IN THE MATTER OF:
WATER QUALITY AMENDMENTS TO
35
III. Adm. Code 302.208(e)-(g),
302.504(a),
302.575(d), 303.444, 309.141(h);
and
PROPOSED 35
III. Adm. Code 301.267,
301.313,
301.413, 304.120, and 309.157
R02-11
(Rulemaking
-
Water)
CL~K’~
~PPfe~
Ji~N
22
20U2
STATE OP
IWNOJS
Pollution
Control
Board
NOTICE OF FILING
Dorothy Gunn,
Clerk
Pollution Control Board
100 West Randolph Street
Suite 11-500
Chicago,
Illinois 60601
Mathew Dunn
Illinois Attorney General’s Office
Environmental
Control Division
James
R. Thompson Center
100 West Randolph Street
Chicago,
Illinois
60601
Marie
E. Tipsord
Illinois Pollution Control Board
James
R. Thompson Center
100 West Randolph Street, Suite 11-500
Chicago,
Illinois 60601
Legal Service
Illinois Department of Natural Resources
524 South Second Street
Springfield,
Illinois 62701-1787
PLEASE
TAKE NOTICE that
I
have today filed with the Office of the
Clerk of the
Pollution
Control
Board the
WRITTEN TESTIMONY OF ROBERT MOSHER, CLARK OLSON, AND
ALAN
KELLER
of the
Illinois Environmental Protection Agency, a
copy of which is herewith
served upon you.
ILLINOIS ENVIRONMENTAL PROTECTION AGENCY
Assistant Counsel
Division of Legal Counsel
Dated:
January
18,
2002
Illinois
Environmental Protection Agency
1021 North Grand Avenue East
Springfield,
Illinois 62794-9276
(217) 782-5544
BEFORE THE
ILLINOIS ~
BOARD
)
)
)
)
)
THIS FILING PRINTED ON RECYCLED PAPER
RECEIVED
CLERR’S
OFF’~
BEFORE THE ILLINOIS POLLUTION CONTROL BOARD
J~N
2 22002
STATE OF
ILLINOIS
Pollution
Control Board
IN
THE MATTER OF:
WATER QUALITY AMENDMENTS TO
)
35
III. Adm. Code 302.208(e)-(g),
302.504(a),
)
R02-1
1
302.575(d), 303.444, 309.141(h); and
)
(Rulemaking -Water)
PROPOSED 35
Ill. Adm.
Code 301.267,
)
301 .31 3, 301.413, 304.120,
and 309.157
)
TESTIMONY OF ROBERT MOSHER
QUALIFICATIONS/INTRODUCTION
My name is Robert Mosher and
I am the Manager of the Water Quality Standards
Section within the Division of Water Pollution Control at the
Illinois Environmental
Protection Agency (“Illinois EPA” or “IEPA”).
I have been with
the
Illinois
EPA in excess of
16 years.
Almost all of that time has been spent in my current capacity where my primary
responsibility is the development and implementation of water quality standards.
I
have a
Masters Degree in Zoology from
Eastern Illinois
University where
I specialized
in stream
ecology.
My testimony will cover three topics.
First,
I will
discuss the background
information concerning development of the instant proposal before the
Illinois
Pollution
Control Board (“IPCB” or “Board”).
Second,
I will provide a brief discussion on the
concepts
contained in various sections of the
Illinois EPA’s proposal.
Third,
I will discuss
the
Illinois EPA’s plans for successful implementation of this proposal.
BACKGROUND
INFORMATION
The Federal Water Pollution Control Act Amendments of 1972, 33 U.S.C. §~1251-
1387,
is commonly known as the Clean WaterAct (“CWA”).
Pursuant to the CWA,
states
2
are required to revise and update their water quality standards to ensure thatthey are
protective of public health and welfare, enhance the quality of water and promote the
purposes of the CWA.
33 U.S.C. §1313(c)(2)(A).
The process of reviewing the state’s
standards is called the triennial water quality standards review.
The changes to the water
quality and effluent standards
in the instant proposal are one element of Illinois EPA’s
current triennial review of water quality standards.
In September 2000, the Agency shared a
packet of information concerning this
rulemaking with a number of stakeholders involved
in water quality standards
affairs.
These entities included municipal and industrial
dischargers, environmentalists and other
governmental agencies.
A few helpful comments were received and were employed to
clarify the intent of this proposal.
There were
no adverse comments, and generally
speaking, the changes to the
Board regulations that encompass this proposal should- not
be controversial since they represent the current state-of-the-art
in water quality standards.
The CLI rulemaking (R97-25) introduced
Illinois stakeholders to several ofthe
concepts
leading to the new and revised standards for the General Use waters proposed
here.
The
instant rulemaking is the result of careful consideration regarding the appropriateness of
selected aspects of the GLI for General
Use waters of the state.
ILLINOIS
EPA’S PROPOSAL
This proposal is divided into five
parts.
Part I proposes adoption of new aquatic life
acute and chronic water quality standards for benzene, ethyl benzene, toluene, and
xylene(s) (“BETX”) for both General
Use waters and the
Lake
Michigan
Basin.
Part II
contains revised acute and chronic water quality standards for zinc, nickel, and weak acid
dissociable cyanide.
Part III proposes that most General
Use metals water quality
3
standards
be specified
in terms of dissolved concentration rather the total concentration
used in
the existing standards.
Part IV contains corrections to the GLI regulations at 35
III.
Adm. Code 302.504(a), 302.575(d), and 309.141.
Part V proposes to update the Board
regulations at 304.120 to reflect that the carbonaceous component of BOD5 be regulated
in treated domestic waste effluents.
I will cover the first four Parts of the
Illinois
EPA’s
proposal and Al
Keller,
Manager of the Agency’s Northern Municipal Permit Unit will testify
to Part V of the proposal.
Part
I:
We intend for all the newly derived standards to either replace existing
General
Use standards or to be added as new listed substances under 35 IAC 302.208(e)
and
(f).
Each substance addressed has both an acute and
a chronic value
proposed.
The
regulatory constructs in 302.208 (a) through
(d) will
apply to newly added or revised
standards.
Several new STORET numbers are necessary because many metals
standards are now proposed to be
in the dissolved rather than total form.
Standards to
protect aquatic life for BETX substances will
also be inserted into the Lake
Michigan Basin
water quality standards where none now exist.
For the
Lake Michigan
Basin, these
standards will be based
on sensitive species from both cold and warm water.
Additionally,
benzene will have a General Use human health standard inserted at 302.208(f) identical to
the
Lake
Michigan
Basin human health standard that already exists.
Part II:
A goal of the triennial review of standards that led to this proposed
rulemaking before the
Board was to update general use water quality standards for toxic
metals found at 35 IAC 302.208(g).
These metals have “one
number” standards
adopted
in the
1970’s as opposed to
“two
number” acute and chronic standards that have been the
preferred method of adopting standards
for the
last
15 years or so.
Nickel and zinc fall
into this category.
Selenium and silver are also considered to be significantly toxic metals
4
and still exist as one number standards in 302.208(g).
New standards for selenium and
silver are not proposed at this time because debate is still ongoing about just how
standards for these metals should
be derived.
USEPA is pursuing these issues and when
a consensus is reached at the national level, IEPA will propose updated standards for
these metals.
National consensus had not been achieved
at the time the Agency filed
its
petition with the
IPCB.
Part III:
The national consensus indicates that the dissolved form of metals
is the
toxic component to aquatic organisms.
It is widely believed that filterable metals are likely
to be complexed with other water constituents and will
have little toxic influence.
For this
reason, GLI water quality standards
for metals were adopted in dissolved form and the
Agency’s petition in this matter lists metals water quality standards as dissolved metal.
Since most researchers
reported total metals when relating the concentrations that
organisms were exposed to in toxicity tests,
USEPA did some experimentation to
determine the percentage of these reported concentrations that was actually dissolved
metal.
The result of this endeavor was a table of metals conversion factors.
These were
published by USEPA under the GLI.
For example, if the final acute value for a given metal
in the total form
is 2.0 mg/L and the conversion factor is
0.8, as determined from
measuring total vs. dissolved metal under the conditions of laboratory toxicity tests, then
the dissolved metal final acute value
is 1.6
mg/L.
The proposed water quality standards
have been converted to dissolved metal concentrations through the
use of the stated
conversion factor.
The BETX substances have no such toxicity relationship between dissolved
and
suspended components.
The total form is presently considered to be that which should be
regulated.
Our proposal designates total BETX substances as the water quality
5
standards.
Federal
regulations at 40 CFR 122.45 require that NPDES permit limits for
metals
be established as total measurable metal.
When water quality based effluent limits
(WQBELs)
are required
in
a permit, this would mean converting the dissolved
metal water
quality standard value
into a total metal value.
A translator factor
is
used for this purpose
and,
in the absence of site-specific data concerning the
ratio of total to dissolved
metal,
consists simply of the
reciprocal of the conversion factor.
This means that if a mixing zone
is not involved
in a WQBEL, the total metal limit would
be what the water quality standard
would have been in the “total metal” form.
That is,
the differential between total and
dissolved
metals in the toxicity tests would not be factored out.
We have
included a site-
specific metals translator provision
in the
proposed
IPCB regulations.
This would allow
dischargers to measure the
ratio of dissolved to total metal
in their effluent and thereby
apply to the Agency for establishment of total metal WQBELs based on this effluent
specific relationship.
Effluents will therefore essentially be regulated
on their potential to
discharge dissolved metals at levels consistent with the water quality standards yet within
the
bounds of the -total metals effluent standards at 35
III. Adm. Code Part 304.
At this time recalculated standards are not being proposed for six metals, arsenic,
cadmium, copper,
lead, mercury and trivalent chromium, found at 35
Ill. Adm.
Code
302.208(e).
Lead and mercury standards
were updated in
1996, there has been no
indication that the arsenic, copper and trivalent chromium
standards are in
need of
revision and cadmium is currently under federal review.
However,
it is appropriate to
convert these standards to the dissolved form to conform to USEPA guidance.
This
simply involves the
application of the correct conversion factor.
The other substances in
302.208(e) are not amenable to regulation
in the dissolved form.
TRC (total residual
6
chlorine) is by nature an inclusive parameter.
Hexavalent
chromium standards
were
adopted as total metal
in the
Board’s CLI rulemaking.
It may be best to continue to
regulate this substance in the total metal form.
Part IV:
Additionally, we propose several corrections to recently adopted Board
regulations.
The GLI rulemaking intended to list metals standards
in the dissolved form.
The conversion factors that accomplish this were inadvertently left out, however.
We now
correct this mistake by inserting the proper conversion factors into 35
III. Adm. Code
302.504(a).
Section 302.575 was missing several pieces of essential information that we
also now correct.
35
III. Adm. Code 303.444
is a site-specific regulation that is no longer
pertinent given the changes to the General
Use cyanide standards and therefore we
propose that the
Board delete this regulation.
We are also proposing to replace language
at 35 III. Adm.
Code 309.141(h)(3) with
a more accurate instruction for implementing the
metals translator in NPDES permits.
ILLINOIS EPA’S FUTURE PLANS
The proposed changes to the standards give rise to several issues regarding the
implementation of water quality standards in NPDES permits and
in other Agency
programs.
The Illinois
EPA intends to provide the
Board a draft Agency rule for
implementing water quality based effluent limits at hearing under R02-1 1.
This rule will
later pass through the JCAR approval process before becoming finalized.
The Agency
rule will allow the
Board and stakeholders to envision how the new Board water quality
standards will
be implemented in the day-to-day activities of the Agency.
7
This concludes my pre-filed testimony.
I will
be supplementing this testimony as needed
during the hearing.
I would be happy to address any questions.
January 29, 2002
Illinois Environmental Protection Agency
1021 North Grand Avenue
East
P.O. Box 19276
Springfield,
Illinois 62794-9276
By:
Robert Mosher
THIS FILING PRINTED ON RECYCLED PAPER
8
CL!RK’~
OP~ICE
BEFORE THE
ILLINOIS POLLUTION CONTROL BOARD
JAN
2.2
2002
STAT~
OF ILLINOIS
PollutIon Control
Board
IN
THE MATTER OF:
WATER QUALITY AMENDMENTS TO
)
35
III. Adm. Code
302.208(e)-(g), 302.504(a),
)
R02-1 I
302.575(d),
303.444, 309.141(h); and
)
(Rulemaking
-
Water)
PROPOSED 35
III. Adm. Code 301 .267,
)
301.313, 301.413, 304.120, and 309.157
)
TESTIMONY OF CLARK OLSON
QUALIFICATIONS/INTRODUCTION
My name
is Clark Olson and
I have
been employed by the
Illinois
Environmental
Protection Agency (“Illinois EPA” or “Agency”) for over 20 years.
I work in the Water
Quality Standards Unit of the
Division of Water Pollution Control as a toxicologist.
I have
been involved with water quality standards
issues throughout my career with the Agency
and have participated
in several previous rulemakings of this type.
I
have
a
PhD in
Biology from
University of Miami (Florida) and have done postdoctoral research
in
toxicology at North Carolina State University.
My testimony will
discuss the development
process of the instant proposal before the
Illinois Pollution Control Board (“IPCB” or
“Board”).
DEVELOPMENT PROCESS
Early in the year 2000,
I began to gather toxicity data for the
instant proposal.
I
developed numeric values suitable for water quality standards for several substances
using USEPA sanctioned methods.
New aquatic life acute and chronic standards were
derived for benzene, ethyl benzene, toluene and xylenes (“BETX”) for both General
Use
9
and Lake Michigan
Basin waters and human health standards were developed for General
Use waters.
New
General Use aquatic life acute and chronic standards were derived for
zinc, nickel and weak acid dissociable cyanide. There are presently single number
standards for zinc and nickel for General
Use waters and current practice recommends
acute and chronic numbers.
In general,
I followed the procedure
laid down
by USEPA in the
Guidelines for
Deriving Numerical National Water Quality Criteria for the Protection ofAquatic Organisms
and Their Uses,
(“the Guidelines”)
1985 (NTIS PB85-227049) which have been followed
in
standards’ development by the USEPA and by other states.
These guidelines have also
been used as a basis of the procedures in
35 III. Adm.
Code Part 302 Subpart E and
Subpart F for deriving water quality criteria.
In the full
USEPA method, often
referred to as “Tier I”, the minimum database
consists of toxicity data for representatives of 8 (reduced to 5
in Subpart F) different
groups of animals.
A statistical procedure then finds the ~
percentile of the distribution of
the data.
That is,
95
of the organisms are considered less sensitive than the one(s) at
the 5th
percentile level. For the acute criterion, this number is divided by 2, and in the
chronic criterion
it is used as is.
However, the chronic criterion
is often derived
by using
an
acute to chronic ratio (“ACR”) obtained from data for several species when
adequate
chronic tests are not available for all the specified groups of organisms.
In the
proposed
standards
presented here, the quality of the databases available does
not always allow
use of the Tier
I procedure for all substances and so a default (“Tier
II”) procedure is used.
The Guidelines process involves several steps.
First, data for each substance was
obtained from the USEPA AQU IRE database and any other sources that were found
coincidentally.
USEPA Ambient Water Quality Criterion documents and Great Lakes
10
Water Quality Standards Initiative documents were also consulted for all substances.
Second, the data was tabulated as directed by the Guidelines.
Third,
much of the original
literature (mostly journal articles) where the original data was
presented was obtained from
our library or other libraries, so that the data could
be verified.
This was especially
necessary for the data for the most sensitive species since this data
is most important in
determining the actual value of the
criterion.
Fourth, statistical calculations were made by
use of a spreadsheet according to the equations
in the
Guidelines.
Finally,
documents
were prepared for each of the substances and are part of the
package submitted.
With the exception of the BETX parameters, the standards for the substances in
this rulemaking are to apply only to General
Use waters.
Therefore,
I used data from only
warm-water organisms in
the derivations for zinc, nickel and cyanide standards.
Trout,
salmon and other cold-water species were
included
in the development of the BETX
standards
for the
Lake Michigan
Basin,
but not for General Use waters,
because these
species do not occur in
Illinois waters outside of Lake Michigan.
Additionally, only species
with
reproducing wild populations in the
Midwest were
utilized
in the derivations.
Metals that have toxicity influenced by water hardness have standards expressed
as an equation containing a factor for slope for the hardness relationship.
Slope values for
nickel and
zinc in our proposed standards are the same values as found
in the
most
recent national criteria documents for GLI standards.
Given that all these substances had
a large database of toxicity test results when the national criteria were
published,
the
additional tests
I found should have very little
impact on the slope value and we therefore
saw
no need to change them.
Of all the substances considered
in this rulemaking, only benzene is believed to
have significant human health effects
—
cancer
-
such that a separate human health
11
standard
is necessary since such standards are lower than those necessary to protect
aquatic life.
I reported human health criteria for the other BETX substances under the
individual summaries for the purpose of demonstrating that these values are much higher
than
the standards
protective of aquatic life.
The metals likewise are not harmful to
humans at the concentrations regulated for aquatic life.
The Human health standard for
benzene is the same as the
Lake
Michigan standard
in 302.504(a).
There are currently acute and chronic General
Use standards under the weak acid
dissociable cyanide form.
The reason they are being readdressed stems from the fact that
they were taken directly from USEPA national criteria
document, which means that cold-
waterspecies such as trout and salmon were
used
in the criteria derivation.
Since
General
Use waters are virtually all warm water habitats, these standards have come
under scrutiny.
The Metropolitan Water Reclamation
District of Greater Chicago obtained
site-specific relief from the
IPCB several years ago for weak acid dissociable cyanide
based on the
premise that warm water species were not as sensitive.
The site-specific
standards they obtained are very similar to the values we propose.
The R88-21
rulemaking (“Toxics”) recognized that total cyanide was not
representative of the toxic component of this substance.
Total cyanide laboratory analysis
measures complexed forms of cyanide, such as some ofthe iron-cyanide compounds that
are known to be nontoxic.
Free cyanide
is a
rough equivalent of dissolved metals, but
unfortunately free cyanide is difficult to measure and other, weakly bound forms of cyanide
not measurable as free cyanide are probably also toxic.
A few analytical methods
measure forms of cyanide that are not all
inclusive as is total cyanide.
One of these, weak
acid dissociable cyanide, was chosen as the best available alternative.
A primary reason
for revising the cyanide standard
is because the original R88-21 two number cyanide
12
standard was derived using cold-water species.
New data from native warm water species
is considered
in this update because no search for new data has been conducted, to our
knowledge, since the early
1980s.
We are retaining weak acid dissociable cyanide as the
best available form to regulate.
This concludes my pre-filed testimony.
I will be supplementing this testimony as needed
during
the
hearing.
I would be happy to address
any questions.
January 29, 2002
By:
62el~4/C~
,S~
o~c2~~~i
Clark Olson
‘2
Illinois
Environmental
Protection Agency
1021
North Grand Avenue
East
P.O.
Box 19276
Springfield,
Illinois 62794-9276
THIS FILING PRINTED ON RECYCLED PAPER
13
RECEIVED
CLERK’S
OFFICE
BEFORE THE
ILLINOIS POLLUTION CONTROL BOARD
JAN
22
2002
STATE OF ILLINOIS
Pollution Control Board
IN
THE
MATTER OF:
WATER QUALITY AMENDMENTS
TO
)
35
III. Adm.
Code 302.208(e)-(g), 302.504(a),
)
R02-1 I
302.575(d), 303.444,
309.141(h); and
)
(Rulemaking
-
Water)
PROPOSED 35
III. Adm. Code 301 .267,
)
301.313, 301.413, 304.120, and 309.157
)
TESTIMONY OF ALAN KELLER
QUALIFICATIONS/INTRODUCTION
My name is Alan
Keller and
I
am the Supervisor of the Northern Municipal
Unit of
the
Permit Section of the
Division of Water Pollution Control.
I have worked for the
Agency since June
1972.
I have worked in
the Permit Section
my entire career with the
Agency and have been
responsible at one time or another with
all of the permit programs.
In my present capacity,
I
manage a
unit, which reviews construction
permits and NPDES
permits for municipal and semi-public facilities
and also perform other duties associated
with
municipalities.
I also serve on two design criteria groups, which establish
the specific
design criteria for sewers,
lift stations and treatment plants for municipal facilities.
One
group
is the Agency Division of Water Pollution Control Design Criteria Committee and the
other group is the Wastewater Design Criteria Committee for the Great Lakes-Upper
Mississippi River Board of State and
Provincial Public Health and Environmental
Managers.
I
have a Bachelor of Science
Degree
in Civil Engineering from the
University of
Illinois concentrating
in
Environmental
Engineering and
I am a Registered
Professional
Engineer in
Illinois.
My testimony will discuss the reasoning
behind
development of the
CBOD5 test.
14
REASONING BEHIND
CBOD5TEST
The Agency has interpreted the intent of 35
III. Adm. Code
304.120, with
respect to
compliance with the respective 5-day biochemical oxygen’~demand(BOD5) effluent
requirements, to be the 5-day carbonaceous biochemical oxygen demand (CBOD5).
35
III.
Adm. Code 309.141
allows the Agency to establish the terms and conditions of each
NPDES permit and directs the Agency to ensure compliance with the effluent limitations
under Sections 301
and 302 of the Clean Water Act.
40 CFR 133 provides for the
use of
CBOD5 for determining compliance with the definition of secondary treatment requirement.
This regulation was
revised in the September 20,
1984 Federal Register to allow for the
use of CBOD5.
The Agency has implemented the
use of CBOD5
in
lieu of BOD5
in
NPDES
permits since 1986 and also incorporates ammonia nitrogen water quality based
effluent limits where appropriate.
At treatment facilities where complete nitrification occurs and treatment facilities
where
no nitrification
occurs, the
CBOD5 would not be substantially less.
The use of the
BOD5test on raw sewage or influent only measures the carbonaceous demand in the
sample because insufficient nitrifying bacteria would be present during the five-day test
period.
It normally takes about ten days for
a sufficient number of nitrifying bacteria to
develop to have a measurable effect on the
BOD5 test.
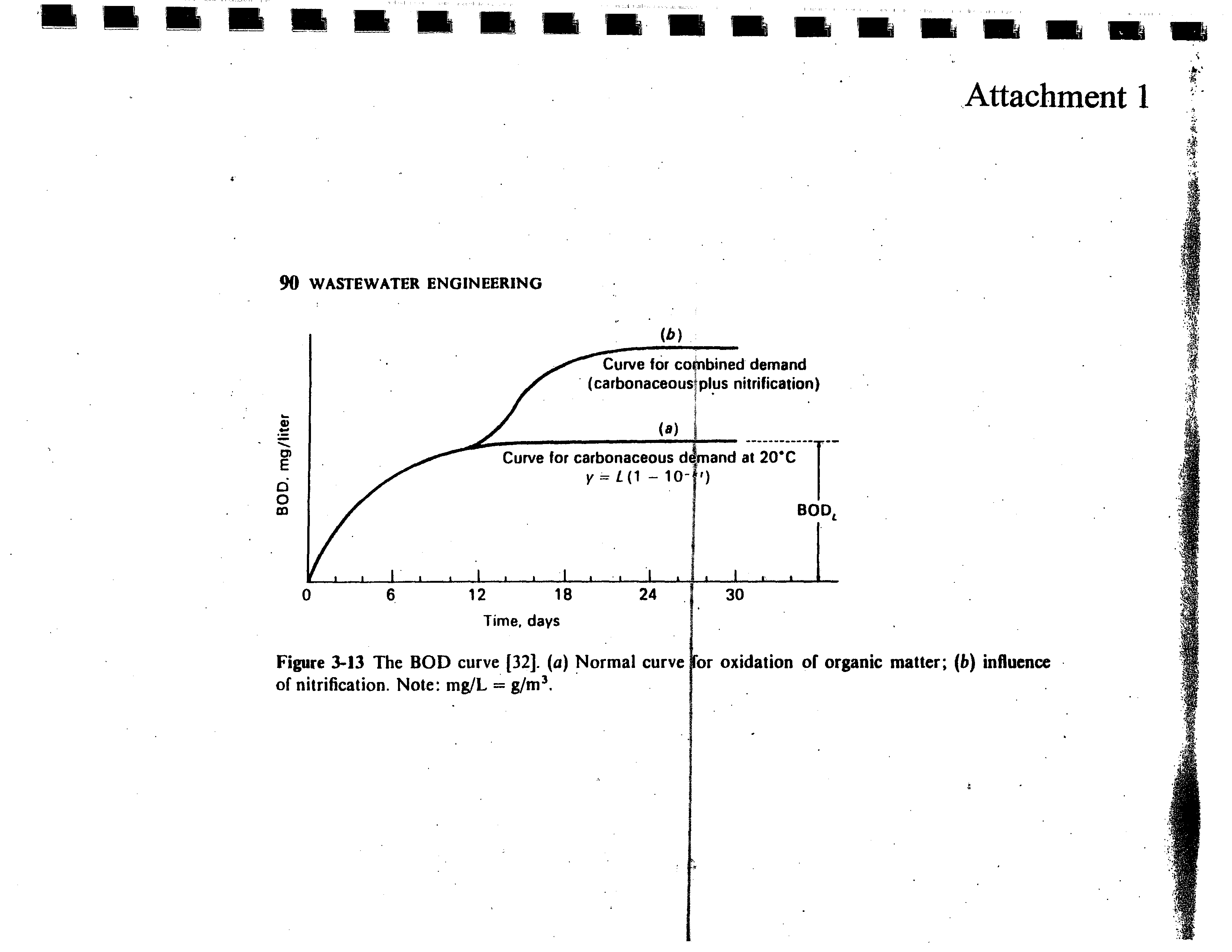
(See Attachment 1).
However, in
a treatment process where partial nitrification occurs,
large numbers of nitrifying bacteria
are present and nitrification can occur during the effluent BOD5test.
The
BOD5 test is
designed to measure the carbonaceous demand in
a sample and to measure the
efficiency of a treatment process by comparing the carbonaceous demand before and
after the treatment process.
In treatment processes that do not nitrify
or completely nitrify,
15
the
use of the
BOD5 test on both the influent and effluent will
provide satisfactory results.
However, in treatment processes that partially nitrify, the
use of the
BOD5test on both the
influent and effluent will compare the carbonaceous demand
in the influent with the
carbonaceous and nitrogenous demand
in
the effluent.
Such
a procedure would provide
no useful information on the carbonaceous removal efficiency in a treatment process.
An
accurate determination of the
removal efficiency of a treatment process
in which partial
nitrification occurs would require the carbonaceous demand of the influent to be measured
by the
BOD5 test and the carbonaceous demand of the effluent to be measured by the
CBOD5 test, which suppresses the nitrogenous demand.
Requiring the
BOD5test on the
influent and the
CBOD5 test on the effluent of all facilities would allow a uniform policy on
carbonaceous removal throughout the state.
The effluent from
a treatment plant consists
of many components, the Agency believes that the quality of the effluent can best be
assessed, and controlled, when each of the components are analyzed
and controlled
individually.
The characteristics of the effluent can
best be assessed when
the CBOD5
test is
used to measure the carbonaceous demand, and where ammonia nitrogen effluent
standards are appropriate,
use the ammonia nitrogen test to measure the nitrogenous
demand.
This procedure would be more logical than trying to measure the combined
carbonaceous and nitrogenous demand with the BOD5 test, which has been proven to
provide inconsistent and misleading
results.
In addition, the attached figures depict the
influence of nitrification on the
BOD test.
Attachment
1 was taken from Metcalf and Eddy’s, “Wastewater Engineering: Treatment
Disposal,
Reuse” Second Edition,
page 90.
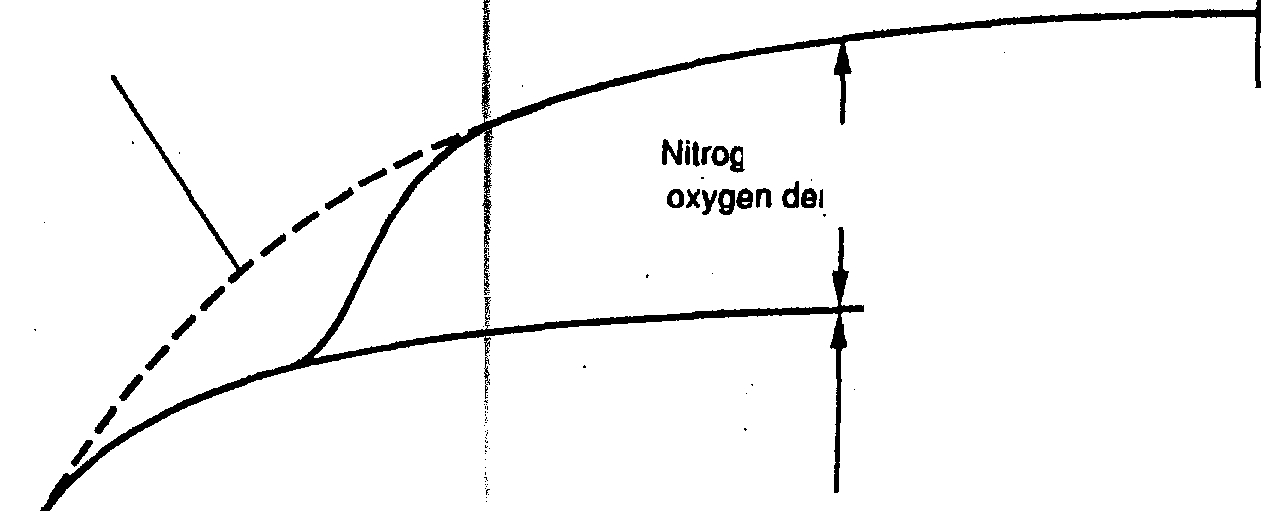
Attachment 2 was taken from Metcalf and
Eddy’s, Third Edition,
Page 76.
The Third
Edition also states the following: “Because the
reproductive rate of the
nitrifying bacteria
is slow it normally takes from 6 to 10 days for
16
them to reach significant numbers and to exert a measurable oxygen demand.
However,
if a sufficient number of nitrifying bacteria
are present initially, the interference caused by
nitrification can be significant.
When nitrification occurs in the BOD test, erroneous
interpretations of treatment operating data are possible.”
The Agency regulates the
nitrogenous biochemical oxygen demand of wastewater by incorporating the ammonia
nitrogen water quality based effluent limits
in NPDES Permits as appropriate under
Sections 304.105 and 304.122 of Subtitle C: Water Pollution.
This concludes my pre-filed testimony.
I will be supplementing this testimony as needed
during the hearing.
I would be happy to address any questions.
By:
(Le4L/
~
Alan
Keller
January 29, 2002
Illinois Environmental Protection Agency
1021 North Grand Avenue
East
P.O.
Box 19276
Springfield,
Illinois 62794-9276
THIS FILING PRINTED ON RECYCLED PAPER
17
lU
~
90
WASTEWATER
ENGINEERING
(b)
Attachment
1
Curve
for co
bined
demand
~~b~ac:ous~Plus
nitrification)
Figure
3-13
The
BOD
curve
132.
(a)
Normal
curve
of nitrification.
Note: mg/L
=
g/m3.
or
oxidation
of organic
matter;
(b)
influence
-S
a)
E
ci
0
Curve
for carbonaceousde
rnand at 20C
I)
BODL
I
.
0
6
12
18
24
Time. days
30
-J
E
0
C
E
Where a sufficient number of nitrilying
organisms are present. nitritication can
occur as shown by the dotted curve.
/
Nitrification
is usually observed to
occur from 5
to 8 days afterlihe
start ol
the ROD incubation period.
Time,
d
Attachment 2
FIGURE 3-15
Definition sketch for the exertion of the carbonaceous and nitrogenous biochemical oxygen demand
in a waste sample.
~
—
—
—
—
—
—
—
enous biochemical
nand, NBOD
Carbonaceous biochemical
oxygen demand,
BOO or
CBOD
1~
)
)
)
)
)
I,
the
undersigned,
on oath state that I have served the attached
WRITTEN
TESTIMONY OF ROBERT MOSHER, CLARK OLSON, AND ALAN KELLER
upon the
person to whom it is directed, by placing a copy in an envelop addressed to:
Dorothy Gunn, Clerk
Pollution Control Board
100 West Randolph
Street
Suite 11-500
Chicago,
Illinois 60601
(Federal Express)
Mathew Dunn
Illinois Attorney General’s Office
Environmental Control Division
James
R.
Thompson Center
100 West Randolph Street
Chicago,
Illinois 60601
(Federal Express)
Marie E. Tipsord
Illinois Pollution
Control Board
James
R.
Thompson Center
100 West Randolph Street,
Suite 11-500
Chicago,
Illinois 60601
(Federal Express)
Legal Service
Illinois Department of Natural
Resources
524 South Second Street
Springfield,
Illinois 62701-1787
(Federal Express)
and mailing
it from Springfield,
Illinois on January 18,
2002, with sufficient postage
affixed
as indicated above.
-~
-.
OFFICIAL
SEAL
BRENDA BOEHNER
X
NOTARY
PUBUC,
STATE
OF
ILLINOIS
$
tMY
COMMISSION
EXPIRES
11.14.2005*
STATE OF ILLINOIS
COUNTY OF
SANGAMON
SS
PROOF OF SERVICE
SUBSCRIBED AND SWORN TO BEFORE ME
this day of January 18, 2002.
Notary Public
THIS FILING PRINTED ON RECYCLED PAPER
18
Albert Ettinger
Environmental Law & PolicyCenter
35 E. Wacker Drive, Suite 1300
Chicago, Illinois
60601-2110
James T.
Hastington
Ross & Hardies
150
North Michigan, Suite
2500
Chicago,
Illinois
60601
Katherine
Hodge
Hodge & Dwyer
3150 Roland Ave., P0 Box
5776
Springfield, illinois
62705-5776
MargaretHoward
Hedinger and
Howard
1225
South Sixth Street
Springfield, illinois
62703
Robert Mesaina
illinois Enviommental Regulatory Group
215 East Adams Street
Springfield,, illinois
62701
Irwin Polls
600lWest Pershing Road
Cicero, illinois
60804-4112