachate is less than 41%. AdditionalIy, the
maximum
MIium
concentration detected in
the
leachate study was below the Illinois Class
U
groundwater standard
(20
ud).
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page
6
Vanadium (totan
It is believed that in aqueous solutions (such as groundwater), vanadium may form ten different
oxides and hydroxides
and
can react with dissolved iron
to
form insoluble precipitates (Fetter,
1
99). Vdium also does not
have
an Illinois Class
I
or Class
I1
groundwater standard.
The parmeters proposed to be monitored quarterly in lieu of the above total
metals
include
general
water
quality parameters as well as dissolved trace metals. Each of the proposed
parameters is listed below along with some
information
about each parameter. in general, these
inorganic parameters are less affected by
natural
pracesses
andlor exist
at
better
concentration
contrast between leachate
and
background groundwater, which
make
them more
effectivg
and
reliable detection monitoring parameters in comparison to total
metals.
Ammonia (dissolved)
Ammonia is a common
component
of anaerobic decomposition
common
to landfills. Due to the
anaerobic
conditions
in landfills (i.e., in the
absence
of
oxygen),
ammonia is present within
leachate at significant concentrations above typical
ambient
background. Exceptions to this
would include areas with
agricultural
sources
or
in
poorly drained areas and swamps.
Arsenic (dissolved)
Arsenic is a
common
trace metal that is typically detected in
both landfill
leachate
ad
natural
groundwater. The detection
fkequency
in landfill leachate for both of
the above
studies
was
92%.
Arsenic
was
reported in Illinois Community Water Supply Wells at an average concentration of
approximately 1 ugll (IEPA
2004),
which is lower than the concentration reported in leachate in
the above
studies.
Boron (dissolved)
Boron is common
in
landfill Ieachate especiaIly where ash
has
been accepted. Boron is also
common1
y
detected in natural groundwater and
was
detected in Illinois Cornmuni
ty
Water
Supply Wells at an
average
concentration of approximately 150 ug/l (IEPA 2004). Chemically
unbound
boron is readily soluble
in
water and behaves similarly to chloride. It is not ~eadily
retarded, adsorbed or chemically transformed in most environments.
Cadmium (dissolved)
Cadmium is considered
a
trace
component
of groundwater with
a
Class
I GQS of
5
ugll.
Although the leachate
study
detection frequency was low (only 8%), the detection frequency
in
the
literature
study
was 74% and the detected concentrations are likely above the Class
I
GQS.
Cadmium sulfate
(CdSOr)
has a
very
low soiubili
ty
product, but cadmium can
be
mobile in
certain conditions (Fetter, 1999).
Chloride (dissoIved)
ChIoride is generally regarded as one of
the
best indicator parameters.
As
stated in Fetter
(1
999),
"chloride
ions
arc
not
reactive.
They do not participate in redox reactions, are not sorbed onto
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page
7
mineral
or organic
surfaces, and do not form insoluble precipitates. Chloride is sometimes used
as
a
tracer
in
groundwater studies because it is conservative."
Chromium (dissolved)
Chromium
is considered a trace component of groundwater. Chromium is not typically detected
in Illinois groundwater
as
less
than
1
%
of samples
from
Illinois
Community
Water Supply Wells
reported chromium above the reporting limit of 5 ugA
@PA
2004).
The detection frequency in
leachate for both studies was moderate, thus indicating that
chromium
is a commonly detected
parameter in landfill leachate.
Cyanide (total)
Cyanide is
a
natural inorganic substarice. of health concern (MacKenzie
et
al,
1998).
Lead
(dissolved)
Lad
is
considered a trace
groundwater
constituent. bad is not typically detected in Illinois
groundwater
as
less than
10%
of samples from Illinois
Community
Water Supply Wells reported
lead above
tbe
reporting limit of
5
ug/l
(IEPA
2004).
Conversely, the detection
fkquency
of lead
in
the
leachate study and literature data
was
greater
than
65%.
Magnesium
(dissolved)
US
EPA funded a research project on the "Flood of
1993".
The study
was
carried out by the
Missouri Department of Natural Resources Solid Waste Management Program.
The
study
identified magnesium
as
one
of
the
best indicators of
ltachate
migration,
Mercury (dissolved)
Mercury is considered
a trace
groundwater constituent. Mercury is
not
typically detected in
Illinois
groundwater
as less than
1%
of samples
hrn
Illinois Community Water SuppIy Wells
reported mercury above the reporting limit of
0.1 ufl
(EPA
2004),
Mercury is
not
commodly
detected
in landfill leachate, however, mercury is proposed
to
be
retained
as
a detection
monitoring
parameter due to its potential effects
on
human health and the environment.
Nitrate (dissolved)
Nitrate is a naturally occurring
form
of nitrogen that
can
be derived from both natural and
manmade sources. The form that nitrogen takes in the environment is dependant
on
the
presence
or absence of oxygen. The nitrogen cycle dictates that nitrate is most prevalent in aerobic
environments, whereas ammonia is present in anaerobic environments. As stated in
Lu
et aJ
(1
9851,
"if the soil/leachate system is aerobic, nitrification (mineralization) of organic nitrogen
sources
occurs
readily, producing nitrate
as
an end product. Nitrate is
mobile,
moving readily
with
the soil solution into the lower vadose zone and ultimateIy
into
groundwater." Because
nitrate
is an anion and
is negatively charged,
it
is
not adsorbed
by clay minerals
or
does not
participate
in
ion
exchange
reactions. Thus, nitrate
can
be om
effective detection monitoring
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page 8
parameter in environments
where
other nitrogen
sources
are minimal and aerobic conditions
are
prevalent.
Sulfate (dhohed)
Sulfate is a naturally occurring form of sulfur, and, similar to the nitrogen discussion above, the
form that sulfur takes
in
the
environment is dependent
upon
the presence or absence of
oxygen
(excluding
elemental
sulfur). In
anaerobic envimnments such
as
during
the
decomposition
process in landfill envkoments, sulfate
is
not
prmat
as
it is converted
to
sulfide. Sulfate
can Be
a usell
parameter in that
in
ambient groundwater
it
will typically decrease if
the
groundwater
is
impacted
by
lachate.
Total Dissolved Solids
Total
dissolved solids
(TDS)
is
a generic
measurement of the
total
amount of minerals and
nutrients that are dissolved
(not
merely suspended)
in
water. Because suspended solids increase
with turbidity, total dissolved solids
(TDS)
is a better measure of the components of
a
solution
as
opposed
to
the suspended solids potentially disturbed during groundwater sampling. Leachates
typically have
a
much higher TDS
as
opposed
to
ambient groundwater providing
a good
contrast
far detection monitoring purposes. It is also useful for
checking
the accuracy of field specific
conductance readings.
Zinc (dissolved)
Zinc is considered
a
trace constituent in groundwater. Zinc is not
typically
detected in Illinois
groundwater
as less than
5%
of samples from Illinois Community Water Supply Wells reported
zinc
above
the
reporting limit of
1 00 ug/l
(IEPA
2004).
Conversely, the detection frequency of
zinc
in
the
leacbate study and literature data
was
greater
than
90%.
Additionally,
the
new proposed program includes expanded VOC monitoring. Volatile organic
compounds {VOCs) are highly detectable and generally non-naturdly
occurring
so as to be
superior detection
monitoring
parameters when cornpqd to the
total
metals.
Given
our
experience
in
administering
the
current groundwater
monitoring
program
in
Illinois,
elimination of
the
above listed
40
CFR Part
258,
Appendix
I,
unfiltered
heavy
metals will not
have
a
deleterious effect
on
groundwater monitoring programs
in
Illinois. Rather
focusing
in
on
certain inorganic parameters
and
VOCs shown
to
be reliable
indicators of
a
reiease wiH enhance
the detection
monitoring
programs by curtailing the false positive rate.
Electronic Filing, Received, Clerk's Office, February 14, 2007
Page
9
Thank
you
for
assistance
and consideration
of
this matter. If you
should
have
any
questions
please feel
free
to
contact
me.
Sincerely,
stepfen F. ~i~htinhle,
P.E.
Manager,
Fennit
Section
Bureau
of
Land
bcc:
DLC-Klm Geving
Steve Nightingale
Chris Liebmn
Gwenyth Thompson
Electronic Filing, Received, Clerk's Office, February 14, 2007
References
Christensen, T.H.,
Kjeldsen, P.,
Albrechtsen, H-J., Heron,
B.
Nielsen, P.H., Bjerg, P.L.,
Holm,
P.E.,
1 994,
Attenuation of
Landfill
Leachate Pollutants
in
Aquifers: Critical Reviews in
Environmental Science and Technology, 24(2):
1
19-202.
Davis, J.A.,
et
al.
1993.
Influence
of
Redox Environment
and
Aqueous Speciation
on
Metal
Transport in Groundwater: Preliminary Results of
Trace
Injection Studies, in:
Metals
in
Groundwater, Allen, H.E., et al. editors. Lewis ~ublishers.
Davis, MacKenzie
L.
and D.
A.
Cornwell,
1998.
Introduction
to
Environmental Engineering.
WCB McGraw-Hill, Boston, Massachusetts.
Dragun,
J.,
1
988.
The Soil Chemistry of Hazardous Materials. Hazardous materials Control
research Institute, Silver Springs, Maryland.
Fetter, C.W.,
1999.
Contaminant Hydrogeology. Prentice Hill, Upper Saddle Hill, New Jersey
Freeze,
R.
Allan
and
J.
Cherry,
1979.
Groundwater.
Prentice-Hall, Inc.,
Englewood Cliffs,
New
Jersey.
Gibbons,
R.D.,
and Sara
M.,
1995.
Statistical comparison of Metal Concentrations in Filtered
and
unfiltered Ground-water Samples,
Ln:
Ground Water Sampling-A Workshop Summary.
EPM6001R-94/205
Illhais Environmeatd Protection Agency, Bureau of Water. Illinois Water Quality Report,
2004.
May
2004
Kjeldsen,
P., Bar&
M.
A., Rooker, A.P., Baun,
A.,
Ledin,
A.,
Christensen,
T.H.,
2902,
"Present
and
Long-Term Composition of MSW
Landfill
Leachate: A Review,
Environmental
Science
and
Technology,
32(4),
297-33
6.
Ls James C.S,
B. Eichenbrger, and
R
Steams, 1985. Leachate From Municipal
Landfills
Production ad Management. Noyes Publications,
Pak
Ridge, New Jersey.
RUST
E&I,
1
995.
Leachate Characterization Study.
Lewis Publishers.
Electronic Filing, Received, Clerk's Office, February 14, 2007
ATTACHMENT I
Parameter
Storet
Acetone
AcroIein
Acrylanitri le
Alachlor
AIdicarb
Aldrin
Alumiaum
Ammonia
(as N) (m&)
Antimony
Arsenic
Atrazine
Barium
Benzene
Benzo(a)Pyrene
Beryllium
BOD
(ma)
Boron
B
romobenzene
Bromoc
hloromethane
(chlorobrornomethane)
Bromodichlorometham
Bromoform vribromomethane)
Bromomethane (Methyl Bromide)
n-Butylbenzene
sec-Butylbemne
tert-Butyl
benzene
Cadmium
Calcium (mgL)
Carbafuran
Carboa Disulfide
Chn Tetrachloride
Chemical Oxygen Demand (COD)
(mglt)
Chlordane
Chloride
(m@)
Chlorobenzene
Chloroethane (Ethyl Chloride)
Chloroform (Trichloromethaae)
Chloromethane (Methy
1
Chloride)
0-Chlomtoluene
p-C hlorotoluene
Chromium
C
hlorodibromomethane (Dibromoc
hIorornethm)
Electronic Filing, Received, Clerk's Office, February 14, 2007
Parameter
Sioret
Cobalt
Copper
p-CresoI
Cyanide (mg/L)
Palapon
PDT
Di
bromomethane (Methylene Bromide)
m-Dichloroknzene (1,3
Dicblorobell~ene)
o-Dichlorobenzene
(1,2
Dichlorohenzene)
p-Dichloxobanzent
[1,4
Dicblorobemne)
Dichlorodifluommethane
Dichlororaethane
(Methylene Chloride)
Dieldrin
Diethy1 Phthalatc
Dimethyl Phthlate
Di-N-Butyl Phthlate
Dinoseb (DNBP)
Endothall
Endrin
Di(2-Ethylhexy1)Phthalate
Ethylbenzene
Etbylene Dibromide
(EDB)(
1,2-Pibrorns ethane)
Fluoride
(m&)
Heptachlor
Heptachlor Epoxide
Hexachllorobutadiene
Hexou:hlorcyc!~pentadiene
lodomzt4ane (Methyl Iodide)
Iron
Isophomne
Isopropyl benzene
p-Isopropyltoluene
Lead
Lindane
Magnesium (mgfL)
Manganese
Mercury
Methoxyclor
Naphthalene
Nickel
Nitrate-Nimgen
(m@)
Oil(Hexane-Soluble or Equivalent)
(m&)
Electronic Filing, Received, Clerk's Office, February 14, 2007
Parameter
Storet
Parathion
Pentachlam phenol
pH
Phenols
Piclaram
PoIychlorinated Biphenyls
Potassium
(mglL)
n-Prop yl
benzene
Selenium
Silver
Simazine
sodium (m&)
sme
Sulfate
(mgk)
TOC
Tetrachloroethylene
m@)
(Perchlo~oethylen)
Tetrahydro
furan
Thallium
Toluene
Toxaphene
Trichloroethy lene
(Trichloroethene)
Trichlorofluoromethane
Vanadium
Vinyl Chloride
Vinyl
Acetate
Xylenes
m-X
ylene
o-Xylene
p-X yiene
zinc
1,1,1
,ZTetrachloroethane
1 ,l ,l -Trichloroethane (Methylchlorofonn)
1,1,2,2-Tetrachloroethane
1,1,2-Trichloroethme
1,1 -Dic hloroethane
1,l -Dichloroethylene
1,l -Dichloropropene
1,2,3
-Trichl oro benzene
1,2,3 -TrichIoropropane
1,2,4-Trichlorobenzene
1,2,4-Trimeth yIbenzene
I
,2-Dibromo-3-Chloropropane
(DBCP)
Electronic Filing, Received, Clerk's Office, February 14, 2007
Parameter
cis-1 $2-Dichloroethylene
trans-
1
,ZDicMoroethylene
1,2-Dichloroehe
1,2-Dichloropropane
(Prop ylene Dichloride)
1,3,5-Trimetbyibenzene
1,3-Dichloropropane
1
$3-Dichloropropne
cis-
l,3-Dichlompropene
trans-
l,3-Dichloropropene
trans-
1,4-Dichlor0-2-Butene
2,2-Dichlarapropane
2,4,5-TP (Silvex)
2,4-Dichlorophenoxyacetic
Acid (2,4-D)
2-Butanone(Methy1
Ethyl
Ketone)
2-Hexanone
(Methyl Butyl
Ketone)
4-Methyl-ZPentanone (Methyl
Isobuty 1
Ketone)
Electronic Filing, Received, Clerk's Office, February 14, 2007
ATTACHMENT
11
Parameter
Acetone
Acrylonitrile
Benzene
Bromobenzene
Bromochlorome~me
Bromodichloromethane
Bromoform; Tri bramomethane
n-Butylbenzene
sec-Butyl
benzene
tert-Butylbenzene
Carbon disulfide
Carbon tetrachloride
Chloroknzene
Chloroethane
Chloroform; Trichloxomethane
o-Chlorotoluene
p-Chlorotoluene
Dibrornochloromethane
1
,ZDibromo-3-chloropropme
1,2-Dibromoethane
1
,ZDichlorobenzene
1,3-Dichlorobenzene
1,4-Dichlorobenzene
tram-
l,4-Dichloro-2-butene
Dichlorodi fluoramethane
1,l-Dichloroethane
1,2-Dichloroethane
1,l -Dichloroethylene
cis-
l,2-Dichloroethylene
trans-
1
2-Dicloroethylene
1,2-Dichloropropane
I ,3-Dichloropropane
2,2-Dichloropropane
I,
1
-Dichloropropene
1,3-Dichloropropene
cis- 1,3-Dichlompropene
trans-
l,3-Dichloropropene
Ethylbenzene
Hexachlorobutadiene
2- Hexanone; Methyl
hutyl
ketone
Isopropylbenzene
Electronic Filing, Received, Clerk's Office, February 14, 2007
Parameter
p-Isoprop
yltoluene
Methyl bromide; Bromomethane
Methyl chloride; ChIoromethane
Methylene bromide; Dibromornethane
Dichloromethaue
Methyl ethyl ketone
Methyl iodide; Iodamethane
4-Methyl-2-pentanone
Naphthalene
Phenols
n-Prop
y
benzene
Styrene
1,1,1,2-Tetrachloroethane
1,1,2,2-Tetrachloloethanel
Tetrachloroethylene
Tetrahydro
furan
Toluene
1,2,3
-Trichlorobewne
1,2,4-Trichlorbenzene
1,1,1
-Trichlom&ane
1,1,2-Trichloroethane
Trichlotoethylene
Trichlarofluorometbane
1,2,3-Trichloropropane
1,2,4-TrimethyIbenzene
1,3,5-Trimethylbenzene
Vinyl
acetate
Vinyl chloride
X ylenes
Electronic Filing, Received, Clerk's Office, February 14, 2007
BEFORE
THE
ILLINOIS POLLUTION CONTROL
BOARD
IN THE
MATIER
OF:
PROPOSED AMENDMENTS
TO
SOLD WASTE DISPOSAL LANDFILL RULES
(35 Ill.
Adm.
Code 810 and
81 1)
1
1
1
R
07-8
)
(Rulemaking
-
Land)
1
1
1
TESTIMONY
OF
CHRISTIAN J, LIEBMAN
My
name is Christian
J.
Liebman. I
am
the Manager of the Solid Waste Unit
in
the
Pmit Section within
the
Bureau of Land of
the
I1Ibois Environmental Protection
Agency.
I
have
been in my
current position since Febsuary
1999.
From
June
1985,
until I assumed
my
current position, I was
a
pennit reviewer in the unit I now manage. In
1984,1
received
a
B.S.
in
Geologicul Engineering from University of Missouri
at
Rolla and, in
2002,
I received
an
M.S. in
Civil
Engineering from
Southem
Illinois University at Carbondate. I
am
licensed in the
State
of
Illinois
as
both
a
Professional Engineer and
a
Professional
Geologist.
My
resume is attached.
Today,
I
wi 11
be testi
fymg
in support of the proposed changes
to
35
Ill. Adm.
Code
Parts
8
10
and
81 1,
specifically Sections
81
1.309(g)I1),
81
1.309(g)(2)(G),
81
1.309(g)(3)(D),
8 1
1.309(g)(4),
8 1 1.309(g)(5)
and 81 1 .Appendix
C.
This tatirnony
also responds to
an issue the Board
raised in
the
first
hearing
on this
rulemaking regarding unpermitted, on-site landfills.
1.
OVERVIEW
Leachate monitoring can help
detmine
the degree
to
which
a
landfill poses
a threat
to
the
groundwater
by
ascertaining what types of
contaminants
are
leaching
out
of the
wastes
that
have
been
disposed in the landfill and in
what
concentrations, The changes
to
the
leachate
monitoring requirements of
35 111. Adrn. Code
Part 8
1 1
proposed
in this rulemaking are intended,
Electronic Filing, Received, Clerk's Office, February 14, 2007
primarily,
to:
I) pnwide clarification
regding
the constituents for
which
leachate should be
rnonitorsd; and
2)
ensure
that
leachate monitoring systems
are
capable of detecting spatial
variability.
These
changes
were
initially
suggested
by the Agency.
*C
.
11.
LEACHATE MONITORING
PARAMETERS
6
in
administering the leachate monitoing
requirements
of 35 Ill. Adm.
Codc
Part 8
1 1, the
Illinois EPA has taken the position
that
the
parameters for which
groundwater
is monitored
should be a subset of the parameters for which leachate is monitd. That is,
we
have required
leachate
hm
permitted solid
waste
landfills to be monitored for
all
the
parameters for which
groundwater must, by regulation, be monitored and essentially all other parameters
that
may,
according
to
the literature,
be
found in leachate.
Since
the
Agency began permitting landfills under 35
Ill.
Adm.
Code
Parts
8 10-8
14
in the
early
1990's,
we have consistently employed the approach described
above.
We
believe that
our
practices
have
a
sound
technical basis, but the current regulatory basis for it
may
be less solid,
While 35 Ill.
Ah. Code
8
1 1.3
19(a)(2)(A)(i) supports our approach,
35
Ill.
Adm. Code
8
1
1.309(g),
as currently
written,
could be read
to
mean that leachate
can
be monitored for a
much shorter list of parameters
than
we
have been
requiring.
The proposed amendments would
codify
tbe
approach
we
have
been using.
The cIarification regarding leachate monitm parameters is made through the proposed
amendments
to
Sections
81 1.309(g)(1), 81
1.309(g)(2)(G),
811.309(g)(3)(D) and the addition of
Appendix
C
[Refmd
to as
Prsposed Amendments
4,5,6
and
9
in
the filing].
Ja.
SPATIAL VARtABILITY
Within
a landfill, leachate
quality
can vary
hm
one area to anather. The causes of
this
spatial
variability
include
differences
in
the age
of
the
wastes
and, therefore,
differences
in the
Electronic Filing, Received, Clerk's Office, February 14, 2007
depe
to
which they have
decomposed and
stabilized, differences in the types of
waste
disposed
from one
area
to another, and differences in the volume of water percolating through
the
waste
--
-
e.g.,
much more water would be
expected
to percolate
through
the waste
at
the
active
face
than
through
waste
in
an
area over which finaI cover
has
been
applied.
Leachate quality data from a leachate monitoring program that is not capable of detecting
spatial variability may underestimate
the
strength of the leachate
from
some
areas
of the landfill.
Also, in
some
cases, constituents
contained in the leachate produced in
one
area
of
the ImdfilI
may not
be
detected at
all due to dilution by leachate from other areas.
Although the
Agency
has long
recognized
that leachate monitoring networks capable of
detecting spatial variability are desirable,
we have
admittedly been less than consistent in
requiring permitted landfills to have such
systems.
As a result, at least one landfill now has
literally dozens of leachate monitoring points, while
others have
only one. The proposed
changes will
ensure
that
new landfills either have the capability of detecting leachate variability
(provided there is
a
minimum of four leachate monitoring points or
one
for
every
25
acres of
waste
footprint)-- or the landfill operator has demonstrated to the
Agency's
sat isfaction that
fewer leachate monitoring points
are
needed due
to
site specific circumstances.
The
change specifying
&e
minimum number of leachate monitoring points is made by
the
addition of Section
8 11.309(g)(4)
[Referred
to as
Proposed
Amendment
7
in
the
filing].
IV,
FREQUENCY OF LEACHATE SAMPLING
Under the current regulations, each leachate monitoring point at a landfill is initially
sampled quarterly,
and
after eight rounds of quarterly sampling the frequency of sampling is
decreased to semi-amuaily. The proposed amendments do not require the initial quarterly
sampling; instead, they require semi-annual leachate monitoring from
the
start, with
each
Electronic Filing, Received, Clerk's Office, February 14, 2007
monitoring point being sampled
at
least once every
two years.
Thus,
under
the
proposed
amendments,
at a
landfill with four &hate monitoring points, every six months one of the
points would be sampled.
The reduction
in
the frequency of leachate
sampling,
as set
forth
in
the
proposed
amendments,
was
offered
by
the Agency in
exchange
for the
NS WM
A's
support of the specified
list of leachate
monitoring
parameters and the minimum number of leachate monitoring points.
The proposal frequency is sufficient to adequately characterize leachate, and it also provida
more equality between landfills that have
many
leachate monitoring points and those with fewer.
For example, under the current regulations, a landfill with four leachate monitoring points must
perform four times
as much
leachate sampling as a landfill with one point, Under
the
proposed
amendments,
two
such landfills would do the
same amount
of
leachate
sampling.
The changes regarding
the
frequency of leachate
monitoring
are
made through
the
proposed amendment
to
Section 81 1,309(gMl) and
the addition
of Section 8 I
1.309(g)(5)
[Referred
to as
Proposed Amendments
4
and
8
in
the
filing].
V.
WERMITTED, ONSITE LANDFILLS
In
the
January
29,2007
hearing
on this
rulemaking, the
Board
asked if the
Agency
could
produce
a
list of
unpennittd
onsite landfills that
are
regulated under
35
111. Ah.
Code
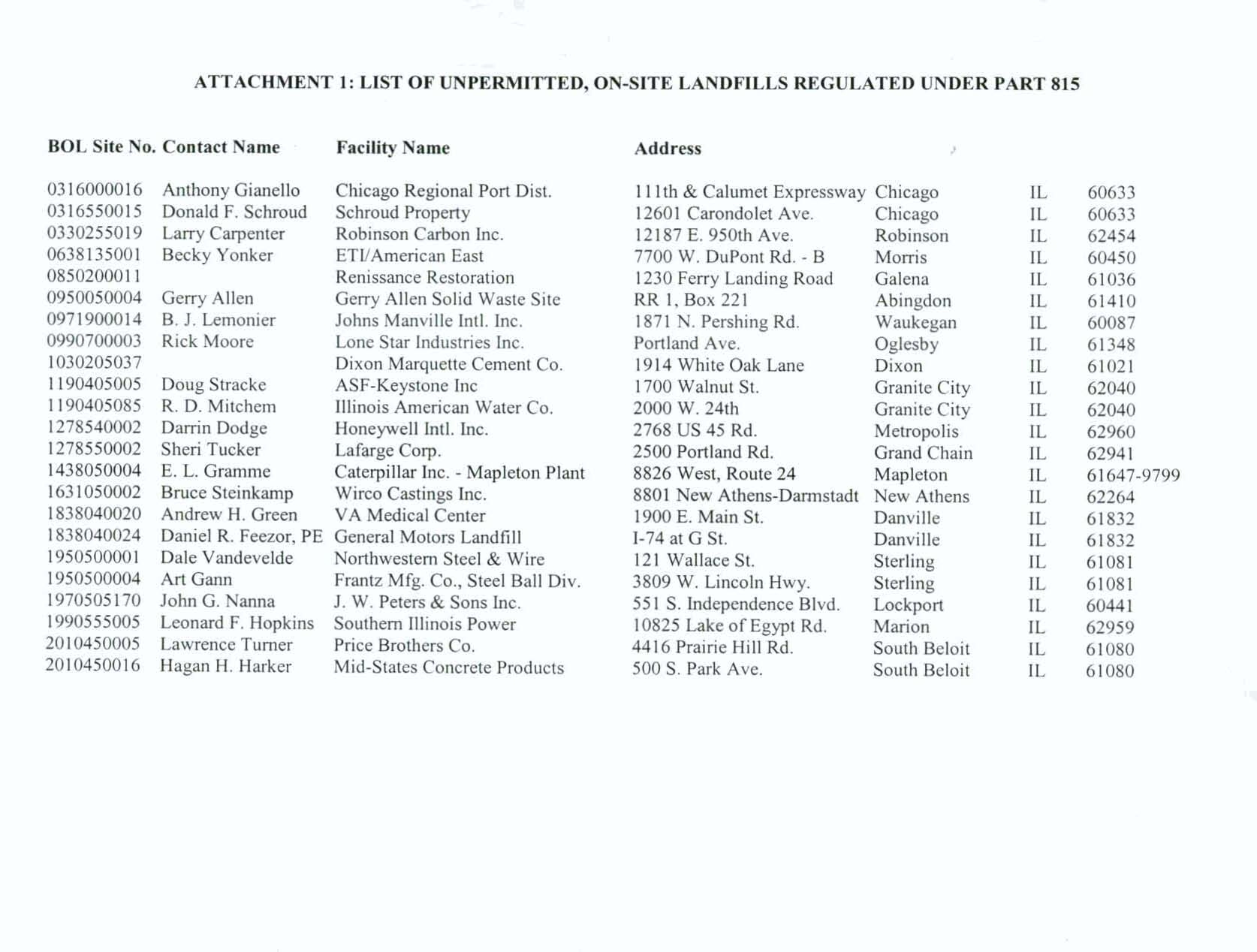
Part 815. Attachment 1 to this
testimony
is offered in response to this request. It is
a
list
of the
unpermittd, onsite landfills
that
the Agency's Bureau
of
LandPIanning and
Reporting
Section
is
aware
of and to which "On-Site Permit Exempt
'8 15'
Facility, Annual Report" forms
are
sent
each year.
VI.
CONCLUSION
In
closing,
I
would like
to
thank
the members
of
the
NSWMA for their hard
work
and
Electronic Filing, Received, Clerk's Office, February 14, 2007
perseverance in pursuing this rule change. I would also like
to
thank. the Board for its
consideration of
these
changes,
ATTACHMENT
1
:
List of Unpennitted, On-Site Landfills Regulated Under
Part
8 1
5
THIS FILING IS SUBMITTED
ON
RECYCLED PAPER
Electronic Filing, Received, Clerk's Office, February 14, 2007
RESUME
OF
CHRISTIAN J. LIEBMAN
102 1
North Grand Ave. East,
P.O.
Box
19276
Springfield, Illinois
62974-9276
(2
17) 524-3294
EDUCATION
M.S., May 2002, Civil Engineering from Southern Illinois Universi ty-Carbondale,
Carbondale, IL, Major: Civil Engineering
B.S., May
1984,
University of Missouri
-
Rolla, Rolla,
1WO,
Major
Geological Engineering
WORK EXPERIENCE
02/99
-
Present
Solid
Waste
Unit
Manager
in
the Illinois Environmatal Protection
Agency's Bureau of land, Division
of
Land
PoIIarfion
Control,
Permif
Section. Tke job
consists of supe~~ising
the I2 engineers who am
responsible
for
reviewing the permit
applications
for all the solid
waste
landfills and
clean camtniction and
demolition debrisjil operalions
in
the
Stde
of
fllinois, subject
to
the BOL pennit procars. The primary job
objective
of
this position
is
to
ewm thal these permit applications
are
given cornistent,
high-quai@
reviaus
in
a
timely manner.
Pennit Reviewer
in
the Illinois Environmental Protection Agency's
Bureau
of Land,
Division
of Land Polluiion Control, Permit Section,
advancing from
an
Environmental Protection Engineer I lo
Environmental Protection
Engineer
III. The job entailed reviewing
permil applications
for
solid
waste
landfills, dransfer stations
and waste
composring
faciliiries,
comparilrg the proposals made in the applications
to the regulatory
and
statutory
requimments
and then
dra3ing
preliminary responses
(either
permits with
conditions
or denials) for
mnageMent
approval.
PROFESSIONAL LICENSES
Licensed Professi~wl Engineer in the
State
of Illinois (License
No.
062-049263).
Licensed Professional Geologist in
the
State of Illinois (License No.
196-000989).
Electronic Filing, Received, Clerk's Office, February 14, 2007
ATTACHMENT
1:
LIST
OF
UNPERMITTED, ON-SITE LANDFILLS REGULATED UNDER PART
815
BOL Site
No.
Contact
Name
Facility Name
Anthony Gianelio
Chicago Regional Port Dist
.
Donald
F.
Schud
Schroud
Roprty
Lany
Carpenter
Robinson Carbon Inc.
Becky Yonker
ETYAmerican East
Renissance Restoration
Gerry Allen
Gerry
Allen Solid Waste Site
B.
J. Lemonier
Johns
Manville Intl.
Inc.
Rick Moore
Lone
Star Industries Inc.
Dixon
Marquette Cement Co.
Doug Stracke
ASF-Keystone Inc
R. D. Mitchem
IIlinois American Water
Co.
Darrin
Dodge
Honeywell
Intl.
Inc.
Sheri Tucker
Lafarge Corp.
E.
L.
Gramme
Caterpillar
hc.
-
Mapleton
Plant
Bruce Steinkarnp
Wirco Castings
Inc.
Andrew H.
Green
VA
Medical Center
Daniel
R.
Feezor,
PE
General Motors Landfill
Dale Vandevelde
Northwestern Steel
&
Wire
Art Gaan
Frantz Mfg.
Co.,
SteeI Ball Div.
John
G.
Nanna
J.
W. Peters
&
Sons
hc.
Leonard F.
Hopkins
Southern
Illinois Power
Lawrence
Turner
Price Brothers
Co.
Hagan H. Harker
Mid-States Concrete Products
1 1
lth
&
Calumet Expressway Chicago
1 260 1
Carondolet
Ave.
Chicago
121
87
E.
950th
Ave.
Robinson
7700 W. DuPont Rd.
-
B
Morris
1230
Ferry Landing
Road
Galena
RR
1,
Box
221
Abingdon
I 871 N. Pershing Rd.
Waukegan
Portland
Ave.
0gI-b~
1914
White
Oak
Lane
Dixon
1 700
Walnut
St.
Granite City
2000 W. 24th
Granite City
2768
US
45
Rd.
Metropolis
2500
Portland
Rd.
Grand
Chain
&826
West,
Route 24
Mapleton
880 1
New
Athens-Dmstadt
New
Athens
1900
E. Main
St.
Danville
1-74 at
G
St.
Danville
121 Wallace St.
Sterling
3809 W. Lincoln
Hwy.
Sterling
55 1 S.
Independence Blvd.
Lockport
1 0825
Lake of
Egypt Rd.
Marion
4416
Prairie Hill Rd.
South
Beloit
500
S. Park
Ave.
South
Beloit
Electronic Filing, Received, Clerk's Office, February 14, 2007
STATE
OF
ILLINOIS
COUNTY OF
SANGAMON
PROOF OF
SERVICE
I,
the
undersigned, on
oath state hat
I
have served the attached Pre-filed
T~dimony of Gwenvtb Thompson
and
Christian J. Liebman
upon
the pawns
to
whom
they
are directed, by placing a copy of each
in
an
envelope address4
to:
Dorothy Gunn, Clerk
Illinois Pollution Control Board
James
R.
Thompson
Center
100
W.
Randolph,
Suite 1 1-500
Chicago, Illinois
6060 1
Matt
Dunn
Environmental
Bureau Chief
Office of the
Attorney
General
James
R.
Thompson
Center
100
W.
Randolph,
lzU
Floor
Chicago, Illinois
60601
Bill Richardson,
General
Counsel
IIlinois Dept
,
of Natural Resources
One
Natural Resources Way
Springfield, Illinois
62702- 1
27
1
Timothy
J.
Fox
Illinois Pollution Control Board
1
00
W.
Randolph
St.
Sitt
11
-500
Chicago,
Illinois
60601
and
mailing them (First Class Mail), with the exception that it
sent via
COOL to the
Clerk of the,
Illinois Pollution Control Bod,
from
Springiield, Illinois on February
t
4,
2007,
with sufficient
postage
affixed
as indicated above.
SUBSCRIBED AND SWORN
TO
BEFORE
This
14th day
of Februarv,
2007.
Notary hblic
THIS FILING SUBMITTED
ON RECYCLED
PAPER
Electronic Filing, Received, Clerk's Office, February 14, 2007