IN THE MATTER OF
WATER QUALITY AMENDMENTS TO
35 Iii. Adm. Code 302.208(e)-(g),
302.504(a)
302.575(d), 303 .444, 309.141(h); and
PROPOSED 35 Iii. Adm. Code 301.267,
301.313, 301.413, 304.120, and
309.157
NOTICE OF FILING
PLEASE TAKE NOTICE that on this date, September 6, 2002, I filed with Dorothy
Gunn, Clerk ofthe Illinois Pollution Control Board, James R. Thompson Center, 100 West
Randolph, Suite 11-500, Chicago, IL 60601, the enclosed Post-Second Hearing Comments of
Environmental Law and Policy Center, Prairie Rivers Network and Sierra Club.
Albert F. Etting
Albert F. Ettinger
Environmental Law and Policy Center
35 East Wacker Drive, Suite 1300
Chicago, IL 60601
(312) 795-3707
BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
rc~
~.
~tiU2
)
~
r~
)
)
~‘
)
R02-11
)
(Rulemaking-Water)
BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
it ~
L.PP~”~
IN THE MATTEROF:
)
SE!)
2UIJ~
)
WATER QUALITY AMENDMENTS TO
)
~
35 Ill. Adm Code 302.208(e)-(g), 302.504(a),
)
R02-1 1
~°~‘~/
~
302.575(d),
303.444, 309.141(h); and
)
(Rulemaking-Water)
PROPOSED 35 Iii. Adm. Code 301.367,
)
-
301.313, 301.413, 304.120, and 309.157
)
POST-
SECOND HEARING COMMENTS
OF
ENVIRONMENTAL
LAW
AND
POLICY
CENTER.
PRAIRIE
RIVERS
NETWORK AND SIERRA
CLUB
The remaining issues in this proceeding are narrow, but important. On those issues, two
conclusions emerge from the record.
First, the Board should allow the Illinois Environmental Protection Agency’s proposal to
weaken the cyanide standards to rest in peace. There is no scientific basis for weakening the
standard and no good reason to change Illinois’ cyanide standard at this time.
Second, JEPA’s effort to have the Board ratify the Agency’s 1980s decision to allow more
deoxygenating wastes into Illinois waters than the regulations allow should not be adopted as
proposed. This was clear before the July
25,
2002 hearing. It became even more clear after July 25
when IEPA released reports indicating that large numbers ofIllinois waters are impaired by
discharges of oxygen demanding waste by municipal wastewater plants and other point sources.
The Board should not adopt changes regarding deoxygenating wastes effluent limits without
taking action to assure that any change does not exacerbate Illinois’ serious problem with violations
ofthe dissolved oxygen standard (35 Ill. Adm. Code 302.206) and the standard against offensive
conditions (35 Ill. Adm. Code 302.203).
II.
There is
no valid reason to weaken Illinois’ cyanide standard
During the July 25 hearing, IEPAreconfirmed that no testing has been done that indicates
the effect ofcyanide on Illinois endangered mussels. Further, no testing has been done that would
support the conclusion that two Illinois endangered fish are not at least as sensitive to cyanide as
salmonid species.
1
There has been no testing on any species ofthe same genus as the Blackchin
Shiner and Iowa darter. (Mosher Testimony, July 25 Tr. 27, 30)
1
The pollution sensitive species that IEPA eliminated from the U.S. EPA cyanide criteria calculation to propose its
weaker standard may well serve as proxies for a number of sensitive species that live in Illinois. Indeed, studies cited
by the United States Fish & Wildlife Service indicate that mussels are more sensitive to some pollutants than the
species IEPA decided did not need to be protectedbecause they do not live in Illinois.
1
In its testimony at the July 25 hearing, the Agency offers two newjustifications for its
cyanide proposal. Neither is persuasive.
The Agency Proposal has not been shown to be Conservative
First, in response to the fact that its proposed chronic standard is only a few parts per billion
under the level know to harm a highly valued Illinois species (the Bluegill), IEPA has claimed that
its standard is actually conservative. IEPA argues this is true because the laboratory toxicity tests,
on which the criterion was based, were performed using free cyanide but the proposed standard is
for weak acid dissociable cyanide (“WAD cyanide”), which measures some forms ofcyanide that
are not free.
However, IEPA admits that its standard does not measure total cyanide, which would
include forms ofcyanide compounds that are not measured by the tests for WAD cyanide. (Mosher
Testimony, July 25 Tr. 11) Further, during the hearing it was determined that little is known ofthe
toxicity of cyanide compounds, except that they are less toxic than free cyanide. (Mosher
Testimony, July 25 Tr. 11-12) The circumstances in which the cyanide compounds break down in
the environment to release free cyanide in Illinois waters are also unclear. The 1984 Criteria
document, on which Illinois EPA selectively bases its argument, points out a wide variety ofways
in which cyanide ions can be liberated from complexes by light, low pH or other factors. (pp.2-3)
In short, using WAD cyanide instead offree cyanide is conservative insofar as it includes
forms ofcyanide that are less toxic than the free cyanide that was used in the toxicity tests. Using
WAD cyanide is not conservative, but risky, in that it does not include forms ofcyanide that may
be toxic or that may release free cyanide under other environmental conditions. Whether on balance
using WAD cyanide is more conservative or less conservative is simply unknown.
The Testing Sensitivity Just~fIcationOfferedfor Weakening the Cyanide Standardfails
factually and logically.
IEPA claims that, even if no discharger in Illinois is having any real problemwith cyanide,
adoption of its proposal is neededbecause the WAD method for testing cyanide is not accurate
enough to test to the current 5.2 micrograms per liter standard.2 In its pre-filed testimony, IEPA
claimed there is no approved method that can reliably test at 5.2 micrograms.
However, as presented at the July 25 hearing, there is a US EPA approved method, OIA
—
1677, capable oftesting for cyanide well below the current standard.3 This method is approved for
NPDES permits. 64 Fed. Reg. 73414 (1999)
Predictably, Illinois EPA will now claim that although the U.S. EPA has approved a method
that would allow testing well the level ofthe current standard, Illinois dischargers should not be
2Unfo~ate1y IEPA has also apparently failed to get its facts straight on whether there is a cyanide problem in
Illinois. The Illinois Water Quality Report 2002, issued after the July
25
hearing by JEPA, shows 110 miles of Illinois
streams as impaired by cyanide. (Ex. A)
~
it does not appear that the WAD method used by Illinois EPA has been approved by U.S. EPA.
2
required to use the method because there are no laboratories available. However, there is a long list
of laboratories that can use the new method. (Ex. B)
The fact that there are not more such laboratories is undoubtedly due to the fact that Illinois
and certain other states do not require that OIA
-
1677 be used. Obviously, there will never be
much demand for more sensitive testing as long as states are willing to accept testing less likely to
reveal that a discharger has a problem.
Leaving aside the fact that IEPA is wrong in claiming that sufficiently sensitive testing
methods do not exist, the fact is that lack ofsufficiently sensitive testing is never a good reason for
adopting a weaker standard. IEPA admits as much by admitting that the mercury should not be
changed although IEPA (wrongly) believes that methods are not available to test down to the level
of sensitivity required to measure mercury at the human health standard level. (Mosher Testimony
July 25 Tr.32). See also 40 CFR Pt. 132 App. F Procedure 8 D. (pollutant minimization program
designed for situation where water quality based effluent limit is below the quantification level for
dischargers to the Great Lakes)
Moreover, IEPA’s claim that the cyanide standard should be weakened because sufficiently
sensitive testing methods are unavailable is somewhat disingenuous given that IEPA does not
require testing sufficiently sensitive to catch violations of the current standard.
Under the current
standard,
EPA does not ask dischargers to test more accurately that 10 microgram per liter.
(Mosher Testimony, July 25 Tr. 30) IEPA is asking the Board to change a standard so that TEPA
will not have to require testing to a level ofaccuracy that it already does not require.
Although IEPA should require testing of sufficient accuracy to determine if water quality
standards for mercury, cyanide and other pollutants are being violated when such accurate testing is
available, it does not do so.
III.
IEPA Must Do More to Prevent Violations ofDissolved Oxygen Standards
The Sierra Club,
Prairie
Rivers
Network
and the Environmental Law
and
Policy Center of
the Midwest (“Environmental Groups”)
have not objected to using
a CBOD5
test instead ofa
BOD5 test for determining
whether sewerage treatment plants are
meeting the secondary
treatment
requirements established by Congress decades ago. The federal rule that defines “secondary
treatment” for technology-based limit purposes states that 25 mgfL
CBOD5 may be substituted for
30 mg/L
BOD5, 40 CFR
§
133.102.
The problem is that IEPA’s proposal ratifies its 1980s decision to measure CBOD5 instead
ofBOD5 with regard to discharges covered by 35 Ill. Adm. Code 304.120(b) and (c). These
provisions are the only mechanisms Illinois has established to protect Illinois waters in the situation
in which secondary treatment is not adequate to protect water quality because the effluent makes up
a large percentage ofthe flow or there are multiple pollutionsources. Because by definition
3
CBOD5 is less than all ofthe BOD5, IEPA’s proposal has the effect ofallowing more
deoxygenatingUnder
thewastesCleantoWaterbe
dischargedAct,
dischargesthan
is authorizedofpollutantsunderto
thethenation’scurrentwatersBoard wererules.4to be
eliminated 15 years ago through a progressive tightening’ of effluent limits as technology improved.
33 USC
§
1251(a)(1);
Adler, R.W, Landman, J.C. and Cameron, D.M., The Clean Water Act 20
Year Later, Island Press (1993) p. 137. Now, however, we see the IEPA seeking to further loosen
effluent limits from what was set by the Board almost 30 years ago
(5
mg/L BOD5). From the level
ofsupport for the IEPA’s proposal from the representatives ofpoint source polluters, one must
assume that the sewerage treatment industry has failed to make any technological advances in the
treatment ofdeoxygenating waste in the last three decades.
That JEPA and sewerage treatment agencies are now asking forhigher effluent limits than
the Board established in 1973 is not merely a sad reflection on the industry. The failure to control
deoxygenating waste has had serious impacts on Illinois waters.
A.
Illinois waters now suffer from discharges ofdeoxygenating waste.
This is not the time to loosen controls on discharges of deoxygenating wastes but instead to
find better ways to control these discharges. After the July 25 hearing, Illinois EPA released two
reports indicating that discharges from point sources are causing violations ofIllinois dissolved
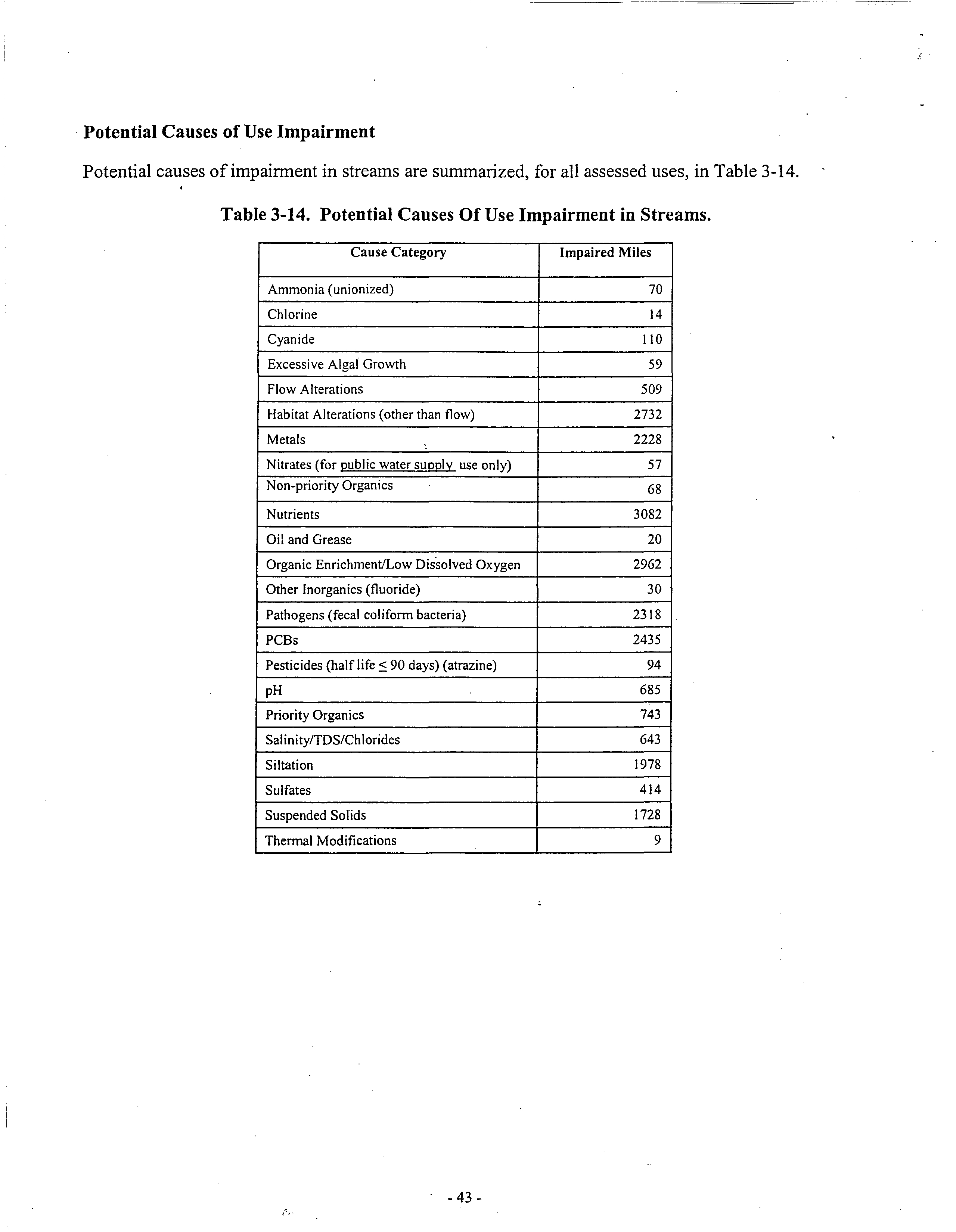
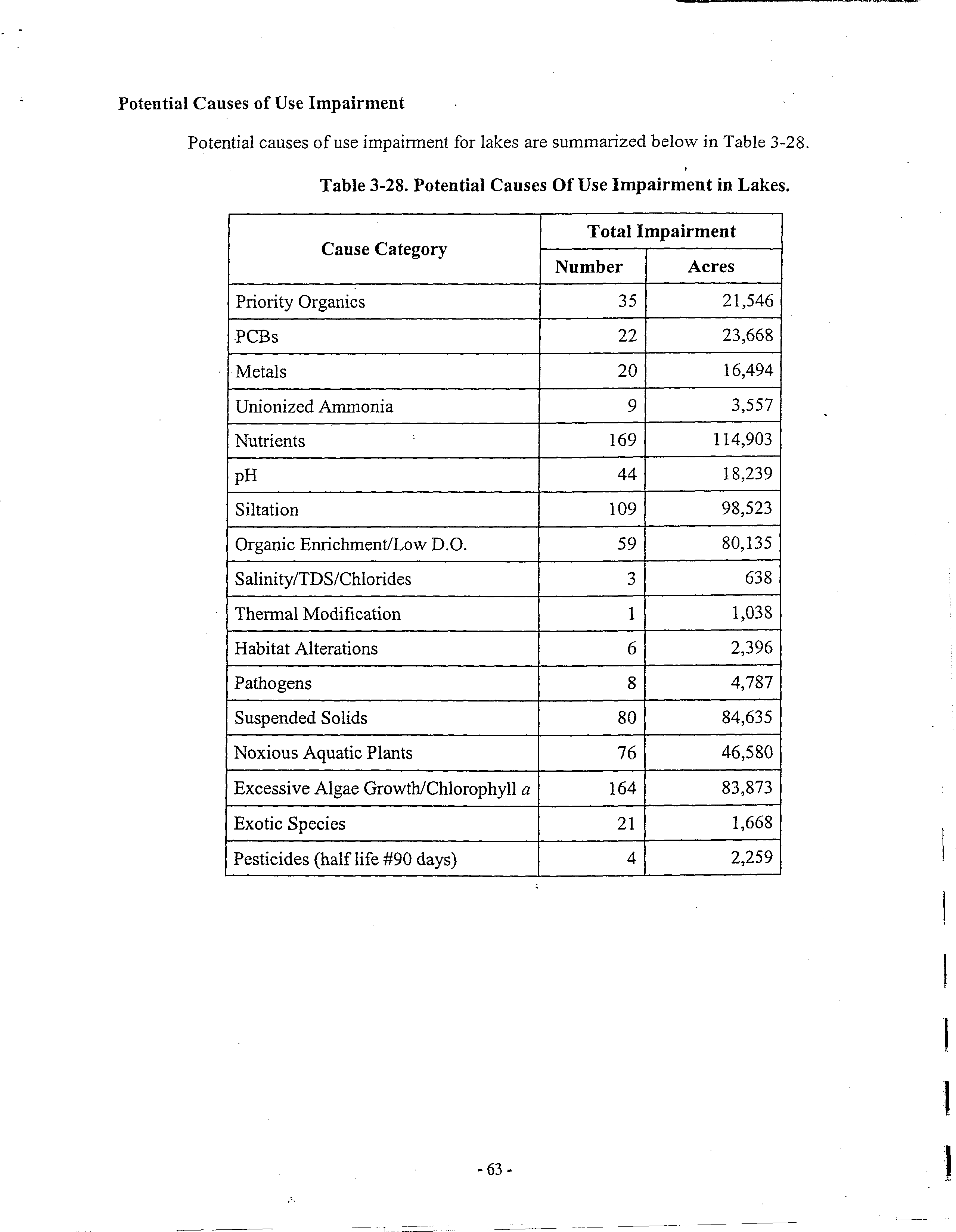
oxygen standards. The Illinois Water Quality Report 2002 states that 2962 miles ofthe 15,993
miles ofstreams assessed are impaired potentially because of“Organic Enrichment! LowDissolved
Oxygen” and 80,135 acres ofIllinois lakes are potentially impaired by this cause. (Ex A)5 Although
other sources ofpollution certainly contributed to this problem, the Illinois 303(d) list ofimpaired
waters (htt~://www.epa.state.i1.us/water/watershed/reports/303d-report/2oo2-
report / 303 d
-
report —2002
.
pdf),
also released after the July 25 hearing, plainly indicates that
industrial point sources (Code 100 in source column) and municipal point sources (Code 200) are
playing a role in the low dissolved oxygen impairment ofmanywaters across Illinois including
Maucopin Creek, Lake Springfield, the Des Plaines River, the Fox River, Salt Creek (Du Page), the
East
Branch ofthe Du Page River,
Marion Lake, the Big Muddy River, the Sangamon River, Rend
Lake, Cedar Creek, and Addison Creek. 81 miles ofthe Illinois River are listed as potentially
impaired by “Organic Enrichment/Low Dissolved Oxygen” with the source ofthis pollution listed
as “unknown.” (p.33). Given the level ofmunicipal discharge to the Illinois River, point sources
certainly constitute at least some part ofthe unknown.
The above figures on dissolved oxygen violations understate the problem. Only 18 of
Illinois waters have been monitored. Further, as we have been informed by IEPA, its monitoring
network was established to avoid taking samples near known point sources.
6
~Adoption of the Illinois EPA proposal will not actually loosen the effluent limits as to municipal discharges as the
Agency did that by itself without Board approval in the 1980s.
~The Illinois Water Quality Report 2002 also has separate listings for waters potentially impaired by nutrients. 3082
miles of Illinois streams and 114,903 acres ofIllinois lakes are potentially impaired by these inadequately regulated
pollutants. (Ex. A) Nutrients not only cause violations of the dissolved oxygen standard, but cause algal blooms that
violate Illinois standards against offensive conditions.
6
Naturally, it is not known how many of the impairments listed in Illinois Water Quality Report would be present if
the dissolved oxygen standards were weakened in the manner that the Illinois Association of Wastewater Agencies has
4
B.
Illinois can practically do more to protect its waters against discharges of
deoxygenating wastes.
That so many Illinois waters are impaired at least in part by regulated point sources is not
the result ofuncontrollable forces. Although there is a sizable contribution to BOD from
nitrogenous compounds, EPA admits that it essentially never regulates ammonia discharges to
prevent violations of dissolved oxygen standards. (Mosher Testimony, March 6, 2002, Tr. 34).
Further, Illinois has not performed modeling or taken other steps to assure that authorized
discharges do not cause or contribute to violations ofdissolved oxygen standards for decades.
(Frevert Testimony July 25 Tr.75-6) Other states do so. As explained by the Michigan Department
ofEnvironmental Quality:
Typically, CBOD5 limits are placed in NPDES permits for all facilities which have
the potential to contribute significant quantities ofoxygen consuming substances to
waters ofthe state. These limits are developed in direct correlation with limits for
ammonia nitrogen and dissolved oxygen.
In determining CBOD5 limits, stream modelers use computer models which
simulate actual stream conditions. Model inputs include the flow ofthe receiving
stream, the quantity of water to be discharged, the decay rate forthe particular type
ofwastewater, the stream’s slope, and temperature. Other upstream or downstream
dischargers are also considered in the model. The modeler determines maximum
limits for CBOD5 and ammonia nitrogen and minimum limits for dissolved oxygen.
These limits are selected to insure that Water Quality Standards for dissolved
oxygenThe
onlyarestepsmetthatin
theIllinoisreceivingtakeswater.to
protect(Ex.C)against7
violations of dissolved oxygen standards
are the provisions ofSection 304.120 (b), which requires effluent limits of20 mg/L BOD5 if a
discharger has an untreated waste load of 10,000 population units or more, and Section 304.120(c),
which generally requires limits of 10 mg/L BOD5 if the dilution ratio is less than five to one. The
Environmental Groups continue to believe that these provisions should not be weakened.
What is really needed are rules that require use ofmodeling or other means to assure that
permits are not issued that allow discharges that cause or contribute to violations ofdissolved
oxygen standards. At a minimum, if “CBOD5” is substituted for
“BOD5”,
adjustments should be
made that recognize that CBOD5 does not make up all ofBOD5.
suggested. However, until the IAWA makes a proposal for new standards and proves their scientificvalidity, the
Board certainly cannot make decisions based on the standards that IAWA wishes existed.
~ It is claimed that Illinois now approaches this problem through use of total maximum daily load studies, but IEPA
has never completed a TMDL. (Frevert Testimony July 25 Tr.76) Even ifIEPA actually did TMDLs, this would not be
an acceptable approach because TMDLs are never attempted in Illinois until an impairment is found. Perhaps naively,
we would like to prevent impairments from occurring in the first place.
5
This is particularly the case as to Section 304.120(b). The Board on numerous occasions has
recognized that CBOD5 limits well below 20 mg/L are readily attainable by almost all dischargers.
See Post Hearing Comments ofELPC, PRN and Sierra Club, filed 4-12-02, p. 8 fn. 12.
At the July
25,
2002 hearing, Mr. Callahan, spokesman for the Illinois Association of
Wastewater Agencies, addressed his testimony made in the March hearing that effluents at the 10
mg/L CBOD5 level are “readily attainable
...
with moderately appropriate user fees and citizens
tax rates” (March Tr. 131), and implied that he had somehow been misquoted. Mr. Callahan
protested that “I am not on the record intentionally ofindicating that a secondary treatment process
can consistently produce a 10 milligram per liter BOD.” (Callahan testimony, July 25 Tr. 43).
Mr. Callahan is correct that he is not on the record as saying a secondary treatment plant can
meet a 10 mg/L BOD standard but then no one said he did. For purposes ofthis proceeding, no one
should care if he ever said that or not. The critical question for the Illinois environment is what can
be done at an economically reasonable expense to address Illinois’s dissolved oxygen problems
(and offensive conditions, as defined by 35 Ill. Adm.302.203, caused by point source discharges).
On that question, Mr. Callahan’s March testimony and numerous other authorities agree that there
is no reason to allow effluents as bad a 20 mg/L CBOD5 if there is any risk that the discharge will
cause or contribute to violations ofIllinois water quality standards. An effluent of 10 mg/L
CBOD5 is readily attainment at a reasonable cost.
CONCLUSION
Consideration ofchanges to Illinois cyanide standard should be left until such time as there
is more information on the potential effects of cyanide on rare Illinois mussels and other
endangered species.
IEPA’s proposal regarding effluent limits fordeoxygenating wastes should be adopted as to
technology-based requirement of35 Ill. Adm. Code 304.120(a). However, the Board should take
action to assure that changes to Illinois regulations are not made that will increase the impairments
to Illinois waters caused by discharges ofdeoxygenating wastes. EPA should be required to use
modeling or other means to analyze the total effect ofdischarges on dissolved oxygen levels.
Alternatively, as has been proposed by the Environmental Groups in the past, the Board should
moderate the effect ofEPA’s proposal by recognizing that CBOD5 BOD5. Lower CBOD5
figures should be used when substituting for BOD5.
Albert F. Ettinger (ARM~C# 3125045)
Counselfor Environmental Law & Policy
Center, Prairie Rivers Network and Sierra Club
6
6
Illinois
Environmental
Protection Agency
m~
AfROW/02-1106
Bureau ofWater
P.O. Box 19276
Springfield, IL 62794-9276
July2002
Illinois Water Quality Report
2002
Back to top
Iffinois Environmental Protection Agency
Back to top
Bureau of Water
EXHIBIT A
~Potential Causes of Use Impairment
Potential causes ofimpairment in streams are summarized, for all assessed uses, in Table 3-14.
Table 3-14. Potential Causes Of Use Impairment in Streams.
Cause Category
Impaired Miles
Ammonia (unionized)
70
Chlorine
14
Cyanide
110
Excessive Algal Growth
59
Flow Alterations
509
Habitat Alterations (other than flow)
2732
Metals
2228
Nitrates (for public water supply use only)
57
Non-priority Organics
‘
68
Nutrients
3082
Oil and Grease
20
Organic Enrichment/Low Dissolved Oxygen
2962
Other Inorganics (fluoride)
30
Pathogens (fecal coliform bacteria)
2318
PCBs
2435
Pesticides (half life ~ 90 days) (atrazine)
94
pH
‘
685
Priority Organics
743
Salinity/TDS/Chlorides
643
Siltation
1978
Sulfates
414
Suspended Solids
1728
Thermal Modifications
9
-43-
Potential Causes of Use Impairment
Potential causes ofuse impairment for lakes are summarized below in Table 3-28.
Table 3-28. Potential Causes Of
Use Impairment in Lakes.
Cause Category
Total Impairment
Number
Acres
Priority Organics
35
21,546
•PCBs
22
23,668
Metals
20
16,494
Unionized Ammonia
9
3,557
Nutrients
169
114,903
pH
44
18,239
Siltation
109
98,523
Organic Enrichment/Low D.O.
59
80,135
Salinity/TDS/Chlorides
3
638
Thermal Modification
1
1,038
Habitat Alterations
6
2,396
Pathogens
8
4,787
Suspended Solids
80
84,635
Noxious Aquatic Plants
76
46,580
Excessive Algae GrowthlChlorophyll
a
164
83,873
Exotic Species
21
1,668
Pesticides (half life ~90 days)
4
2,259
1
-
63
-
I
AUG ~1 ‘~217:31 FR SCC—SAMFLE CONTROL CE 7034618056 TO 13127953730
P. 02/03
Laboratories Capable
of
Perforrnin~tMethod 1677 Analysis
These laboratories are identified below for informational purposes only. This does
not
representan
exhaustive list, nor does it constitute endorsement by either EPA
or
DynCorp ofany laboratory appearing
on the list.
Analytical Services Laboratory, Inc.
1988
Triumph St.
Vancouver,
B.C.,
Canada VSL1KS
Contact: Blair Easton
Phone: (604) 253-4188
Bayer Environmental Testing Services
StateRoute 2 North,
P.O. Box
500
New Martinsville, WV
26155
Contact: John Sebroskie
Phone: (304) 455-4400
Fax: (304)
455-5134
DegussaCorporation
4
Pearl Ct.
Allendale, NJ 07401
Contact: JagChattopadhyay
Phone: (201) 818-3700
DeltaFaucetCompany
Chemistry Laboratory
Address Unknown
Contact: Mike Mosely
Phone: (812) 663-4433
Frontier (3eoscienccs.
414 Pontius North, SuiteB
Seattle,
WA 98109
Contact: Nicolas Bloom
Phone: (206) 622-6960
Fax: (206)
622-6870
Email: nicolasb@frontier.wa.com
Golden SunlightMines
453 MT Hwy. 2 East
Whitehall, MT
59759
Contact:
Neil Gallagher
Phone: (406)287-3257
Newmont Exploration Limited
Metallurgical Services
412
Wakara
Way, Suite 210
Salt Lake City,UT 84108
Contact: Tom Patten
EXHIBIT B
AUG 01 ‘02 17:31 FR SCC—SAMPLE CONTROL CE 7034618056 TO 13127953730
P.03/03
Phone: (801) 583-8974
01 Analytical
1556 Spring Street
Saint Helena, CA 94574
Contact:
Mike
Straka
Phone: (707)963-1069
Fax: (707) 963-3335
Email: mstraka~’oico.com
University ofNebraska
Department of Chemical Engineering
Beadle Center
Lincoln, NE 68588-0668
Contact: Dr. Ljiljana Solujic
Phone: (402)472-4784
University of Nevada
-
Reno
Department ofChemical
and
Metallurgical Engineering
Mackay School of
Mines/MS
168
Reno, NV 89557
No contact
name
or phone
number
(previous contact is Dr. Solujic atUniversity ofNebraska)
**
TOTAL PAGE.03
**
Biochemical Oxygen Demand
Page 1 of3
State of Michigan_____
D~U
=
F
—
Department of EnvironmentalQuality
Surface Water Quality Division
Permits Section
Biochemical Oxygen Demand (BOD)
Biochemical Oxygen Demand, or BOD, is a measure of the quantity of
oxygen consumed by
microorganisms during the decomposition of organic matter. BOD is the most commonly used
parameter for determining the oxygen demand on the receiving water of a municipal or
industrial discharge. BOD can also be used to ~valuate the efficiency of treatment processes,
and is an indirect measure of biodegradable organic compounds in water.
Imagine a leaf falling into a stream. The leaf, which is composed of organic matter, is readily
degraded by a variety of microorganisms inhabiting the stream. Aerobic (oxygen requiring)
bacteria and fungi use oxygen as they break down the components of the leaf into simpler, more
stable end products such as carbon dioxide, water, phosphate and nitrate. As oxygen is
consumed by the organisms, the level of dissolved oxygen in the stream begins to decrease
Water can hold only a limited supply of dissolved oxygen and it comes from only two sources-
diffusion from the atmosphere at the air/water interface, and as a byproduct of photosynthesis.
Photosynthetic organisms, such as plants and algae, produce oxygen when there is a sufficient
light source. During times of insufficient light, these same organisms consume oxygen. These
organisms are responsible for the diurnal (daily) cycle of dissolved oxygen levels in lakes and
streams.
If elevated levels of BOD lower the concentration of dissolved oxygen in a water body, there is a
potential for profound effects on the water body itself, and the resident aquatic life. When the
dissolved oxygen concentration falls below 5 milligrams per liter (mg/I), species intolerant of low
oxygen levels become stressed. The lower the oxygen concentration, the greater the stress.
Eventually, species sensitive to low dissolved oxygen levels are replaced by species that are
more tolerant of adverse conditions, significantly reducing the diversity of aquatic life in a given
body of water. If dissolved oxygen levels fall below 2 mg/I for more than even a few hours, fish
kills can result. At levels below 1 mg/I, anaerobic bacteria (which live in habitats devoid of
oxygen) replace the aerobic bacteria. As the anaerobic bacteria break down organic matter, foul-
smelling hydrogen sulfide can be produced.
BOD is typically divided into two parts- carbonaceous oxygen demand and nitrogenous oxygen
demand. Carbonaceous biochemical oxygen demand (CBOD) is the result of the breakdown of
organic molecules such a cellulose and sugars into carbon dioxide and water. Nitrogenous
oxygen demand is the result of the breakdown of proteins. Proteins contain sugars linked to
nitrogen. After the nitrogen is “broken off” a sugar molecule, it is usually in the form of
ammonia, which is readily converted to nitrate in the environment. The conversion of ammonia
liup
::
\\
\V\VdC~1.St~tlt..flii.USi’S\\ q’penhl
its/1)aralllCtCrs/bod.htlll
EXHIBIT C
2/19/02
Biochemical Oxygen Demand
Page 2 of
3
to nitrate requires more than four times the amount of oxygen as the conversion of an equal
amount of sugar to carbon dioxide and water.
When nutrients such as nitrate and phosphate are released into the water, growth of aquatic
plants is stimulated. Eventually, the increase in plant growth leads~toan increase in plant decay
and a greater “swing” in the diurnal dissolved oxygen level. The result is an increase in microbial
populations, higher levels of BOD, and increased oxygen demand from the photosynthetic
organisms during the dark hours. This results in a reduction in dissolved oxygen concentrations,
especially during the early morning hours just before dawn.
In addition to natural sources of BOD, such as leaf fall from vegetation near the water’s edge,
aquatic plants, and drainage from organically rich areas like swamps and bogs, there are also
anthropogenic (human) sources of organic matter. If these sources have identifiable points of
discharge, they are called point sources. The major point sources, which may contribute high
levels of BOD, include wastewater treatment facilities, pulp and paper mills, and meat and food
processing plants.
Organic matter also comes from sources that are not easily identifiable, known as nonpoint
sources. Typical nonpoint sources include agricultural runoff, urban runoff, and livestock
operations. Both point and nonpoint sources can contribute significantly to the oxygen demand
in a lake or stream if not properly regulated and controlled.
Performing the test for BOD requires significant time and commitment for preparation and
analysis. The entire process requires five days, with data collection and evaluation occurring on
the last day. Samples are initially seeded with microorganisms and saturated with oxygen
(Some samples, such as those from sanitary wastewater treatment plants, contain natural
populations of microorganisms and do not need to be seeded.). The sample is placed in an
environment suitable for bacterial growth (an incubator at
200
Celsius with no light source to
eliminate the possibility of photosynthesis). Conditions are designed so that oxygen will be
consumed by the microorganisms. Quality controls, standards and dilutions are also run to test
for accuracy and precision. The difference in initial DO readings (prior to incubation) and final
DO readings (after 5 days of incubation) is used to determine the initial BOD concentration of
the sample. This is referred to as a BOD5 measurement. Similarly, carbonaceous biochemical
oxygen test performed using a 5-day incubation is referred to as a CBOD5 test.
Water Quality Standards for BOD
Although there are no Michigan Water Quality Standards pertaining directly to BaD, effluent
limitations for BOD must be restrictive enough to insure that the receiving water will meet
Michigan Water Quality Standards for dissolved oxygen.
Rule 64 of the Michigan Water Quality Standards (Part 4 of Act 451) includes minimum
concentrations of dissolved oxygen that must be met in surface waters of the state. This rule
states that surface waters designated as coldwater fisheries must meet a minimum dissolved
oxygen standard of 7 mg/I, while surface waters protected for warmwater fish and aquatic life
must meet a minimum dissolved oxygen standard of 5 mg/I.
Biochemical Oxygen Demand Limitations in NPDES Permits
Typically, CBOD5 limits are placed in NPDES permits for all facilities which have the potential to
contribute significant quantities of oxygen consuming substances to waters of the state. These
limits are developed in direct correlation with limits for ammonia nitrogen and dissolved oxygen.
The nitrogenous oxygen demand is computed separately because of the difference in oxygen
demand (as explained above) and because the rate of oxygen consumption over time varies
hip \\ ~ \\
.dL’q.st~iIc.Ih1I.us.swqiperniits/puranic1ers/bod.ht~n
2/19/02
Biochemical Oxygen Demand
Page 3 of3
from carbonaceous oxygen demand. Ammonia is further considered separately because in
sufficient levels (dependant upon several variables) it can also be toxic to living organisms.
In determining CBOD5 limits, stream modelers use computer models which simulate actual
stream conditions. Model inputs include the flow of the receiving stream, the quantity of water
to be discharged, the decay rate for the particular type of wastewater, the stream’s slope, and
temperature. Other upstream or downstream dischargers are also considered in the model. The
modeler determines maximum limits for CBOD5 and ammonia nitrogen and minimum limits for
dissolved oxygen. These limits are selected to insure that Water Quality Standards for dissolved
oxygen are met in the receiving water.
Permit-related questions and comments? Contact Fred Cowles, cowlesf(~michigan.gov
Web page maintained by Sean Syts, sytss(ä~michician.gov
Last revision: April 30, 2001
http ://www.deq .state.mi. us/swq/permits/parameters/bod . html
11 ~ w~
Idea I
I
~
I
Home
littp ://www.deq state. ml.us/swq/pcrm I is/parameters/bod. htm
2/19/02
CERTIFICATE OF SERVICE
I, Albert F. Ettinger, certify that I have filed the above Notice ofFiling together with an
original and 9 copies ofthe Post-Second Hearing Comments of Environmental Law and Policy
Center, Prairie Rivers Network and Sierra Club, on recycled paper, with the Illinois Pollution
Control Board, James R. Thompson Center, 100 West Randolph, Suite 11-500, Chicago, IL
60601, and served all the parties on the attached Service List by depositing a copy in a properly
addressed, sealed envelope with the U.S. Post Office, Chicago, Illinois, with proper postage
prepaid on September 6, 2002.
Albert F. Ettinler
Albert F. Ettinger, Senior Attorney
Environmental Law and Policy Center
35
East Wacker Drive, Suite 1300
Chicago, IL 60601
SERVICE LIST
Mike Callahan
Bloomington Normal Water Reclamation District
P.O. Box 3307
Bloomington, IL 61702
Larry Cox
Downers Grove Sanitary District
2710 Curtiss Street
Downers Grove, IL 60515
Dennis Daffield
Department ofPublic Works
City of Joliet
921 East Washington Street
Joliet, IL 60433
Lisa M. Frede
Chemical Industry Council
9801 West Higgins Road
Suite
515
Rosemont, IL 60018
James T. Harrington
Ross
& Hardies
150 North Michigan, Suite 2500
Chicago,
IL 60601
Roy M. Harsch
Gardner, Carton & Douglas
321 N Clark Street
—
Suite 3400
Chicago,
IL 60610
Ron Hill
Metropolitan Water Reclamation District
100 East Erie
Chicago, IL 60611
Katherine Hodge
Hodge Dwyer Zeman
3150 Roland Avenue
P.O. Box 5776
Springfield, IL 62705-5776
Margaret P. Howard
Hedinger & Howard
1225 South Sixth Street
Springfield, IL 62703
Robert A. Messina
Illinois Environmental Regulatory Group
215 East Adams Street
Springfield, IL 62701
Tom Muth
Fox Metro Water Reclamation District
682 State Route 31
Oswego, IL 60543
Irwin Polls
Metropolitan Water Reclamation District ofChicago
6001 West
Cicero, IL 60804
Sanjay Sofat
Illinois Environmental Protection Agency
1021 North Grand Avenue East
Springfield, IL 62794-9276
Marie Tipsord
Attorney,
Pollution Control Board
100 West Randolph Street, 11-500
Chicago, IL 60601k