BEFORI~THE ILLINOIS POLLUTION CONTROL BOARD
iN THE
MATTER OF:
WATER QUALITY AMENDMENTS TO
35
Iii. Adna. Code 302.208(e)-(g),
302.504(a),
3 02.575(d), 303.444, 309.141(h); and
PROPOSED 35 111. Adm. Code 301.267,
301.3 13, 301.413, 304.120, and 309.157
NOTICE OF FILING
~~CEIVEj~
CLERK’S O~’~
SEl-’ 062002
S~TEOF ILLINOIS
Pollution Control Board
Dorothy Gurm, Clerk
Pollution Control Board
100 West Randolph Street
Suite 11-500
Chicago, Illinois 60601
Mathew Dunn
illinois Attorney General’s Office
Environmental Control Division
James R. Thompson Center
100 West Randolph Street
Chicago, Illinois 60601
Attached Service List
Marie E. Tipsord
Illinois Pollution Control Board
James R. Thompson Center
100 West Randolph Street, Suite 11-500
Chicago, Illinois 60601
Legal Service
illinois Department ofNatural Resources
524 South Second Street
Springfield, Illinois 62701-1787
PLEASE TAKE NOTICEthat I have today filedwith the Office ofthe Clerk ofthe Pollution Control
Board the
COMMENTS OF ILLINOIS
ENVIRONMENTAL
PROTECTION AGENCY,
copiesof
which are herewith served upon you.
ILLINOIS ENVIRONMENTAL PROTECTION AGENCY
By:___________________________
SanjayK Sofat
Assistant Counsel
Division ofLegal Counsel
Dated: September 6, 2002
Illinois Environmental Protection Agency
1021 North Grand Avenue East
Springfield, Illinois 62794-9276
(217) 782-5544
)
)
)
)
)
R02-l1
(Rulemaking
-
Water)
~Z7
THIS FILING
PRP~TED
ON RECYCLED PAPER
BEFORE
THE
ILLINOIS POLLUTION CONTROL BOARD
RECEIVEDCLERK’S
OFETCE
SEP
0
62002
IN THE MATTER OF:
Pollution
STATE OF
Control
ILLINOIS
Board
WATER QUALITY AMENDMENTS TO
)
35 ill. Adm. Code 302.208(e)-(g), 302.504(a),
)
R02-l 1
2-
7
302.575(d),
3 03.444, 309.141(h); and
)
(Rulemaking
-
Water)
17 ~
PROPOSED 35111. Adm. Code 301.267,
)
301.313, 301.413, 304.120, and 309.157
)
AGENCY’S COMEMNTS
THE ILLiNOIS ENVIRONMENTAL PROTECTION AGENCY (the “Agency” or “Illinois
EPA”) respectfully submits these comments on the hearing held on July
25,
2002, in the Illinois
Pollution Control Board’s (the “Board”) R02-1 1 rulemaking proceeding. The Agency filesthe
comments to provide additional information on the Agency’s proposed cyanide standard and address
the issues raised by Albert Ettinger, attorney forthe Environmental Law & Policy Center, the Sierra
Club, and Prairie Rivers Network atthis hearing. The Agency is thankful to the Board for holding
the third hearing on this important rulemakingproceeding and providing this opportunity to file
comments.
Inrulemaking proceedings, the Agency has always ensured that the Board has access to the
necessary and available information for its consideration. This rulemaking proposal was no
different. To ensure that the Agency has the most current information, it contacted several
organizations including industry groups, agencies, and environmental groups fortheir comments on
the Agency’s draft proposal. (See Public Participation section ofthe Original Petition) Based on
the comments received, the Agency made essential changes to its draft before filing it with the
Board. The first time the Agency heard about the USEPA’s new laboratory method for cyanide was
2
at the July 25 hearing. At that hearing, Albert Ettingerintroduced the document, Method OIA-1677
Available Cyanideby Flow Injection, LigandExchange, and Amperometry, EPA-821-R-0l3,
August 1999, into the hearing record as Exhibit 18. The Agency wishes that in the future
rulemakings, the stakeholders bring the new information to the Agency’s attention at the earliest
possible time rather than wait until the First Notice period. However, since the hearing, the Agency
has been gathering facts regarding this new laboratory method.
NEW LABORATORY METHOD FOR CYANIDE
-
The new method was promoted several years ago by USEPA as the solution to the difficulties of
cyanide laboratory analysis and correlationto the USEPA national criteriafor cyanide. Within
USEPA RegionV (the six Westernmost Great Lakes states), Michigan is the only stateto adopt this
method. For some reason, Region V water quality coordinator, who usuallypasses such information
down to the states, was not aware ofthis new method. The Michigan representatives indicated that
the newmethod, in theory, was attractive for use in cyanide standards, but due to budgetary
concerns, the state had not proceeded to use the method. Further, the representatives did not know
of any discharger using the new method in Michigan, nor were theyaware ofany consulting
laboratories capable ofperformingthe analysis.
To find out about the capabilities ofthe new method, the Agency contactedtwo sources that
were key players in the development, testing, and USEPA adopting process. Mr. Jim Boiani of
Dyncorp participated in the testing process as a USEPA contractor. Ten laboratories were given the
instruments and reagents necessary for the newmethod and were instructed in its use. The
laboratories also participated in an evaluation process. USEPA found that the new method did
indeed have fewer problems with interferences and had a better minimum detection limit than
previously approved methods. As the method passed USEPA criteria for reliability, it was adopted
as an approved method.
3
The Agency also contacted Mr. Jason Gray of01 Analytical Co., the company that actually
developed the method. According to Mr. Gray, the new method measures the same forms of
cyanide as does the weak acid dissociable method. Essentially, both methods, the new method and
weak acid dissociable method, exclude the iron-cyanide complexes that are not toxic to aquatic life,
but commonly are found in cyanide solutions. The tightly bound iron-cyanide complexes do not
revert to free cyanide, the toxic form, unless strong-acidic conditions are present or, as explained
later, they are exposed to ultraviolet light. All other cyanide present in the solution, i.e., the cyanide
directly toxic to aquatic life or what maybe conceivably be converted to a toxic form, is measured
by both methods. Both methods measure the bound forms ofcyanide present in other metal
complexes, e.g., copper, zinc, silver, etc.
01
Analytical makes the proprietyreagents forthe test and sells the instruments neededto
run the test. Mr. Gray indicatedthat these instruments are also available from other manufacturers.
According to Mr. Jim Boiani ofDyncorp, the cost ofthe instrumentation is about $34,500 before
discounts. The reagent cost for approximately 100 samples is $320. Mr. Boiani believes that that
private laboratories would charge about $50 per sample analyzed. The approximate cost for
analyzing a sample with the weak acid dissociable method is about
$35.
The City ofNew York and Cincinnati Metro Water District and a number ofindustrial
laboratories are currently using the new method. The newmethod is said to be much easier to
perform than the old weak acid dissociable or cyanide amenable to chlorinationmethods. The
minimum detection limit of2 ~.tg/Lhas been achieved with this new method. Mr. Pfeifer, Region V
standards coordinator, has indicatedthat USEPA would have no objection to states adopting the new
method since it came from USEPA’s research arm.
One practical problem that exists with the newmethod, as well as with all newmethods, is
that most commercial laboratories in Illinois are not equipped to run the test. The Agency surveyed
4
three major commercial laboratories (Suburban Labs, EMT, Inc., and PDC Laboratories, Inc.) in
Illinois and foundthat none ofthese laboratories conduct the cyanide test using this new method. In
fact, these laboratories do not even have the necessary equipment to do so. All echoedthe sentiment
that unless their clients ask for this method or show a tremendous interest in the newmethpd, the
laboratories would not be making such a considerable investment.
It is clear from the above discussion that sufficient benefits exist from the use ofthe new
method. Therefore, the Agency supports the use ofthis newmethod. The Agency recommends that
both the weak acid dissociable method and the new cyanide method should be referenced as
appropriate tests in the Board’s cyanide standard. As the new method provides less interference and
a lower detection limit, the dischargers, given the option ofthe new method, may beginto demand
that laboratories use this method instead ofthe weak acid dissociable method. As the demand grows
for the newmethod, the commercial laboratories mayconsider it worthwhile to invest in the
necessary equipments to runthe cyanide test.
ENVIRONMENTAL IMPACTS
OF
IRON-CYANIDE COMPLEXES
At the July 25 hearing, Albert Ettinger raised issues concerning the impact ofiron-cyanide
complexes on the aquatic system. Neitherthe weak acid dissociable method nor the new method
measure the iron-complexed forms ofcyanide. A publication by the US Fish& Wildlife Service
addresses the issues raised by Mr. Ettinger at the hearing. (Cyanide Hazards to Fish, Wildlife, and
Invertebrates: A Synoptic Review, Ronald Eisler, Biological Report 85 (1.23) December, 1991).
The author reports that cyanide in general does not persist very long in surface waters, at most a few
days. Volatilization and microbial decomposition are two ofthe main ways that waters are naturally
cleansed of cyanide. The publication refers to the ferricyanides and ferrocyanides as “sparingly
decomposable” and that these iron complexes “do not release free cyanide unless exposed to ultra
violet light.” Further, the author states, “~t~hemuch lower toxicities ofthe ferrocyanide and
5
ferricyanide complexes- which are ofhigh stability but subject to extensive and rapid photolysis,
yielding free cyanide on direct exposure to sunlight- and the nickelocyanide ion complexes are not
likely to be ofpractical importance.”
(~
Attachment A)
Clearly, the iron-cyanide complexes are more persistent in the environment than other forms
ofcyanide. Any cyanide that becomes free from the iron-cyanide complexes due to extensive
exposure to ultraviolet light would not persist very long in surface waters. As the author indicates,
the iron forms ofcyanide are not likely to be ofpractical importance because actual photolysis
(chemical decomposition due to exposure to sunlight) would occur at relatively slow rates. The
National Criteria Document for cyanide explains that “rlease ofcyanide ion by
photodecomposition might be important in relatively clear waters.” Apparently, the relative clarity,
depth, and exposure to sunlight (i.e., degree ofshading) all would be significant factors in
photolysis. Since most waters in illinois are not sufficientlyshallow, clear and unshaded, photolysis
is probably not ‘rapid’ in illinois waters. Therefore, there is no need to consider the impact ofthe
iron-cyanide complexes on the aquatic system. The Agency recommends the use ofweak acid
dissociable standard, the existing standard. This recommendation is further supported by the fact
that even the USEPA’s new cyanide method does not measure the iron form ofcyanide.
HUMAN HEALTH IMPACTS OF CYANIDE
The issues related to human health impacts ofcyanide were also raised at the July 25 hearing. The
USEPA MCL for total cyanide is 0.2 mgfL, several times the level ofthe proposed aquatic life
standards.
CONCLUSION
The Agency wishes to remind the Board that this rulemaking concerns the proposing ofnew
standards for cyanide. The Agency has explained in detail the process for updating the General Use
standards for cyanide, a process that has nothing to do with laboratory analytical methods. The
6
addition ofa better laboratory method does not change the appropriateness ofthe numeric values of
a standard that have been found to be protective ofaquatic life. While supplemental reasons for
changing the water quality standard for cyanide no longer exist, given the existence ofnew
laboratory method, the primaryreason is still pertinent. The warm water aquatic organisms found in
General Use waters are not as sensitive to cyanide as the existing standard implies; and therefore,
the cyanide standards should be updated to reflect “the latest scientific knowledge.”
Proposed Update:
Constituent
STORET
AS
CS
Number
(j.tg/L)
(~.tg/L)
Cyanide
00718
49
11
(Weak acid dissociable
or Available Method OJA-l677)
The Agency appreciates this opportunity to comment in this proceeding. As set forth in detail
above, the Agency urges the Board to adopt the proposed cyanide standard.
Respectfully Submitted
ILLINOIS ENVTRONMENTALPROTECTION AGENCY
By:______________________
Sanjay K Sofat
Assistant Counsel
Division ofLegal Counsel
DATED: September 6, 2002
Illinois Environmental Protection Agency
1021 North Grand Avenue East
P.O.Box 19276
Springfield, Illinois 62794-9276
(217) 782-5544
7
Exhibit A
Biological Report 85(1 23)
December 1991
Contaminant Hazard Reviews
Report 23
Back to top
CyanideHazards to Fish, Wildlife, and
Back to top
Invertebrates: A Synoptic Review
574 526321j
and Wildlife Service
23) ~ Department of the Interior
I
• ..
.-
. -
LIBRARY
EnV1roflm~talprotec~ttøfl
~
-.
.
State
of
UILno(9
~pr1ngftetd1WlnO~ ~:
6
BIoLoGIcAL REPORT 85(1.23)
and is also one ofthe most toxic cyanide species, it acrylonitrile, propionitrile, and succinonitrile, are
is noteworthy that the toxicity of simple cyanides nitrile-containing materials of varying complexity
will not be affected measurably below pH 8.3. and lability, and can liberate free and toxicologi-
Acidification of dilute (milligrams per liter) cya- cally available amounts of cyanide. But the non-
nide solutions will not intiate any greater release nitrile portion ofthe cyanogenmoleculemay exert
of HCN, but acidification of concentrated (grams an independent or interactive toxicity, causing a
per liter) solutions promotes HON formation and complex response.
release.
Cyanates contain the OCN group. Inorganic
Complex cyanides are compounds in which cyanates that are formed industrially by the oxida-
the cyanide anion is incorporated into a complex or tionofcyanide salts hydrolyze inwaterto form am-
complexes; these compounds are different in monia and bicarbonate ion. Alkyl cyanates are
chemical and toxicologic properties from simple insoluble in water and form cyanurates. Alkyl
cyanides. In solution, the stability of the cyanide isocyanates contain the OCN radical, are formed
complex varies with the typeofcation andthe com- from cyanates, and, like cyanates, are readily hy-
plex that it forms. Some ofthese are dissociable in drolyzed. Thiocyanates (SCN group) are formed
weak acids to give free cyanide and a cation, while from cyanides and sulfur-containing materials
other complexes requiremuch stronger acidic con- and are relatively stable.
ditions for dissociation. The least-stable complex
Total cyanidesrefers to all cyanide-containing
metallocyanides include Zn(CN)42, Cd(CN)i~,and compounds, including simple and complex cya-
Cd(CN)42-; moderately stable complexes include nides, cyanoglycosides, and free cyanide. Total
Cu(CN)2, Cu(CN)32,Ni(CN)42, andAg(CN)2; and cyanides is a chemical measurement of free cya-
the most stable complexes include Fe(CN)6~and nide p’~’~
-
- ~
~
Co(CN)&1. The toxicity ofcomplex cyanidesis usu- or dige
ally related to their ability to release cyanide ions
~
~-~------—-
in solution, which then enter into an equilibrium 1
~c~ircumstances, the concentra-
with HON; relatively small fluctuations in pH sig- tion of total cyanide will exceed that ofHON. In
nificantly,affect their biocidal properties.
some waters, however, the total cyanide concen-
Cyanogen (CN)2~is the simplest compound tration may consist almost entirely offree cyanide,
containing the cyanide group. Cyanogen is an ex- or it may contain cyanides that readily photo-
tremely toxic, flammable gas that reacts slowly decompose ordissociate to yield HON. The relation
with water to form HCN, cyanic acid, and other between total cyanide and free cyanide in natural
compounds; it is rapidly degraded in the environ- waters varies with receiving-water conditions,
ment. Cyanogen and its halide derivations are type ofcyanide compounds present, degree ofexpo-
comparable in toxicity to hydrogen cyanide.
sure to daylight, and presence of other chemical
Nitriles are defined as organic compounds compounds.
(RCN) containing the cyanide group. Cyanide
Hydrogen cyanidehas frequently been associ-
bound to carbon as nitriles (other than as cyano- ated with the odor of bitter almonds (Ballantyne
genic glycosides) are comparatively innocuous in 1983; Gee 1987). The threshold odor for olfactory
the environment, and are low in chemical reac- detection ofatmospheric HCN is 1 mWL, but the
tivity and are biodegradable. For simple mono- odor may not be detected for various reasons, in-
nitriles there is a clear progression,with more cya- cluding the presence of other odors and the fact
nide being released as chain length increases. ,A that only 20 to 40 ofthose tested could detect a
similar pattern exists in diñitriles, but corres- cyanide odor.
ponding compounds require a longer carbon chain
Analytical methods for determining free and
than mononitriles before free cyanide is produced. bound cyanide and cyanogenic compounds in bio-
Based on studies with chicken liver homogenates logical materials are under revision. Current
(Davis 1981), mononitriles were more toxic than methods include chromatography; enzymic post-
dinitriles, and within each group the order oftoxi- column cleavage; electrochemical detection; and
city was OH3 O2H5 C3H7 04119 C5H11 C7H15. ultraviolet, infrared, proton, and carbon-13 nu-
Cyanohydrins R2O(OH)CNJ
and cyanogenic clear magnetic resonance spectroscopies (Brimer
glycosides R1R2C(0R3)CN are special classes of 1988). Proposed newer analytical methodologies
nitriles, in that under appropriate conditions they include chemiluminescence (Wu et al. 1989);
will decompose to HON and cyanide ions. Cyano- deproteinization techniques (Krynitsky et al.
gens (not to be confused with cyanogen), such as 1986); thin film dissociation coupled with prefer-
18
BIoLoGIcAL REPORT 85(1.23)
low in winter owing to dilution by high runoff, but
peaked in summer because of cyanide production
by plants (Leduc 1984). Cyanides do not seem to
persist in aquatic environments. In small, cold
oligotrophic lakes treated with 1 mg NaCN/L,
acute toxicity was negligible within 40 days. In
warm shallow ponds, toxicity disappeared within 4
days after application of 1 mg NaCNIL. In rivers
and streams, toxicity rapidly disappeared on dilu-
tion (Leduc 1984). Cyanide was not detectable in
water and sediments ofYellowknife Bay, Canada,
between 1974 and 1976, although the bay receives
liquid effluents containing cyanides from an oper-
ating gold mine. Nondetection was attributed to
rapid oxidation (Moore 1981). Severalfactors con-
tribute to the rapid disappearance ofcyanide from
water. Bacteria and protozoans may degrade cya-
nide by converting it to carbon dioxide and ammo-
nia. Chlorination of water supplies can result in
conversion to cyanate (EPA 1980). An alkaline pH
favors oxidation by chlorine, and an aôidic pH fa-
vors volatifization of HON into the atmosphere
(EPA 1980).
Persistence in Water, Soil,
andAir
In water, cyanides occur as free hydrocyanic
acid, simple cyanides, easily degradable complex
cyanides such as Zn(CN)2,~
-
. - -
v
~
ation is the dominant mechanism for re-
moval offree cyanide from concentrated solutions
and is most effective under conditions ofhigh tem-
peratures, high dissolved oxygen levels, and at in-
creased concentrations, of atmospheric carbon
dioxide (Leduc et at 1982; Simovic and Snodgrass
1985). Loss of simple cyanides from the water col-
umn is primarily4 oigla~dimentation,micro-
bial degradation, and volatilization (Leduc et al.
1982; Marrs and Ballantyne 1987)1
-~
1982; Simovic and Snodgrass 1985; Marrs and Bal-
lantyne 1987).
Alkaline chlorination ofwastewaters is one of
the most widely used methods of treating cyanide
wastes. In this process, cyanogen chloride, (CNC1)
is formed, which at alkaline pH is hydrolyzed to
the cyanateion(CNO). Iffree chlorine is present,
CNO can be further oxidized (Way 1981; Leduc et
al. 1982; Simovic and Snodgrass 1985; Marrs and
Ballantyne 1987). Other methods used in cyanide
waste management include lagooning for natural
degradation, evaporation, exposure to ultraviolet
radiation, aldehyde treatment, ozonization, acidi-
fication—volatilization—reneutralization, ion ex-
change, activated carbon absorption, electrolytic
decomposition, catalytic oxidation, and biological
treatment with cyanide-metabolizing bacteria
(Towill et al. 1978; EPA 1980; Way 1981; Marrs
and Ballantyne 1987). In the case of Canadian
gold-mining operations, the primary treatment for
cyanide removal is to retain gold millwastewaters
in impoundments for several days to months; re-
moval occurs through volatilization, photo-
degradation, chemical oxidation, and, to a lesser
extent, microbial oxidation. Microbial oxidation of
cyanide is not significant in ‘mine tailing ponds,
whichtypically have pH 10, a low number of mi-
croorganisms, low nutrient levels, large quiescent
zones, and cyanide concentrations 10 mgfL
(Simovic and Snodgrass 1985).
Cyanide seldom remains biologically avail-
ablein soilsbecause it is either complexed by trace
metals,metabolized byvariousmicroorganisms, or
lost through volatifization (Towill et al. 197.8;
Marrs and Ballantyne 1987). Cyanide ions are not
strongly adsorbed or retained on soils, and leach-
ing into the surrounding ground water will prob-
ably occur. Under aerobic conditions, cyanide salts
in the soil are microbially degraded to nitrites or
form complexes with trace metals. Under anaero-
bic conditions, cyanidesdenitrif~rto gaseousnitro-
gen compounds that enter the atmosphere.
Volatile cyanides occur only ‘occasionally in
the atmosphere, due largely to emissions from
plating plants, fumigation, and other special op-
erations (Towill et al. 1978). Under normal concli-
tions cyanide has relatively low persistence in air,
usually between 30 days and 1 year (Way 1981), al-
,‘
though some atmospheric HCN maypersist forup
to 11 years (Marrs and Ballantyne 1987). Data are
lacking on the distribution and transformation of
cyanide in the atmosphere (Towill et al. 1978) and
should be acquired.
-
~t maylead
to
-J
~ iormation in wastes containing iron—
cyanide complexes (Towill et al. 1978; Leduc et al.
20
BIoLOGICAL REPORT 85(1.23)
resistant to 65 mg KCNIL at low temperatures
(13°C) than were seedlingsfrom cold-susceptible
cultivars (25°C), as judged by respiratory activity
of mitochondria (Van De Venter 1985). Results
suggest that cyanide-resistant respiration may
play arole in coldresistance in maize seedlings, al-
though more evidence is needed to demonstrate
that cold-resistant plants actually use their
greater potential foralternative respiration at low
temperatures (Van De Venter 1985).
The cyanogenic system comprising cyano-
genic glycosides, cyanohydrins, betaglucosidases,
and nitrile lyases is widespread in plants, but also
occurs in several species of arthropods,. including
the tiger beetle
(Megacephala virginica),
leaf bee-
tie
(Paropsis atomaria),
zygaenid moths, and cer-
tain butterflies (Nahrstedt 1988). In
Zygaena
trifolii,
cyanide compounds seem to function as
protection against predators (Nahrstedt 1988). De-
fensive secretions of cyanide have also been re-
ported in polydesmid millipedes, and these
organisms seem to be more tolerant than other
species whenplacedinkillingjars containingHON
(Towiul et al. 1978). In amillipede
(Apheloria
sp.),
cyanide is generated in atwo-compartment organ
by hydrolysis of mandelonitriie; cyanide genera-
tion occursoutside the glandwhen the components
ofthe two compartments are mixed during ejection
(Towill et al. 1978).
-
Highly toxic substances, such as cyanides, are
sometimes feedingcues and stimulants for special-
ized insects. For example, instar larvae of the
southern armyworm
(Spodoptera eridania)$
strongly prefer cyanogenic foods, such as foliage of
thelima bean, a plant with comparativelyelevated
cyanide content—up to 31 mg/kg in some varie-
ties—in the form of linamurin (Brattsten et al.
1983). Feeding was stimulated in southern ar-
myworms at dietary levels up to 508 mg KCN/kg
(208 mg HON/kg) for first to fourth ‘instar larval’
stages, and between 1,000 and 10,000 mg KCN/kg
diet for fifth and sixth instar larvae (Brattsten‘et
‘al. 1983). Sixth instar larvae preexposed to diets
containing 5,000 mg KCN/kg showed no adverse
affects at dietary levels of 10,000mgKCNIkg; how-
ever, previously unexposed larvae showed revers-
ible signs of poisoning at 10,000 mg/kg diet,
including complete inhibition of oviposition and
83 reductionin adult emergence (Brattsten et al.
1983). Experimental studies with southern ar-
myworm larvae and tbiócyanate—one of the in
vivo cyanide metabolites—showed that 5,000 mg
thiocyanate per kilogram diet reducedpupationby
77,
completely inhibited oviposition, and re-
duced adult emergence by 80 (Brattsten et al.
1983), strongly suggesting that thiocyanate poi-
soning is the primary effect ofhigh dietary cyanide
levelsin southern armyworms.
Resistant species, such as southern ar-
myworins, require injected doses up to 800 mg
KCN/kg BW (332 mg HON/kg BW) or diets of
3,600 mg KCN&g for 50 mortality (Brattsten et
ai. 1983), but data are scarce for other terrestrial
invertebrates. Exposure to 8 mg HCN/L air inhib-
its respiration in the granary weevil
(Sitophilus
granarius)
within 15 mm and kills 50 in 4 h;
some ‘weevils recover after cessation of 4-h expo-
sure (Towill et al. 1978).
Aquatic Organisms
Numerous accidental spills of sodium cyanide
or potassium cyanide into rivers and streams have
resulted in massive kills of fishes, amphibians,
aquatic insects, and aquatic vegetation; sources of
poisonings were storage reservoirs ofconcentrated
solutions, overturned rail tank cars, or discharge
of substances generating free HON in the water
from hydrolysis or decomposition (Leduc 1984).
Data on the recovery of poisoned ecosystems are
scarce. In one case, a large amount ofcyanide-con-
taining slag entered a streamfrom the reservoir of
a Japanese gold mine as a result ofan earthquake
(Yasuno et al. 1981). The, slag covered the
streambed for about 10 km from the point ofnip-
ture, killing all stream biot~d
~
covering the above-water stones, but there was lit-
tle underwater growth.After 6—7 months, popula-
tions of fish,, algae, and invertebrates had
recovered, although species composition of algae
was altered (Yasuno et al. 1981).
Fish were the most sensitive aquatic organ-
isms tested under controlled conditions. Signifi-
cant adverse nonlethal effects, including reduced
swimming performance and inhibited reproduc-
tion, were observed in the range of 5.0—7.2 pg free
cyanide per liter; deaths were recorded for most
species between 20 and 76 pgfL (Table 3). Among
invertebrates, adverse nonlethal effects were
documentedbetween 18 and 43 ~ig/L,and lethal ef-
fects between 30 and 100 ~ig/L—although some
deaths were recordedin the range 3—7 pg/L for the
amphipod
Gammarus pulex
(Table 3). Algae and
macrophytes were comparativelytolerant;adverse
effects were reported at 160 pg free cyanide per
liter (Table 3).
CYANIDE
27
C
C)
U
C)
C.
C
Ui
0
z
.~
0
U.
Ui
0.
w
I-
-J
Ui
100
FREE CYANIDE, in ug/L
hepatic damage. Exposure of fish for 9 days to 10
pg HCN/L was sufficient to induce extensive
necrosis inthe liver, although gill tissue showedno
damage. Intensification ‘of liver histcipathology
was evident at dosages of 20 and 30 ~igHCN/L and
exposure periods up to 18 days (Leduc 1984). Cya-
nidehas a strong, immediate, andlong-lasting in-
hibitory effect on the swimming abifity of fish
(Leduc 1984). Free cyanide concentrations as low
as 10 ~ig/Lcan rapidly and irreversibly impair the
swimming ability of salmonids in well-aerated
water (Doudoroff 1976). Osmoregulatory distur-
bances recorded.at 10 pg HCNIL may affect migra-
tory patterns, feeding, and predator avoidance
(Leduc et al. 1982; Leduc 1984). In general, fish ex-
perience a significant reduction in relative per-
formance (based on osmoregulation, growth,
swimming, and spermatogenesis) at ‘10 pg HCN/L,
and although fish can survive indefinitely at 30 jig
HCN/L in the laboratory, the different physiologi-
cal requirements necessary to survive in nature
could not be met (Leduc 1978, 1981; Leduc et al.
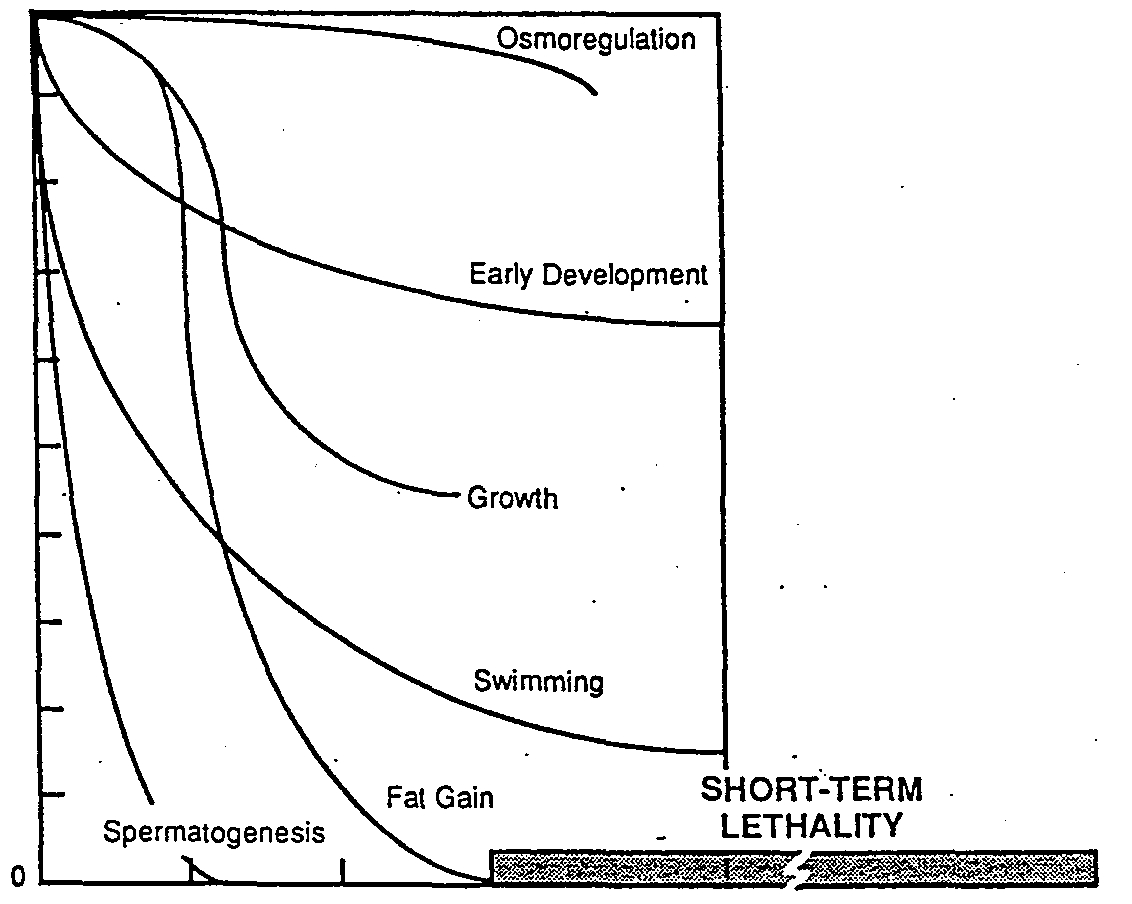
1982; Fig re~.Increase’dpredatIoii~bygreen sun-
fish
(Lepomis cyanellus)
on fathead minnows
(Pimephalespromelas)
was noted at sublethal con-
centrations of HCN, but it was uncertain if fat-
heads became easier prey or if green sunfish had
greater appetites (Smith ,et al. 1979).
-
Sodium cyanide has stimulatory effects on
oxygen-sensitive receptors in lungfish, amphibi-
ans, reptiles, birds, and mammals (Smatresk
Figure. Summary of lethal and sub-
lethal effects of free cyanide on fresh-
water fish. Modifiedfrom Leduc etal.
(1982).
1986). Facultative and aquatic air breathers ap-
pear to rely on air breathing when external•
chemoreceptors are stimulated, whereas obligate
air-breathing fish are more responsive to internal
stimuli (Smatresk 1986). Gill ventilation fre-
quency oflongnose gar
(Lepisosteus osseus),
for ex-
ample, was little affected by external cyanide’
application, but responded strongly when cyanide
was administered internally by injection
(Smatresk 1986Y. Cyanide, like many other chemi-
cals, can stimulate growth of fish during exposure
‘to low sublethal levels. This phenomenon, referred
to as hormesis, is little understood and warrants
additional research (Leduc 1984).
The observed toxicity to aquatic life of simple
and complex cyanides was attributed ‘almost en-
tirely to molecular (undissociated) HCN derived
from ionization, dissociation, and photodecomposi-
tion ofcyanide-containing compounds. The toxic-
ity of the cyanide ion, CN, which is a minor
component of free cyanide (HON
+
CN) in waters
that are not exceptionally alkaline is of little fin-
portance (Doudoroff 1976;Towill et al. 1978;Smith
et al. 1979; EPA 1980). The acute toxicity ofstable
silver cyanide andcuprocyanide complexanions is
much less thanthat ofmolecular HCN, but is nev-
ertheless important; these ions can be the princi-
ts~eveninjj&lut~o~ions.
r~—~’~
~
~c~©
90
80
70
60
50
40
30
20
10
10
20
30
40
-
50
100
150
BIoLoGIcAL REPORT 85(1.23)
1910). ‘1
~
~~isms
of organic
cyanide compounds, suchaslactonitrile,is similar
to that of inorganic cyanides because theyusually
undergo rapid hydrolysis in water to free cyanide
(Towill et al. 197~’
-
~sanal.,
rde-
t~1In6..~.
~entration in cyanide-polluted
waters is considered to be the mostreliableindex of
toxicity (Doudoroff 1976; Smith et al. 1979; EPA
1980; Abel and Garner 1986).
Cyanide acts rapidlyin aquatic environments,
does not persist for extended periods, and is highly
species selective; organisms usually recover
quickly on removal to clean water. The critical
sites for cyanide toxicity in freshwater organisms
include the gills, egg capsules, and other sites
where gaseousexchange and osmoregulatory proc-
esses occur. On passing through a semipermeable
membrane, the HON molecules are usually dis-
tributed by way of the circulatory system to vari-
ous receptor sites where toxic action or
detoxification occurs (Leduc 1984). Once in the
general circulation, cyanide rapidiy inhibits the
electron transport chain ofvital organs. Signs of
distress include increased ventilation, gulping for
air at the surface, erratic swimming movements,
muscular incoorclination, convulsions, tremors,
sinking to the bottom, and death with widely ex-
tended gill ,covers (Leduc 1981, 1984). The acute
mode of action of HCN is limited to binding those
porphyrins that contain Fe~3,such as cytochrome
oxidase, hydroperoxidases, and methemoglobin.
At lethal levels, cyanide is primarilya respiratory
poison and one of the most rapidly effective toxi-
cants known (Leduc et al. 1982). The detoxi-
fication mechanism of- cyanide is mediated by
thiosuifate sulfur transferase, also known as
rhodanese. This enzyme is widely distributed in
animals, including fish liver, gills, and kidney.
Rhodanese plays a keyrole in sulfur metabolism,
and catalyzes the transfer of a sulfane—sulfur
group to a thiophilic group (Leduc 1984). Thio-
sulfate administered in the water with cyanide re-
duced the toxicityofcyanide to fish, presumably by
increasing the detoxification rate of cyanide to
thiocyanate (Towill et al. 1978).
Additive or more-than-additive toxicity offree
cyanide to aquatic fauna has beenreported in com-
bination with ammonia (Smith et al. 1979; Leduc
ret al. 1982; Alabaster etal. 1983; Leduc 1984) or ar-
senic (Leduc 1984). However, conflicting reports
on the toxicity of mixtures of HON with zinc or
chromium (Towiul et ai. 1978; Smith et al. 1979;
Leduc et al. 1982; Leduc 1984) require clarifica-
tion. Formation of the nickelocyanide complex
markedly reduces the toxicity of both cyanide and
nickel at high concentrations in alkaline pH. At
lower concentrations and acidic pH, solutions in-
crease in toxicity by more than 1,000 times, owing
to dissociation of the metallocyanide complex to
form hydrogen cyanide (Towill et al. 1978). Mix-
tures of cyanide and ammonia may interfere with
seaward migration of Atlantic salmon smolts un-
der conditions of low dissolved oxygen (Alabaster
etal. 1983). The 96-h toxicity ofmixtures ofsodium
cyanide and nickel sulfate to fathead minnows is
influenced bywateralkalinity and pH. Toxicity de-
creased with increasing alkalinity and pH from
0.42 mg CN/L at 5 mg CaCOaIL and pH 6.5; to 1.4
mg CN/L at 70 mg CaCO3/L and pH 7.5;to 730 mg
CN/L at 192 mg CaCO3/L and pH 8.0 (Doudoroff
1956).
-
Numerous biological and abiotic factors are
knownto modif~-the biocidal propertiesoffree cya-
nide,includingwaterpH, temperature, and oxygen
content; life stage, condition, and species assayed;
previousexposure to cyanide compounds; presence
ofother chemicals; and initial dose tested. There is
general agreement that cyanide is more ‘toxic to
freshwater fish under conditions of low dissolved
oxygen (Doudoroff 1976; Towill et al. 1978; Smith
et ai. 1979; EPA 1980; Leduc 1984); that pH levels
within the range 6.8—8.3 had little effect on cya-
nide toxicity but enhanced toxicity at acidic pH
(Smith et al. 1979; EPA 1980; Leduc et al. 1982;
Leduc 1984); that juveniles and adults were the
most sensitive life stages tested and embryos and
sac frythe most resistant (Smith et al. 1978, 1979;
EPA 1980; Leduc 1984); and that substantial in-
terspecies variability exists in sensitivity to free
cyanide (Smith et al. 1979; EPA 1980). Initial dose
and water temperature both modif~rthe biocidal
properties ofHCN to freshwater teleosts. At slowly
lethal concentrations (i.e., 10 j.tgHCN/L), cyanide
was more toxic at lower temperatures; at high, rap-
idly lethal HCN concentrations, cyanide was more
toxic at elevated temperatures (Kovacs and Leduc
1982a, 1982b; Leduc et al. 1982; Leduc 1984). By
contrast, aquatic invertebrates were most sensi-
tive to HCN at elevated water temperatures, re-
gardless of dose (Smith et al. 1979). Season and
exercise modif~rthe lethality of HCN to juvenile
)
STATE OF ILLINOIS
)
)
SS
COUNTY
OF SANGAMON
)
)
PROOF OF SERVICE
I, the undersigned, on oath statethat I have served the attached COMMENTS
OF
TilE
ILLINIOS ENVIRONMENTAL
PROTECTION AGENCY
upon the person to whom it is
directed, by placing a copy in an envelope addressed to:
Dorothy Gunn, Clerk
Pollution Control Board
100 West Randolph Street
Suite 11-500
Chicago, illinois 60601
(OVERNIGHT MAIL)
Mathew Dunn
Illinois Attorney General’s Office
Environmental Control Division
James R. Thompson Center
100 West Randolph Street
Chicago, Illinois 60601
(FIRST CLASS MAIL)
Attached Service List
(FIRST
CLASS MAIL)
Marie E. Tipsord
illinois Pollution Control Board
James R. Thompson Center
100 West-Randolph Street, Suite 11-500
Chicago, Illinois 60601
(OVERNIGHT MAIL)
Legal Service
Illinois Department ofNatural Resources
524 South Second Street
Springfield, illinois 6270 1-1787
(FIRST CLASS MAIL)
and mailing it from Springfield, Illinois on September 6, 2002, with sufficient postage affixed as
indicated above.
OFFICIAL SEAL
~3REND~BOEHNER
NC1AI~PUBLIC.
STATE OF ILLINOIS ..
+r,4y
COMMISSION EXPIRES ll-14.20051•
SUBSCRIBED AND SWORN TO
BEFORE ME
this day ofSeptember 6, 2002.
3~ (B~Q~\QX’
Noi’ary Public
TillS FILING PRINTED ONRECYCLED PAPER
8
R02-l1
Rulemaking
Notification List
September 6, 2002
Mike Callahan
Bloomington Normal Water Reclamation District
Post Office Box 3307
Bloomington, illinois 61702-3307
Larry Cox
Downefs Grove SanitaryDistrict
2710 Curtiss Street
Downer’s Grove, Illinois 60515
Dennis Daffleld
Department of Public Works City of Joliet
921 East Washington Street
Joliet, illinois 60433
Matthew Dunn Chief,
Environmental Bureau
Qffice ofthe Attorney General
188 West Randolph Street, 20th Floor
Chicago, illinois 60601-3218
Albert Ettinger
Environmental Law andPolicy Center
35 East Wacker Drive, Suite 1300
Chicago, illinois 60601-2110
Lisa Frede
ChemicalIndustry Council
9801 West Higgins Road, Suite 515
Rosemont, Illinois 60018
James T. Harrington
Ross & Hardies
150 North Michigan, Suite 2500
Chicago, illinois 60601
Roy Harsch
Gardner, Carton andDouglas
321 North Clark Street, Suite 2400
Chicago, illinois 60610-4795
Ron Hill
MetropolitanWater Reclamation District of Chicago
600 iWest Pershing Road
Cicero, illinois 60804-4112
Katherine Hodge
Hodge, Dwyer and Zeman
3150 Roland Avenue, Post Office Box 5776
Springfield, illinois 62705-5776
Robert Lawley
Chief Legal Counsel
illinois Department of Natural Resources
524 South Second Street
Springfield, illinois 62701
RobertMessina
illinois Enviommental RegulatoryGroup
215
East Adams Street
Springfield, illinois 62701
Tom Muth
Fox Metro Walter Reclamation District
682 State Route 31
Oswego, illinois 60543
Irwin Polls
Metropolitan Water Reclamation District of Chicago
600 lWest Pershing Road
Cicero, illinois 60804-4112
Page 1 of 1