| | - BEFORE THE ILLNOIS POLLUTION CONTROL BOARD
- OF THE STATE OF ILLINOIS
- NOTICE OF FILING
- PRINTED ON RECYCLED PAPER
- TESTIMONY OF RICHARD P.COBB, P.G.
- FOR THE PROPOSED MTBE GROUNDWATER QUALITY STANDARDS
- QUALIFICATIONS/INTRODUCTION
- BACKGROUND
- CWS Facilities With MtBE Detections
- TREATMENT SUMMARY
-
- RIBBON MTBE PANEL FINDINGS
- SDWA UNREGULATED CONTAMINANT MONITORING REQUIREMENT
- FOR MTBE
- ILLINOIS EPA’S PROPOSAL TO AMEND THE GROUNDWATER QUALITY
- STANDARDS
- CONCLUSION
- CURRICULUM VITAE OF RICHARD P. COBB, P.G.
- I. Personal
- II. Education
- III. License
- IV. Certification
- V. Summary ofExperience
- VI. Summary of Computer Skills
- W. VIII. Professional Affiliation
- IX. Chronological Experience
- A. Legislation and Legislative Development Documents
- Co-Author
- B. Regulations
- Co-Author
- Principal Author
- C. Groundwater Quality and Hydrogeology
- Principal Author
- D. Groundwater Protection Program Documents
- Principal Author
- Co-Author
- E. Geology
- Principal Author
- EXHIBIT Il—Maps of Community Water Supplies with MTBE Detections
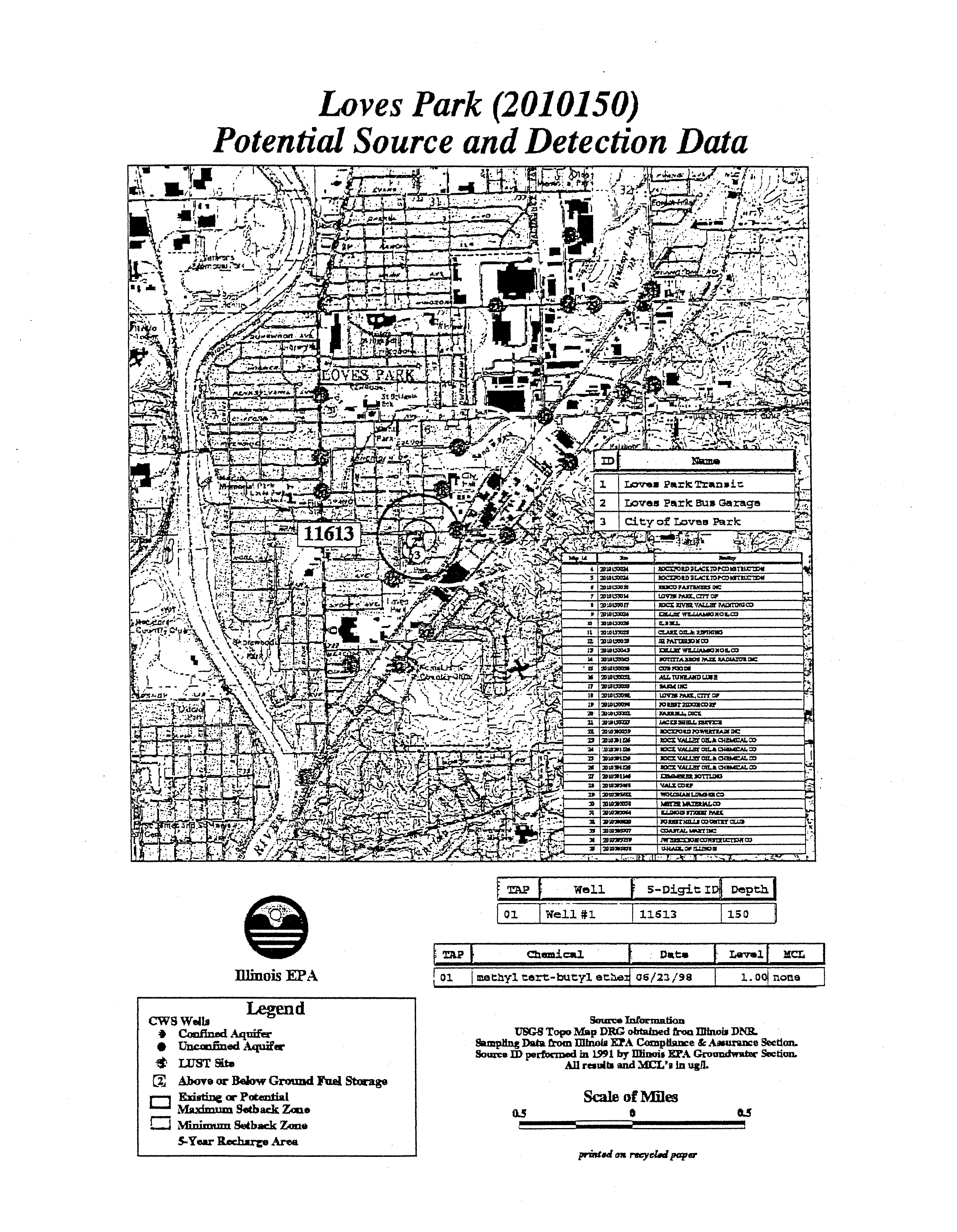
- T&C Mobile Estates (0015815)
- Potential Source and Detection Data
- Germantown (0270350)
- Grafton (0830200)
- Illinois EPA.
- South Elgin (890800)
- Potential Source and Detection Data
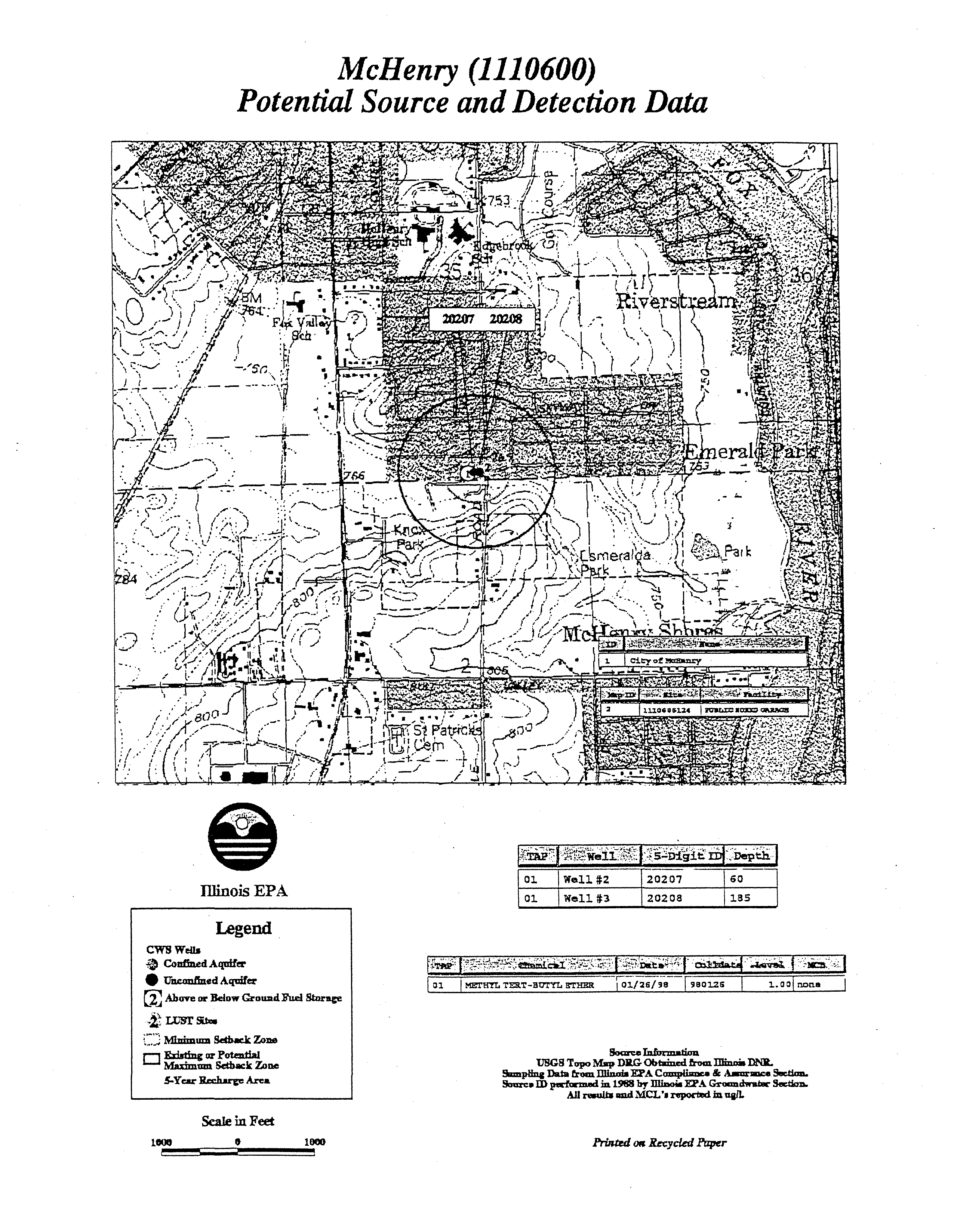
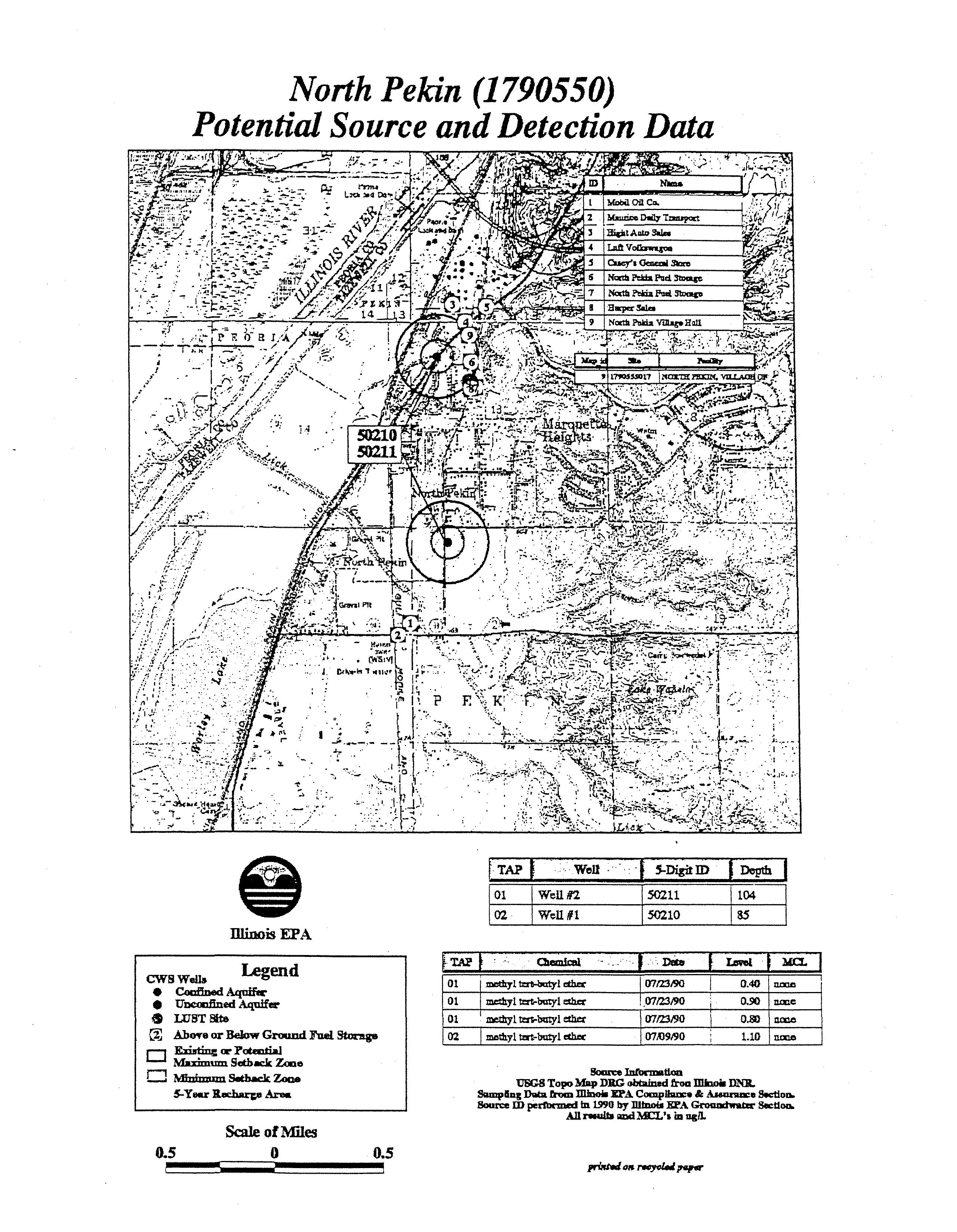
- Marengo (1110650)
- Well#13 01191 36Legend
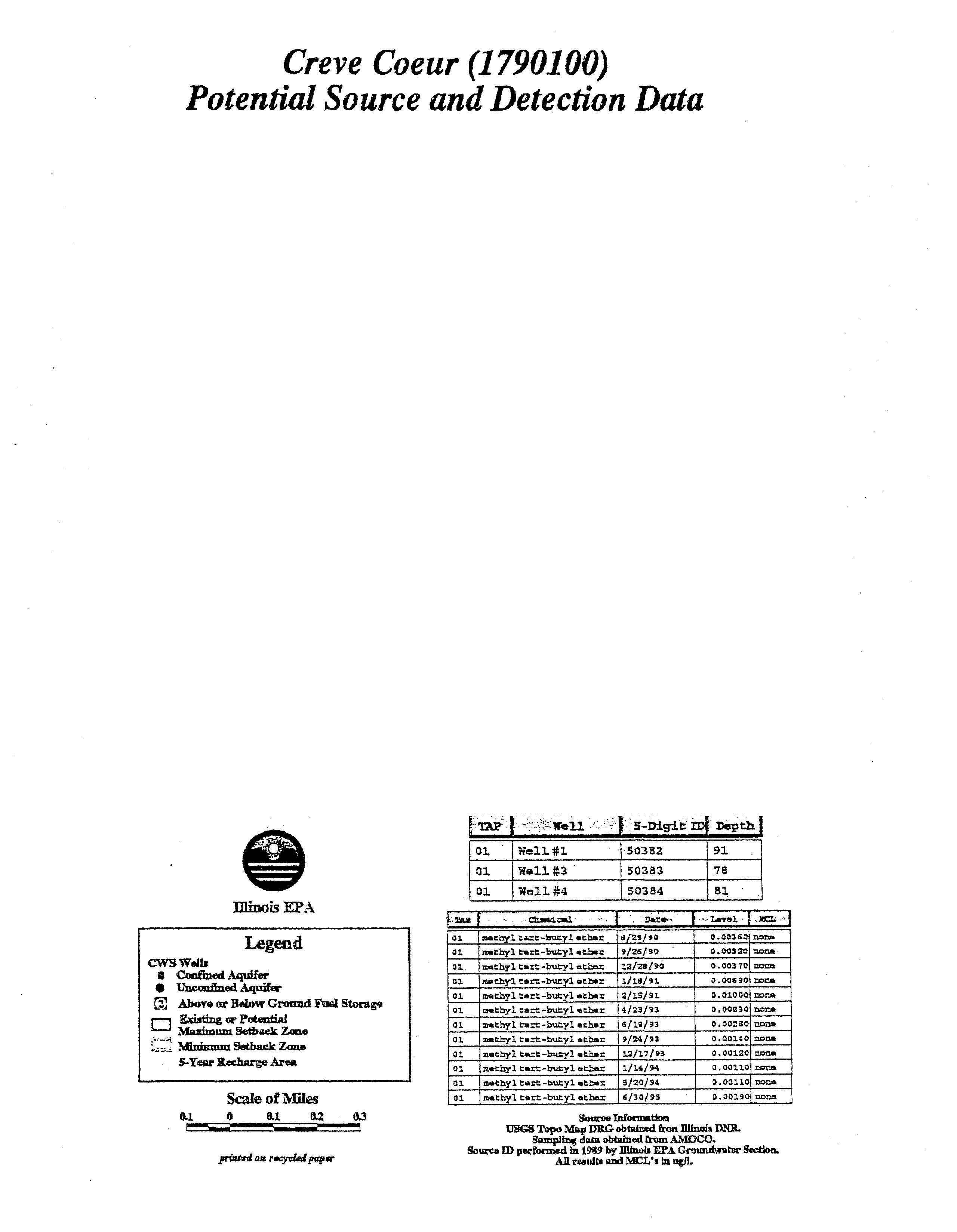
- Marquette Heights (1790400)
- Potential Source and Detection Data
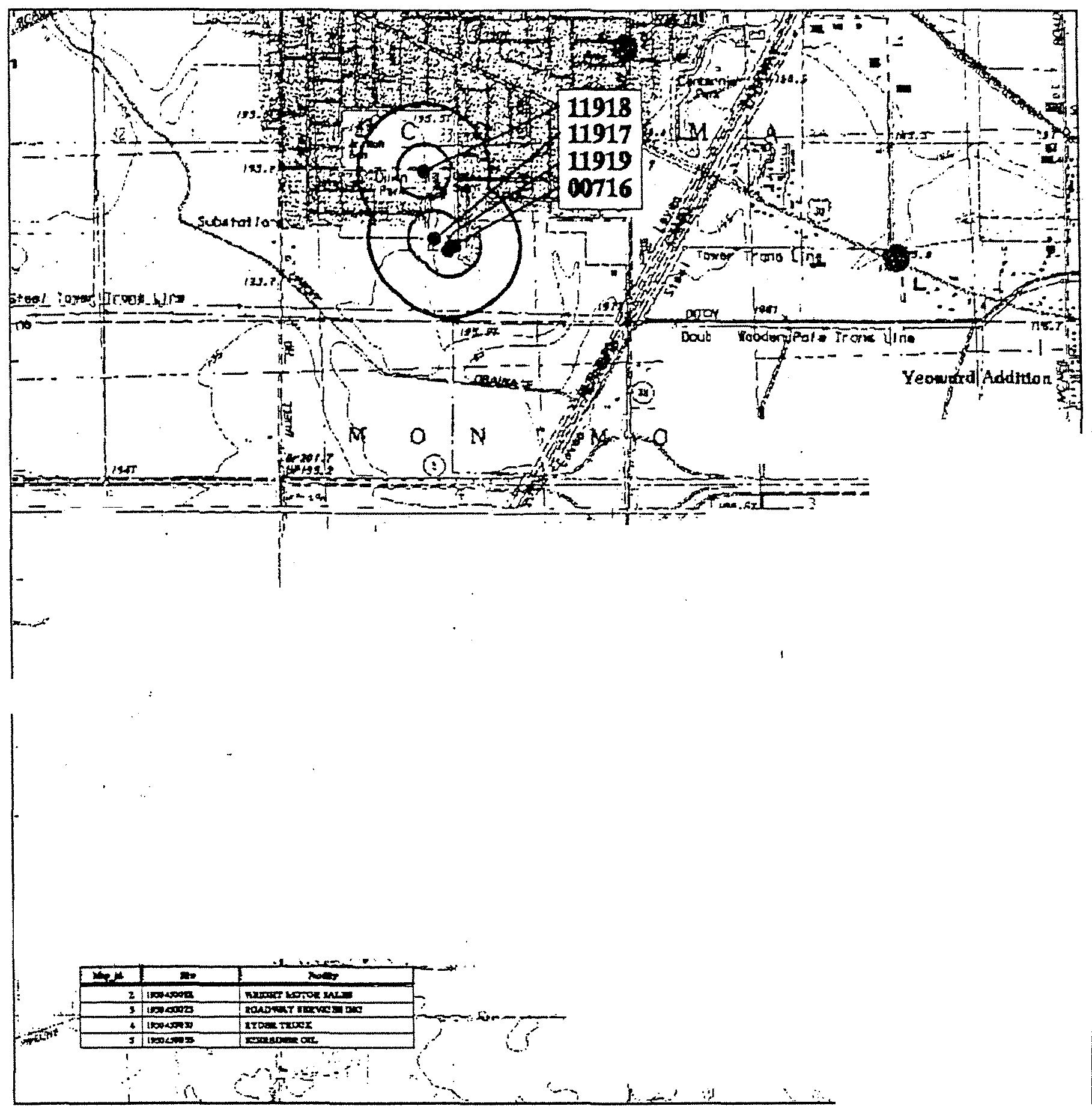
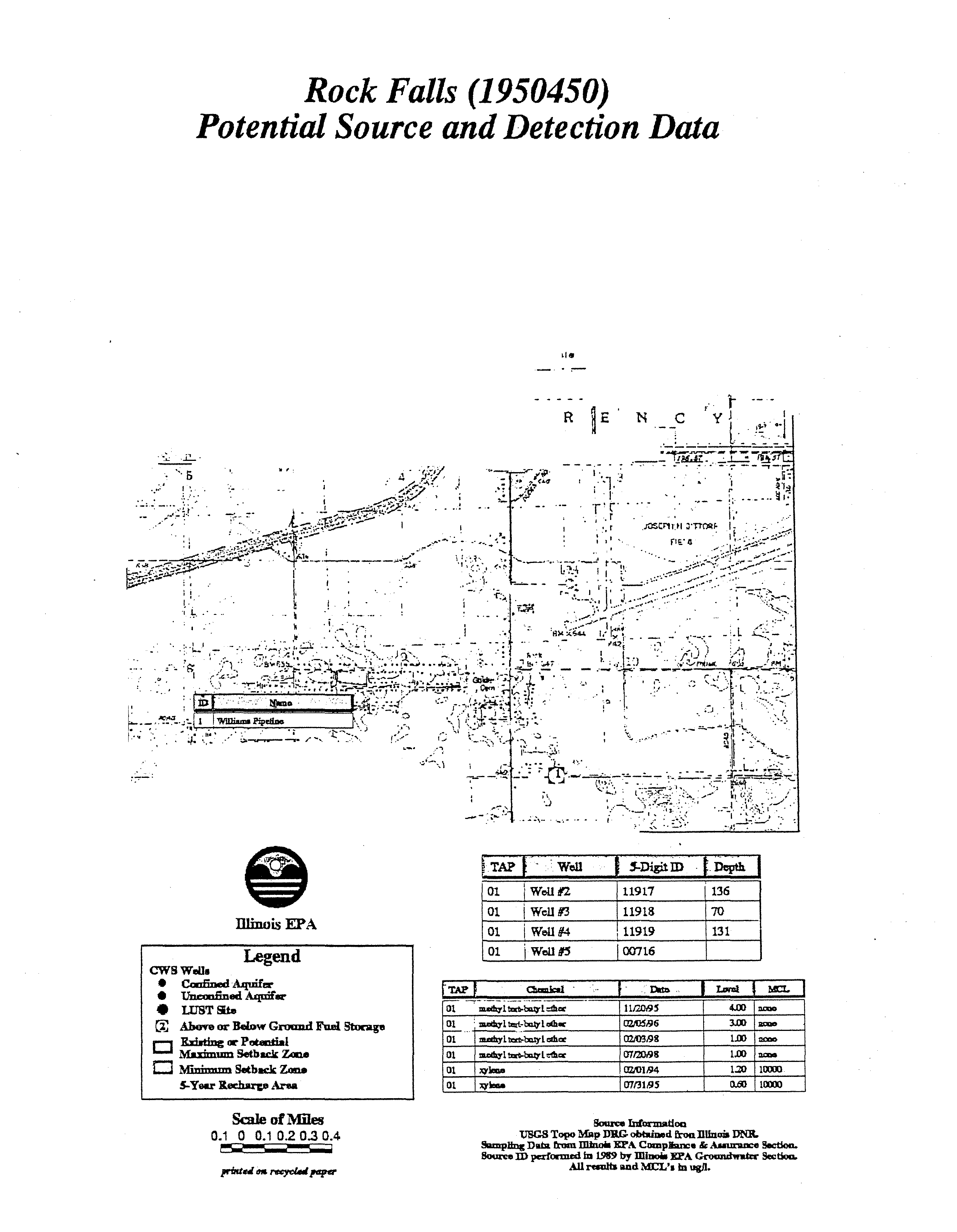
- Rock Falls (1950450)
- Scale of Mi~o~s e
- EXHIBIT ffl —MTBE Taste and Odor Thresholds
- -- Effects Analysis on
- Methyl Tertiary~Buty1 Ether (MtBE)
- Conclusion and Recommendation
- Characterization Summary
- 1.0 INTRODUCTION
- December 1997
- Home Page at: http ://www.whitehouse.govIWHJEQP/OSPIhtmIIOSTP_Home.htrnl.
- 3.0 CHEMICAL AND PEYSICAL PROPERTIES
- 4.0 TOXICOK1NETICS
- 4.1 Dosimetry: Route-to-Route Extrapolation
- December 1997
- 4.2 NSTC’s Extrapolatioa ofDose from Inhalation Exposure
- 24.O4Lx 70kg
- 5.0 HEALTH E~CTS DATA
- 5.1 HumanStudies
- December 1997
- 5.2.1 Noncancer Effects
- December 1997
- 5.2.1.2 Reproductive and Developmental Studies
- Reproductive Studies
-
- Developmental Studies
- December 1997
- 5.2.1.3 Neurotoxicity Studies
- December 1997
- 5.2.1.4 Mutagenicity Studies
- 5.2.2 Cancer Effects
-
- Gavage Study
- December 1997
- December 1997
- December 1997
- Formaldehyde
- 6.0 ORGANOLEPTIC PROPERTIES
- December 1997
- 7.1 Hazard Characterization
- December 1997
- December 1997
- December 1997
- December 1997
- December 1997
- November 4, 1994
- of from 1-4 liters per day.
- which becomes after substitution:
- Manage. Assoc. 40:282.
- Morrow, Leslie D. 1994. Personal communication.
- Development. November, 1993.
- CERTIFICIATE OF SERVICE
- I, the undersigned, CERTIFY that I have served a copy of the TESTIMONY OF
- Springfield, Illinois 62701-1199
- Stephen DavisIllinois Department ofNatural Resources524 South Second Street
- Law Dept. 125 S. Clark St.
- Chicago, Illinois 60603
- Woodstock, Illinois 60098
-
- Georgia VlahosDept ofthe Navy, Naval Training Center260lA Paul Jones Street
- Great Lakes, Illinois 60088-2845
|
BEFORE THE
ILLNOIS POLLUTION
CONTROL BOARD
OF THE STATE
OF ILLINOIS
rN THE
MATTER OF:
PROPOSED MTBE GROUNDWATER
QUALITY
STANDARDS AMENDMENTS:
35
ILL. ADM. CODE
620
)
)
)
R0l-14
)
(Rulemaking
-
Water)
)
NOTICE OF FILING
Dorothy M. Gunn, Clerk
Clerk ofthe Board
Illinois
Pollution Control Board
James R. Thompson Center
100 West Randolph Street, Suite 11-500
Chicago, Illinois 60601
Robert Lawley, ChiefLegal
Counsel
Dept. ofNatural Resources
524
south Second Streeet
Springfield, IL
62706
Service List
Matthew J. Dunn, Chief
Environmental Bureau
Office ofAttorney General
188 w. Randolph,
20th
Floor
Chicago, Illinois 60601
Joel J. Stemstein, Esq.
Hearing Officer
Illinois Pollution Control Board
James R. Thompson Center
100 West Randolph Street, Suite 11-500
Chicago, Illinois 60601
PLEASE
TAKE
NOTICE
that I have filedtoday with the Clerk ofthe Illinois Pollution
Control Board the
TESTIMONY OF
RICHARD
P.
COBB, P~G.
with Exhibits by the illinois
Environmental Protection Agency, a copy ofwhich is herewith served upon you.
DATE:
February 16,2001
Illinois Environmental Protection Agency
1021
North Grand Avenue East
Post Office Box
19276
Springfield, Illinois
62794-9276
(217) 782-5544
Respectfiully submitted,
by:
Stephen C. Ewart
Deputy Counsel
Division ofLegal Counsel
PRINTED
ON
RECYCLED PAPER
BEFORE THE
ILLNOIS POLLUTION CONTROL BOARD
N
THE
MATTER OF:
GROUNDWATER
QUALITY STANDARDS
AMENDMENTS:
35
ILL. ADM.
CODE
620
)
)
)
R0l-14
)
)
(Rulemaking Water)
)
DATED:
February
16, 2001
1021
North Grand Avenue Northeast
P.O. Box
19276
Springfield,
IL
62794
—9276
217/782-5544
Stepheri~
C. Ewart
Deputy Counsel
Division of Legal Counsel
TESTIMONY OF RICHARD P.
COBB, P.G.
The
Illinois
Environmental
Protection
Agency
hereby
prefiles
the
attached
TESTIMONY of RICHARD
P.
COBB,
P.G.
This
testimony
will
be
presented by
Mr.
Cobb
at the Illinois Pollution Control Board hearing to
be held on
March
1
and 22, 2001.
Illinois Environmental Protection Agency
By:
____________
TESTIMONY OF RICHARD
P.COBB, P.G.
FOR THE PROPOSED MTBE
GROUNDWATER QUALITY STANDARDS
ROl
—
14
QUALIFICATIONS/INTRODUCTION
My name is Richard P.
Cobb and
I am Manager ofthe Groundwater Section of
the Illinois Environmental Protection Agency’s (“Illinois EPA”) Bureau ofWater.
For
further detail on my qualifications I have enclosed a copy ofmy
Curriculum Vitae in
Exhibit I.
This testimony, the statement ofreasons, and exhibits included with this
testimony describe the basis for the proposed amendments to the groundwater quality
standards.
The Illinois EPA is proposing a preventive notice and response level, and
Class
I, and II groundwater standard for Methyl Tertiary-Butyl Ether (“MTBE”).
In
addition we are proposing amendments to
the compliance
determination section.
Illinois EPA is proposing these amendments consistent with the Illinois
Groundwater Protection Act (“IGPA”) policy and program statement; in accordance with
the requirements in Section
8 ofthe IGPA;
and in response to the Illinois Pollution
Control Board’s (“Board”) request to
continually update the groundwater standard
BACKGROUND
Community
water
supplies
(“CWS”)
in
Illinois
routinely
sample
for
volatile
organic
chemicals
as
a
result
of
Safe
Drinking
Water
Act
monitoring
requirements.
Under Illinois’
CWS
Laboratory Fee Program,
analyses for MTBE have been reported
as.
a part of standard
laboratory
methods
since
1994.
Therefore,
we have been receiving
SDWA compliance samples that are taken at the entry point to
a community water supply
distribution
system.
These are also referred to as (“finished water samples”).
Since
1994
26
CWS
have been impacted by MTBE
contamination.
Another factor to
consider is that
these
are
finished
water
samples
and
they
are
collected
after
treatment.
Thus,
the
contamination
level
in
the source
water
could be
higher.
In addition,
there
is
also
the
potential
risk to other potable
wells,
including
private,
semi-private and non-community
water supply wells.
The
Illinois EPA has evaluated each of these 26 CWS
with MTBE detects
as
shown in Figure
1. The monitoring conducted at
over
1,200 CWS
participating in the
program (just over
1,100 of these facilities are groundwater dependent) has resulted in 26
facilities with detections of MTBE.
Four CWS have had
to
discontinue use ofwells
as a
result ofMTBE contamination:
•
Oakdale Acres Subdivision (and two other small subdivisions
served by private
wells), located in Kankakee County,
had to
discontinue use oftheir wells and
connect
to a nearby CWS;
•
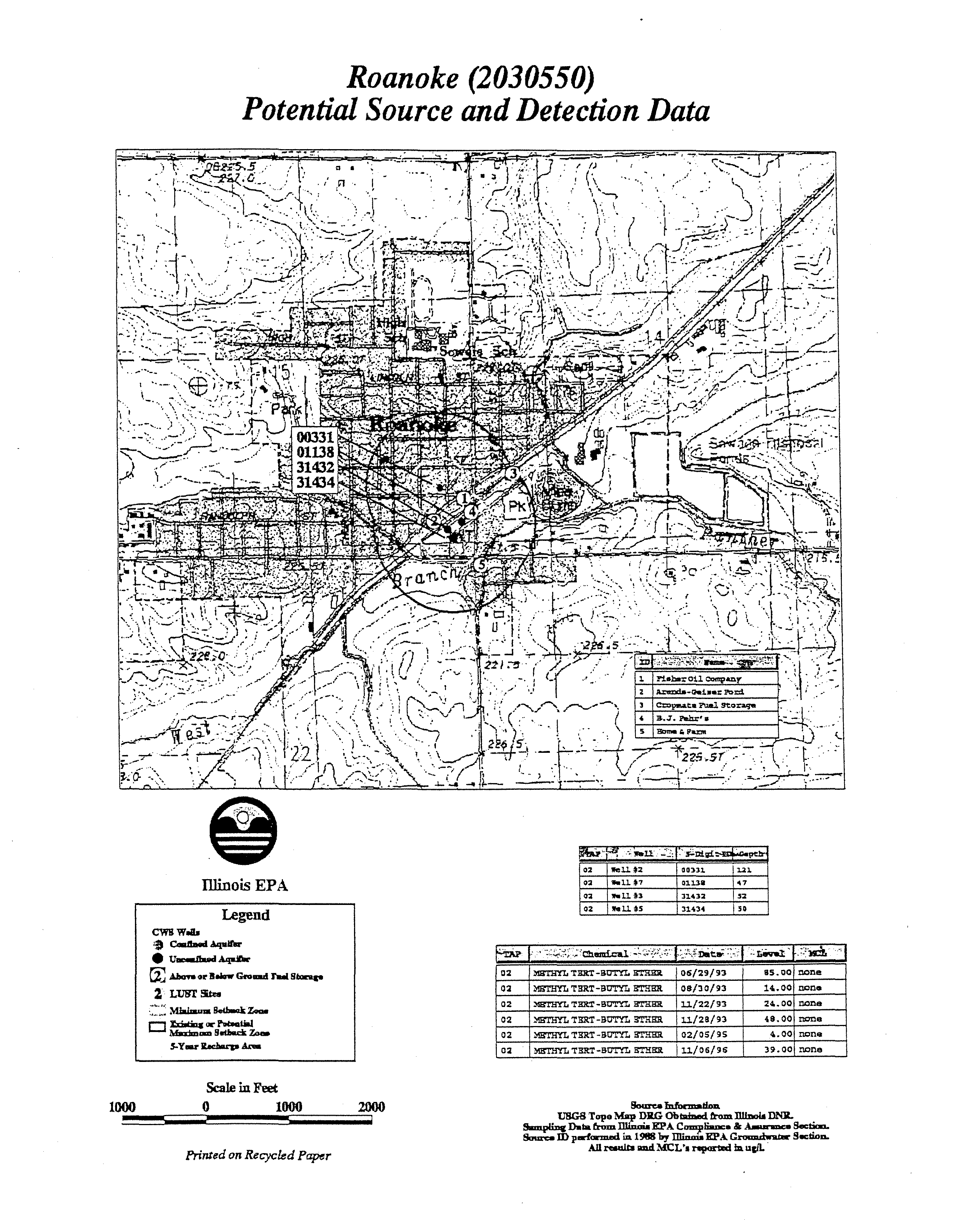
Roanoke located in Woodford County has had to shut down
wells due to high levels
ofMTBE;
•
East Alton located in Madison County has had to use one oftheir wells as a hydraulic
containment well with treatment and discharge to
surface water to protect their well-
field from a MTBE plume with a concentration exceeding
1,000 parts perbillion
(“ppb”); and
•
The community ofIsland Lake had to take a well out of service as a result ofelevated
levels of MTBE.
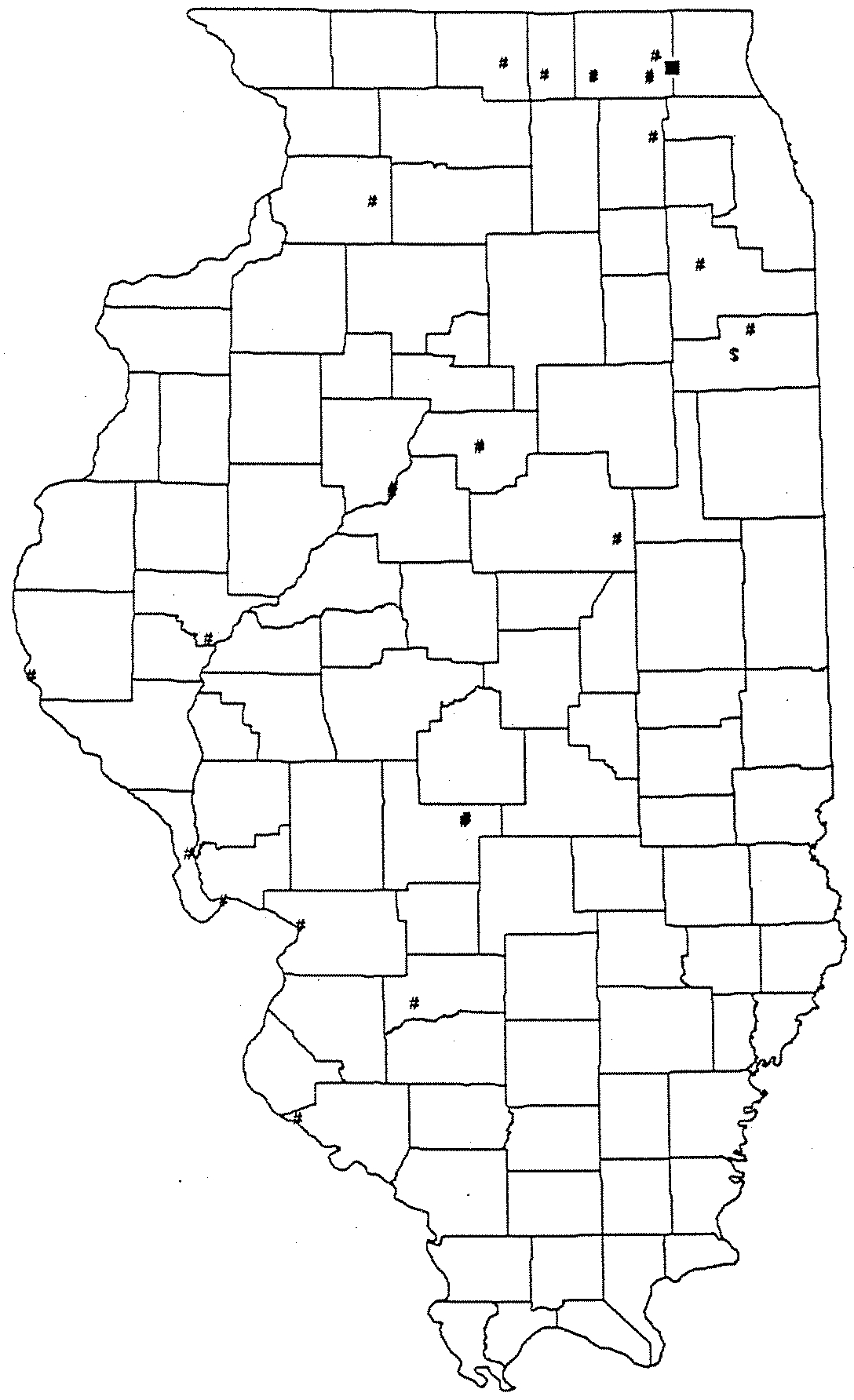
Maps
of each of these communities
has also
been prepared showing:
the CWS;
the
type
of aquifer
being
used;
CWS
well
depth;
MTBE
and
BETX
concentrations;
the
location of potential
contamination
sources
surveyed
by
Illinois
EPA
staff
under
the
IGPA well site survey requirements; the location of reported leaking underground storage
tank sites,
the setback zone established
under the IGPA;
and, if delineated, the recharge
area of the well(s).
These maps are contained
in Exhibit II.
CWS
Facilities
With
MtBE
Detections
T&C
MOBILE
ESTATES
Adams
BELVIDERE
Boone
HARDIN
Calhoun
GERMANTOWN
Clinton
GRAFTON
Jersey
OAKDALE
ACRES
SUBD
Kankakee
SOUTH
ELGIN
Kane
MANTENO
Kankakee
CRYSTAL LAKE
Lake
ISLAND
LAKE
McHenry
MC
HENRY
McHenrv
MARENGO
McHenry
BETHALTO
Madison
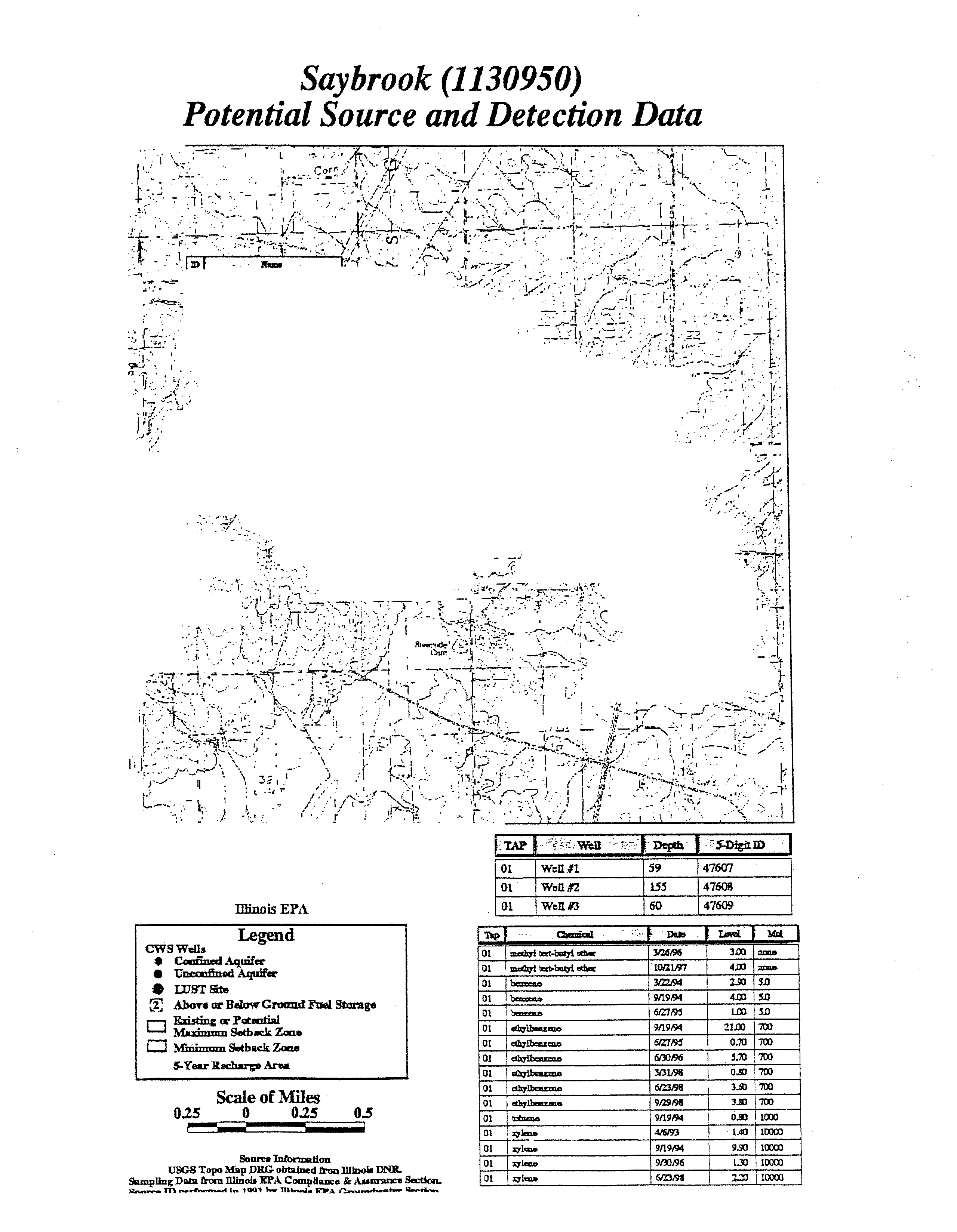
SAYBROOK
McLean
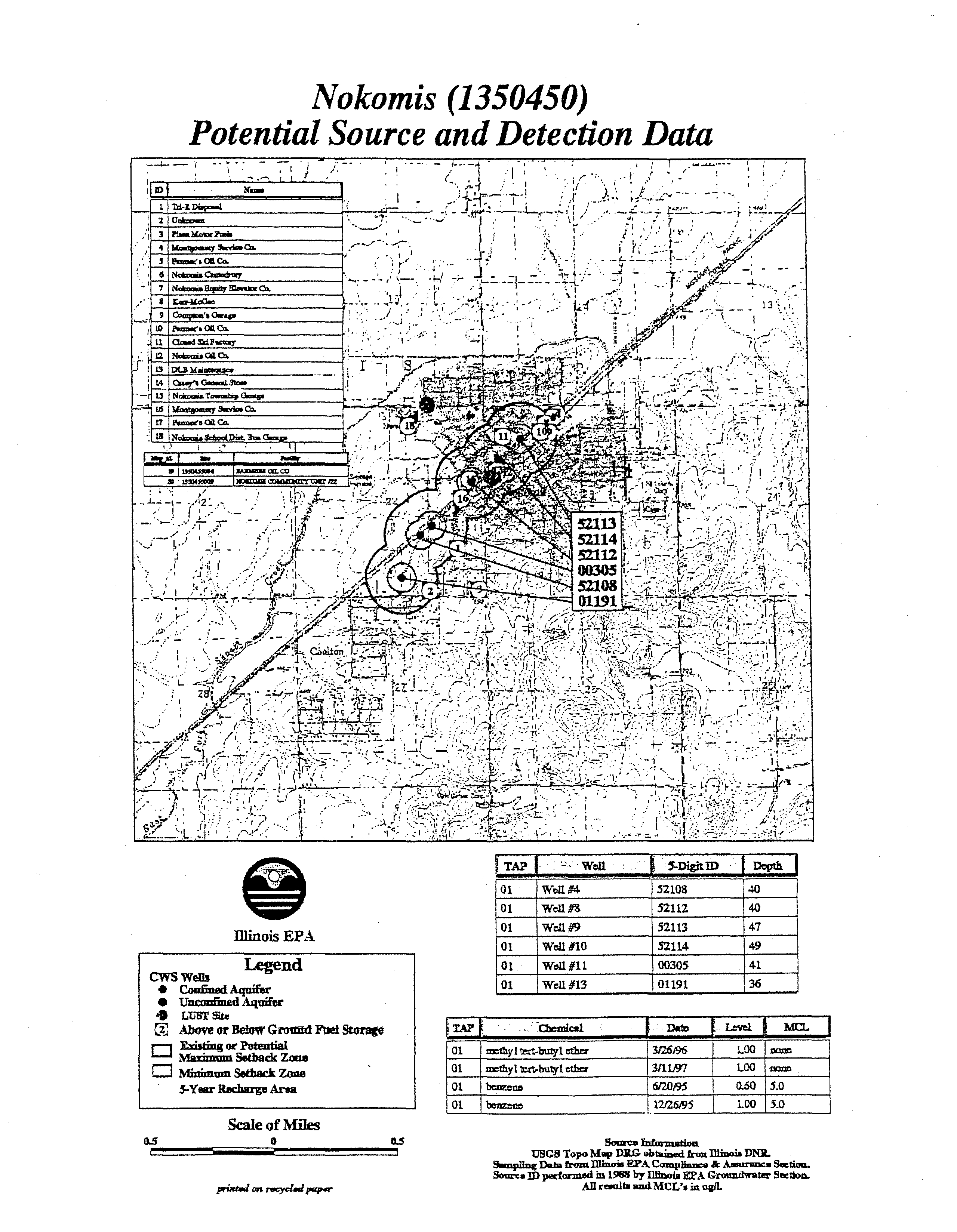
NOKO MIS
Montgomery
PRAIRIE DU
ROC HER
Randolph
RUSHVILLE
Schuyler
NORTH
PEKIN
Tazewell
MARQUETTE HTS
Tazewell
CREVE
COEUR
Tazewell
ROCK
FALLS
Whiteside
CLEARVIEW
SBDV
Will
LOVES
PARK
Winnebago
ROANOKE
Woodford
Legend
~
Active
CWS
Facility w/MtBE
Detections
~
Active
CWS Facility w/Closed
Wells
Due
To MtBE Dections
~
Closed CWS
Facility w/MtBE
Detections
Figure
1. Conimunit)’ Water
Supplies with MTBE Detections
MTBE is an organic chemical, specifically an
ether.
Ethers, especially those of
low molecular weight such as MTBE, are significantly soluble in water1.
MTBE in
drinking water can be detected by the senses oftaste and smell at extremely low
concentrations of 20 to 40 ppb2.
MTBE
is primarily manufactured and isolated for use as
a fuel additive.
It is used
in gasoline to increase the octane rating, in effect causing the
fuel to burn more completely and therefore create less pollution in
the exhaust.
MTBE
was used in
small amounts from the late
seventies primarily in California to help
curtail
the air pollution problems
due to hydrocarbon emissions in large urban areas.
In recent
years, however, its
use has spread throughout the country in response
to
increased air
pollution control laws.
MTBE is raising increasing concerns because it is being found in
many water supply wells across the country3’4
Some states such as California and Maine have taken the initiative to regulate or
ban MTBE use within its borders.
With increasing detection at fairly high levels in
community and private water supply wells, MTBE has been raised as a contaminant of
concern for its possibility to
cause cancer and its disagreeable taste and odor2
MAJOR ISSUES
Solubility and Dispersal
-
MTBE is a high solubility for an organic compound.
When in
an organic solution such as gasoline, a high percentage ofMTBE
can transfer
into water that is in contact with the organic phase.
Once in the aqueous phase,MTBE
‘United States Environmental Protection Agency, Office ofUnderground Storage Tanks.
April
1988.
Cleanup of Releases from Petroleum
USTs: Selected Technologies.
EPAJ53OTLJST-88/OOl.
2
Drinking
Water Advisory:
Consumer Acceptability Advice and Health Effects Analysis on
Methyl
Tertiary-Butyl Ether (MTBE), U.S. Environmental Protection Agency Office of Water, December
1997.
Squillace, P.J., D.A. Pope, and C.V. Price,
March
1995, Occurrence of the Gasoline Additive
MTBE in
Shallow Ground Water
in Urban and Agricultural Areas, U.S. Geological Survey, Fact
Sheet FS-1 14-95.
‘~
United States Environmental Protection Agency.
1999. Blue Ribbon Panel
on
Oxygenates
in Gasoline,
Executive Summary, Executive Summary and Recommendations (including a statement by Carol
Browner
on
the
Findings)
4
can disperse in the water, and migrate at the same rate as the water in underground
aquifers.5
Environmental Fat
-
MTBE
is readily broken down in the presence of high UV such
as direct sunlight.
In its pure form, on the surface, or in shallow surface water, it
volatilizes rapidly
or
is broken down by sunlight with sufficient time5.
Natural
degradation of MTBE
in groundwater, however, is not
as effective.
The primary method
ofattenuation for MTBE in
groundwater is through dispersion.
Biodegradation is also
not an effective method
ofnatural breakdown ofMTBE in a groundwater setting.
MTBE
is resistant to
natural forms ofdegradation.
According to
research by the United States
Geological Survey (“USGS”)
biodegradation rate constants for MTBE are estimated to
be several orders ofmagnitude lower than for other gasoline components such as benzene
and toluene.6’
1
MTBE vs. BTEX
-
Detections ofMTBE in groundwater can often be traced to above
ground bulk terminals and underground petroleum storage tanks (“USTs”), both of which
have been leaking fuel materials to the groundwater surface.
With releases or leaks of
petroleum products, two
components of concern often detected are MTBE and BTEX
(benzene, toluene, ethylbenzene, and xylenes).
BTEX plumes are very organic in nature,
tend to “float” on the surface of groundwater, and the soluble components principally
BTEX dissolve in
the water layer.
MTBE has a much higher solubility index than the
BTEX components ofpetroleum
products”7’8. Therefore, a larger proportion ofMTBE is
expected to
be in the water layer, relative to
the proportional amounts ofBTEX in the
water layer.
~Squillace, P.J., J.F. Pankow, N.E. Korte and J.S. Zogorski. September 1997. Review of the Environmental
Behavior and fate of Methyl Tert-Butyl
Ether,
Environmental Toxiciology and Chemistry,
Vol.
16,
no.9.
6
Moran, M.J., J.S. Zogorski, and P.J. Squillace, 1999, MTBE in Ground Water ofthe United
States
—
Occurrence, Potential Sources,
and
Long
Range Transport, Proceedings of the Water Resources
Conference, American Water Works Association.
7Landmeyer, J.E., Chapelle,
F.H.,
Bradley, P.M., Pankow, J.F., Church, C.D. and P.G. Tratnyek,
1998, fate
of MTBE Relative to Benzene
in
a Gasoline-Contaminated aquifer (1993-1998). Ground Water Monitoring
Review,
Fall Issue,
pps. 93-102.
‘USEPA, Office of Underground Storage Tanks. April
1988.
~Buxton, H.T., J.E. Landmeyer,
and
A.L. Baehr,
1997, Interdisciplinary
Investigation of Subsurface
Contaminant Transport and Fate
at
Point- Source Releases of Gasoline Containing
MTBE,
United
States
Petroleum Plumes as MTBE
Reservoirs
-
MTBE is both soluble
in organic as well
as aqueous liquid phases.
It is more soluble, by roughly an order of magnitude,
in the
organic phase. When releases or leaks ofpetroleum products containing MTBE float on
the surface of the groundwater, the petroleum plume may act as an MTBE reservoir
allowing MTBE to dissolve into the water layer so long as MTBE concentration are
available in the organic phase ofthe petroleum plume.
Thus, in considering the treatment
ofMTBE, the remediation must remove the original petroleum plume
containing MTBE
as a reservoir ofthe MTBE
while any
necessary
MTBE
treatment is taking place for a
CWS
at the entry point of its
distribution system.
Without attending to
the petroleum
plume as an MTBE reservoir, the treatment ofMTBE at a CWS
may become a lengthy
process.
The recharge of the groundwater with MTBE from the original petroleum plume
can
occur for long periods oftime.
The half-life of MTBE is listed as between 4 months
and2
years7’8.
MTBE
is
a
Progressive
Problem
-
As
discussed
earlier,
MTBE
has
a
very
long
residence
time
in
groundwater.
The source
of MTBE
contamination
is
often
leaking
USTs.
With
many known
and
unknown
aging USTs
still
in the ground
and
potentially
leaking,
the
increasing
contribution of MTBE
to
groundwater
seems
inevitable.
Since
MTBE
resists
breakdown,
any
addition
of
MTBE
to
groundwater
will
most
likely
increase the concentration of MTBE
detected in the downstream aquifer at some time
in
the future.23’4’5”°
Geological Survey
and Oregon
Graduate Institute of Science and Technology, Petroleum
Hydrocarbon
Conference
Proceedings.
9Keller,
A. A.,
O.C. Sandal!,
R.G. Pinker, M.M. Mitani,
B.
Bierwagen, and M.J. Snodgrass,
1998, Cost and
Performance Evaluation of Treatment Technologies for MTBE-
Contaminated Water, Bren School of
Environmental Science and Management and Department of Chemical Engineering, University of
California Santa Barbara.
‘°
RFG/MTBE
Findings
and Recommendations, August
1999, Northeast States for Coordinated Air Use
Management.
6
CURRENT
TREATMENT METHODS
COMPARED
Natural Attenuation /
Biodegradation
-
Scientific studies have been performed
that show natural attenuation of MTBE in groundwater is negligible.
MTBE is
considered persistent, or recalcitrant, in groundwater and degrades very
slowly by natural
chemical or biological
degradation.
With the recent
introduction ofMTBE into the
underground environment, sufficient microbial organisms do not exist in most natural
settings to
degrade MTBE7’8.
Acidic chemical breakdown ofMTBE can occur, but at
lower pH levels than typically observed in nature. A study by Lawrence Livermore
National Laboratory in California determined that very limited
evidence exists that
natural attenuation ofMTBE is occurring in the field.’2
Chlorination
/
Sodium
Hypochlorite
-
The
typical
chlorination process
used
to
disinfect
drinking
water supplies has been shown to have no
noticeable effect on MTBE
concentrations.~
Ultraviolet
Irradiation
-
High-energy
ultraviolet
light
can be
used
in
a
similar
manner
as
chlorine
to
disinfect
drinking
water supplies.
The
UV
light
disrupts
DNA
function and is
designed to
effectively kill
all organic life in the water stream.
However
effective
this
method
is
on microbial
life in
the potential
drinking
water,
it is ineffective
on MTBE.
Experiments performed at the University of California Davis confirmed that
there was
no evidence of MTBE degradation in water upon
exposure to
UV
light emitted
by a low-pressure mercury lamp.’1
Reverse
Osmosis
(RO)
-
This
process
utilizes
a
semi-permeable
membrane,
which allows only small particles to pass through.
For instance, reverse osmosis has been
used to
filter
salinity
(salts)
out of seawater
to provide fresh drinking water for areas with
extreme
water
supply
problems.
For
large
pumping
rates,
this
method
can
be
very
expensive,
depending
on
the
constituents
in
the
water.
To
date,
most
membrane
technologies
are
not
applicable
to
volatile
organic
chemicals.
Little
information
is
“Chang,
P.
and T. Young. Reactivity
and
By
Products of methyl Tertiary Butyl Ether Resulting
from
Water Treatment
Processes, Department of Civil
and environmental Engineering University of California
Davis. (http:tsrp.uedavis.edulmtbreptlvol55.pdf)
7
available
concerning
removal
of
MTBE
using
RO
filtration.
Ultimately,
the
high
equipment cost,
maintenance,
and
filter
replacement costs
would
cause
this
method
to
lose
cost-effectiveness.
These systems
are expensive even for home use, which
in
most
cases is purification ofalready treated water.
Even under those conditions,
filters must be
replaced periodically.
For the cleansing ofraw water for a CWS,
filter replacement costs
would
make this
method
impractical unless
the
source water influent
into the treatment
system
was
fairly
clean
to
start
with
and
the
flow of water through
the
system
was
moderate
to low9.
Granular Activated Carbon (GAC)
-
Most concentrations oforganic chemicals in
a water phase are effectively reduced when treated with GAC.
With MTBE, however,
GAC is not as effective treatment medium due to the limited adsorption capability of
GAC for MTBE.
When used alone, removal of MTBE by GAC is not considered cost
effective for treating the large volumes ofwater used by a CWS.
Cost prohibitively large
units or multiple pass GAC systems may be
necessary to reduce the levels ofMTBE to
desired concentrations.
GAC will also
be reduced in its efficiency to remove MTBE if
the influent water contains TDS, metals or especially organics.
If benzene or other
organic chemicals are present with MTBE, MTBE adsorbed on a GAC
filtration
unit
could be
dislodged by the benzene or other organic compound sending a large spike of
MTBE through the treatment system.
To protect from such an
occurrence would require
careful monitoring ofthe
GAC system when GAC is used as a
primary
method of
treating MTBE.
Such monitoring of the GAC system will also increase costs.
Studies
have shown that MTBE
may be treated cost-effectively with GAC only at low
concentrations.
GAC may be
useful
and
cost
effective as a means ofsecondary
treatment
as a polishing step following some other forms ofMTBE removal9.
Air Stripping
-
Air stripping
one
ofthe most cost-effective approaches for
removing VOCs from groundwater.
Since MTBE is a volatile organic chemical with a
moderately high vapor pressure, one would expect it to be
susceptible to
air
stripping.
MTBE, however, is not
an efficiently
air stripped under moderate conditions due
to its
high solubility in water and its low Henry’s Law constant.
The high solubility ofMTBE
‘~
Happel, A.M., E.H.
Beckenbach,
and
R.U. Halden,
1998, An Evaluation of MTBE Impacts to California
Groundwater Resources,
Lawrence Livermore National Laboratory,
University of California.
8
requires the construction ofmuch larger air stripping units
than constructed
for
conventional VOCs,
which would
impart higher capita! and operating costs
for MTBE
treatment.
However,
if the temperature of the
influent
water
containing MTBE
contaminants
can
be raised significantly at reasonable cost, the size of the stripping unit
can be
reduced with the same or similar removal efficiency9.
In various field studies, MTBE has been air stripped effectively, but it requires
very
high
air to
water ratios, the use ofinfluent water heating to
facilitate volatilization,
and the use of a packed tower
with
appropriate
media.
In
one
study,
at
44:1,
75:1,
125:1,
and 200:1
ratios of
air-to-water
the following removal
efficiencies were achieved,
respectively: 44,
51,
61,
93-99,
At such high
air-water
ratios, however, the
media in the stripping tower can become clogged with precipitating scale and freezing
problems can occur in cold months.
One study found that heating the influent water from
10°Cto 27°C
increased
the efficiency of removal by a factor oftwo.
This
would require
pre-heating the water, which would add additional
cost.
The cost of air stripping is
approximately one-halfthat ofGAC, but this does not include treatment ofthe resulting
gas stream containing the MTBE vapors.
Ifthe facility
is in an air pollution non-
attainment area and cannot release MTBE into the atmosphere, treatment ofthe gas
stream will be required.
This will roughly double the cost, thus decreasing the cost-
effectiveness ofair stripping as a treatment option
~.
If MTBE vapor treatment is not necessary, packed tower air stripping may be
coupled with GAC treatment and
air!watei’
stream heating as a cost-effective method of
reducing concentrations ofthe contaminant.
Currently, this appears to be the most cost-
effective
method oftreatment compared to other proven methods
~.
~Keller,
A. A.,etal.,
1998.
9
TREATMENT SUMMARY
With
the limited
field-tested data available
for most recently
researched
methods
of MTBE
treatment,
few viable
options
exist that
have
wide
applicability
and
are cost-
effective.
It
is
important
to
note that
for traditional
technologies such
as
GAC or air
stripping, the average costs for treating MTBE-contaminated water is 40-80
higher than
treating waters
containing benzene or other organic chemicals.
Air stripping is
the lowest
cost technology for high flow rates (100-1000 gpm), if no
air
treatment
is
required.
Air
treatment
can
be required.
Hollow fiber membranes
are the
lowest
cost
technology for
low
flow
rates (10-100
gpm), if no
air
treatment
is
necessary
(which
is
normal
at low
flow rates).
GAC
will be most cost-effective
at all flow rates if air
treatment
is required
and
the
influent
water has
low
levels
of other
organic
chemicals.
If air
treatment
is
required and high levels of other organic compounds
are detected,
air stripping
is
more
cost-effective
than GAC
at flow rates of 100
or greater.
Advanced oxidation
processes
(“AOP”) are in all
cases
more expensive than the alternative technologies, and there are
sufficient uncertainties at this point with respect to by-products of AOP to warrant further
study
of this
technology before accepting full
utilization.
At
high flow rates, however,
AOP
may
become
cost-effective
compared
to
other
technologies, pending
further full-
scale field tests.
Various forms ofbiodegradation may in fact soon take precedence over
some these methods, but at this time there is not enough field study
completed to
warrant
full implementation.
Most
sources claim that
treatment
options for MTBE
in
groundwater should
be
conducted
on a case-by-case
basis.
Each well may have different sets ofparameters with
respect to
other wells,
Factors
such as pH, pumping rate,
facility design,
water hardness,
inorganic
levels, level
of MTBE
contamination,
and
the
level
of interference
by
other
organic contaminants will differ by well ortreatment application point.
10
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY’
BLUE
RIBBON MTBE PANEL FINDINGS
On November 30,
1998, Carol Browner, Administrator of the United States
Environmental
Protection Agency (“U.S. EPA”) appointed a Blue Ribbon Panel of
leading experts to
investigate concerns raised by the discovery ofMTBE,
a gasoline
additive,
in some water supplies.
According to the report produced from the Blue Ribbon
Panel, U.S.
EPA recommended that:
Recommended a comprehensive set ofimprovements to
the nation’s water
protection programs, including over 20 specific actions to
enhance Underground
Storage Tank, Safe Drinking Water, and private well protection programs.
Review
ofthe Blue Ribbon Panel recommendations and findings supports
inclusion ofa groundwater standard for MTBE4.
SDWA UNREGULATED CONTAMINANT MONITORING REQUIREMENT
FOR MTBE
U.S.
EPA recently adopted new revisions to the Unregulated Contaminant
Monitoring Regulation (“UCMR”) under the SDWA.
MTBE
is one of 13
chemicals
included in
this regulation.
One ofthe Blue Ribbon Panel recommendations consisted of
accelerating the UMCR for MTBE prior to the implementation date ofJanuary
1,
200113.
‘
USEPA.
1999.
“
Federal Register 40
CFR Part 9,
141
and
142. September,
17
1999.
Revisions to the
Unregulated
Contaminant Monitoring Regulation for Public Water Systems, Final Rule.
Vol
64, No.
180.
Ii
ILLINOIS EPA’S PROPOSAL TO
AMEND THE
GROUNDWATER QUALITY
STANDARDS
Section 620.31 0(a)(3)(A)(i)
Preventive Response Activities
This subsection has been amended to
include a preventive response level MTBE
based
on its taste and odor threshold.
Exhibit III details information on the taste and odor
threshold for MTBE.
Section 620.410(b)
This subsection has been amended to include a Class
I:
Potable Resource
Groundwater Standard for MTBE.
This standard is based on a draft Illinois EPA health
advisory,
developed pursuant to 35
Ill. Adm.
Code 620.605, and a
review
ofwhat other
states are doing.
Exhibit
IV details information on the health advisory information for
MTBE.
Section 620.420(b)
This subsection has been amended to include a Class
II: General Resource
Groundwater Standard for MTBE.
In the original regulatory proceeding, R89-14(B), the
Class II:
General Resource Groundwater standard for organic constituents was based on
the capability oftreatment technology to
achieve the Class I standard.
The treatment of
MTBE
is very difficult once it has dissolved into the groundwater.
The Henry’s law coefficient for MTBE is very low making it difficult to remove.
Granular activated carbon is also
not effective because MTBE does not readily adsorb.
Thus, the Class II standard is also
proposed at 0.070 mg/i.
Section
620.505(a)(5)
This subsection has been amended to not exclude compliance points that are valid
for determining groundwater quality,
and in certain instances may be existing potable
water supply wells.
12
CONCLUSION
This concludes my testimony.
I will be happy to
address any questions.
L:/epa3 I 88/docs/regulatory/IG PAIMTBE/MTBEtest,
February
15,
2001
‘3
EXHIBIT I
—
Curriculum Vitae of Richard
P. Cobb
14
CURRICULUM VITAE
OF RICHARD P. COBB, P.G.
I. Personal
A. Present Position:
Manager, Groundwater Section,
Illinois Environmental Protection
Agency
II. Education
1981
B.S.
Illinois
State University (Geology)
1984
Illinois State
University (Hydrogeology and Engineering Geology)
1986
United States Geological Survey National Training Center (Geochemistry for
Groundwater Systems)
1986
Illinois
State University Graduate Geohydrology Program (Hydrogeology of Waste
Disposal Sites)
1987
Illinois State University Graduate Geohydrology Program (Hydrology of Glacial
Deposits in Illinois)
1992
United States Geological Survey (MODFLOW and MODPATH groundwater
modeling)
1994
24 Hour Occupational Health & Safety Training
1995
Illinois
State University Graduate Geohydrology Program (Computer Modeling of
Groundwater Systems)
III. License
Licensed Professional Geologist
196-000553, State of Illinois, expires 3/3 1/2001
IV. Certification
Certified Professional
Geologist
7455,
Certified by the
American
Institute ofProfessional
Geologists 4/88
Certified Total Quality Management Facilitator
Certified by Organizational Dynamics Inc., 5/92
V. Summary ofExperience
15
More than twenty years ofexperience of working as a professional geologist in hydrogeolôgy,
environmental
geology and petroleum geology. Twelve years of diversified, interdisciplinary
experience as a senior manager, junior manager of a technical hydrogeology unit, and
lead
worker for Illinois’
statewide groundwater protection program. Three years of experience as a
consulting well site geologist for major and
independent oil companies
conducting petroleum
exploration and
development in Arkansas, Kansas, Louisiana, Montana, North Dakota,
Oklahoma and Utah. Two years of undergraduate teaching assistant experience for several
geology courses.
VI. Summary of Computer Skills
I use the following computer programs: WordPerfect,
8.1, Microsoft Word 2000, QuattroPro,
FoxPro, Power Point, Freelance Graphics,
ARC VIEW II, Aqtesolv, SURFER, WFIPA,
DREAM, AQUIFEM, MODFLOW, MODPATH,
and Visual MODFLOW.
VII.
Professional Representation
A.
Illinois Environmental Protection Agency (Agency) liaison to the Governor appointed
Groundwater Advisory Council
(GAC).
B.
Agency representative on the Interagency Coordinating
Committee on Groundwater
(ICCG).
C.
Agency representative on the Senate Working Committee on Geologic Mapping.
D.
Agency representative on the State Certified Crop Advisory Board, and chairman ofthe
ethics and regulatory subcommittee
established in association with the American Society of
Agronomy/American Registry ofCertified Professionals in Agronomy, Crops and
Soils.
E.
Chairman of the Agency Geographic Information System Users Group.
F.
Member ofthe Agency Cleanup Objectives Team from
1988 to
1993 that established soil
and groundwater cleanup objectives on a site-by-site basis.
G.
Member of technical work group that developed Illinois
groundwater
quality
standards
regulations.
H.
Project leader for a special Agency work group that utilized vadose zone and solute
transport modeling to develop soil
cleanup objectives under different hydrogeologic settings
for the leaking underground storage tank program.
I.
Agency representative on a special subcommittee ofthe ICCG charged with the
development of a State Pesticide Management Plan for the protection ofgroundwater.
16
J.
Member ofAgency
task group involved with
developing the siting criteria for a low level
radioactive waste site
in.
Illinois.
K.
Environmental regulatory representative from Illinois
on the Fresh Water Foundation’s
Groundwater Information System (GWIS) project in
the great lakes basin.
L.
Agency representative on four priority regional
groundwater protection planning
committees
designated by the Director to
advocate groundwater protection programs at the
local
level.
M.
Representative on the Groundwater Subcommittee ofthe National
Section 305(b) Report,
ofthe Clean Water Act, Consistency Workgroup.
N.
Bureau ofWater representative on the Agency’s Locational Data Policy Workgroup.
0.
Bureau ofWater representative
on the Agency GIS Steering Committee.
P.
Member ofthe Ground Water Protection Council’s Wellhead Protection Subcommittee.
Q.
Elected Co-Chair ofthe Groundwater Division ofthe GWPC on September 1997.
GWPC
is a national, not for profit organization whose members are interested in the protection ofthe
nation’s ground water supplies. The mission ofthe GWPC
is to promote the safest methods and
most effective regulations regarding comprehensive ground water protection and underground
injection techniques.
GWPC’s meetings, workshops, seminars, and symposia provide forums,
educational resources, open communication, and active participation by its
members. GWPC’s
membership includes local, state, and federal governments, citizen groups, industry, academia,
and other parties interested in responsible protection and management of ground water
resources.
R.
Chairman ofIllinois’
Source Water Protection Technical and Citizens Advisory
Committee.
S.
United States Environmental Protection
Agency National Ground Water Report work
group member.
One of 10 state
representatives
serving
on a work group sponsored by U.S.
EPA headquarters charged with development of a national report to be submitted to the U.S.
Congress on the status and needs for groundwater protection programs across the country.
January
1999 to present.
T.
Northeastern Illinois Planning Commission Water Supply Task Force member.
The
purpose
ofthis task
force is to assist the Commission in the development of a Strategic Plan for
Water
Resource Management. March
1999
to
present.
U.
GWPC/1J.S.
EPA Futures Forum Work Group providing input on source water protection
for
the next 25 years.
January
1999 to present.
17
V.
GWPC/ASDWA work group providing input into the U.S.
EPA Office ofGround and
Drinking Water Strategic Plan for Source Water Protect. June 2000.
W. VIII. Professional Affiliation
National Groundwater Association
Illinois Groundwater Association
Association ofGroundwater Scientists and Engineers
American Institute ofProfessional
Geologists
The Society ofSigma Xi
Ground Water Protection Council
IX. Chronological Experience
9/92-Present
Title: Manager ofthe Groundwater Section in Bureau of Water at the
Illinois Environmental Protection Agency.
I
also serve periodically as Acting Manager for the
Division ofPublic Water Supplies.
My primary responsibilities include development and
implementation ofIllinois statewide groundwater quality protection, USEPA approved
wellhead protection program, and
source water protection program.
My responsibilities
include development and implementation ofIllinois statewide groundwater quality protection,
USEPA approved wellhead protection program, and the source water assessment and
protection program for surface and groundwater public drinking water supplies.
These duties
include extensive coordination with federal, state and local stakeholders that include the
Governor appointed Groundwater Advisory Council, the Interagency Coordinating Committee
on Groundwater, four Priority Groundwater protection planning Committees,
Illinois Source
Water Protection Technical and Citizens Advisory Committee and through being co-chair of
the GWPC Ground Water Division.
Additionally, work with the Bureau ofWater permit and
Mine Pollution Control Program staff to develop source water protection, groundwater
monitoring and aquifer evaluation and remediation programs.
I have also
served as a primary
Agency witness at Illinois Pollution Control Board proceedings in the matter ofgroundwater
quality standards, technology control regulations,
and
water
well setback zone exceptions.
Furthermore, I have served
as an Agency witness
in enforcement matters.
7/91-9/92
Title: Acting Manager of the Groundwater Section in Bureau ofWater at the Illinois
Environmental Protection Agency. My responsibilities
include continued development and
implementation of Illinois statewide groundwater quality protection and USEPA’s approved
wellhead protection program. Additionally, work with the Bureau of Water permit and Mine
Pollution Control Program staff to develop groundwater monitoring and aquifer evaluation,
remediation and/or groundwater management
zone programs. I also served as a primary
Agency
witness
at Illinois Pollution Control Board proceedings in the matter ofgroundwater
quality standards and technology control regulations. Additionally, serve
as an Agency total
quality
management
(TQM) facilitator,
and TQM
trainer.
18
Manage a statewide regulatory compliance program for activities located within setback zones
and regulated recharge areas ofpotable water supply wells.
7/88-7/91
Title:
Manager of the Hydrogeology Unit, Groundwater Section
in the Bureau of
Water.
Manage
a staff ofgeologists and
geological engineers that apply hydrogeologic and
groundwater modeling principals to
statewide groundwater protection programs. Oversight the
development, integration and
application ofGeographic Information System, global
positioning system, geostatistical, optimization, vadose zone, solute transport, groundwater
flow and particle tracking computer hardware/software programs
for groundwater protection
and remediation projects.
Provide
administrative support to
the Section manager in
coordination, planning, supervision,
grant application and management, regulatory and legislative
development in relation to the
statewide groundwater quality protection program. Establish soil
and groundwater cleanup
objectives on the Agency Cleanup Objectives Team.
7/85-7/88
Title: Environmental Protection Specialist in the Groundwater Section ofthe
Illinois
Environmental Protection Agency. Lead worker and senior
geologist in the
development and implementation ofIllinois statewide groundwater quality protection program.
3/81-12/83 Title: Consulting Well Site Geologist for Geological Exploration Consultants of
Denver Colorado. Worked as a consulting well site geologist
in petroleum exploration and
development for major and independent oil companies. Responsible for the geologic oversight
oftest drilling for the determination and presence ofpetroleum hydrocarbons.
Prepared
geologic correlations and performed analysis ofgeophysical logs, drilling
logs and drill
cuttings. Supervised and analyzed geophysical logging. Made recommendations for conducting
and assisted with the analysis ofdrill stem tests and coring operations. Provided daily
telephone reports and final written geologic reports to
clients.
1/79-3/81
Title:
Undergraduate Teaching Assistant for Illinois
State
University
Geology
Department. Responsible for teaching and assisting with lecture sessions, lab sessions,
assignment preparation and grading for petrology, stratigraphy and
geologic field techniques.
X. List of Rulemaking or Cases in Which Expert Witness
Experience Has Been
Gained
IN THE MATTER OF:
GROUNDWATER QUALITY STANDARDS
(35
ILL. ADM. CODE
620). R89- 14(B) (Rulemaking).
Subject:
I served as the principal Illinois EPA witness
recommending adoption of this Agency proposal.
R89-14(B) was adopted by the Board.
IN
THE MATTER OF: GROUNDWATER PROTECTION:
REGULATIONS FOR
EXISTING AND NEW ACTIVITIES WITHIN SETBACK ZONES AND REGULATED
RECHARGE AREAS
(35
ILL. ADM. CODE
601. 615, 616 and 617). R89-5
(Rulemaking).
Subject:
I
served
as the principal Illinois
EPA witness
supporting adoption of this Agency
proposal.
R89-5
was adopted by the Board.
19
IN THE MATTER OF: GROUNDWATER QUALITY
STANDARDS (35
ILL. ADM. CODE
620). R93-27 (Rulemak~g)~
Subject:
I served as the principal Illinois EPA witness
recommending amendments of
new
constituent standards
in
this Agency
proposal.
IN THE MATTER OF: PROPOSED REGULATED RECHARGE AREAS
FOR PLEASANT
VALLEY PUBLIC WATER DISTRICT,
PROPOSED AMENDMENTS TO (35 ILL. ADM.
CODE 617), R00-17
(Rulemaking)~Subject:
I served
as the principal Illinois EPA witness
supporting adoption ofthis Agency proposal.
IN THE MATTER OF:
NATURAL GAS-FIRED, PEAK-LOAD ELECTRICAL
GENERATION FACILITIES (PEAKER PLANTS).
ROl-lO (Informational Hearing) Subject:
I served as a supporting Illinois EPA witness to discuss the impact ofpeaker plants
on
groundwater.
IN THE MATTER OF: PROPOSED AMENDMENTS
TO TIERED
APPROACH TO
CORRECTIVE ACTION OBJECTIVES
(35
Ill. Adm.
Code 742). (R00-19(A) and R00-19(’B))
(Rulemaking)~Subject:
I served as a supporting Illinois
EPA witness recommending inclusion
ofMTBE in this Agency proposal.
STATE OIL COMPANY
vs.
DR. KRONE, McHENRY COUNTY and ILLINOIS EPA. PCB
90-102 (Water Well Exception). Subject: This case involved obtaining an
exception from thern
owner
ofa non-community
water
supply well for placing new underground gasoline storage
tanks within the 200 foot setback zone ofwell.
I served as the principal witness for Illinois
EPA on this case.
The Board granted the exception with conditions.
SHELL OIL COMPANY vs. COUNTY ofDuPAGE and THE ILLINOIS
ENVIRONMENTAL PROTECTION AGENCY, PCB
94-25
(Water Well Setback Exception).
Subject: A new underground gasoline storage tank was seeking an exception from the Illinois
Pollution Control Board in relation to
a private
drinking water supply well setback zone.
The
DuPage County and the Illinois EPA held that the tank would be a significant hazard and
opposed the exception.
I served as the principal Illinois EPA witness.
Shell withdrew the
petition from the Board after hearings were held.
People ex rel. Ryan
v. STONEHEDGE,
INC.. 288 Ill.App.3d
318,
223
Ill.Dec. 764, 680
N.E.2d 497
(IlLApp. 2 Dist.
May
22,
1997).
Subject:
State brought Environmental Protection
Act action against company engaged in business ofspreading deicing salt, alleging that salt
stored on company’s industrial property leaked into area’s groundwater supply, thereby
contaminating it.
The Circuit Court, McHenry County, James C. Franz, J., granted company’s
motion for summary
judgment.
State appealed.
The Appellate Court, Colwell, J., held that:
(1)
wells existing before Illinois
Water
Well Construction Code was enacted are not
“grandfathered”
in
as being
in
compliance with Code, so as to be automatically subject to
testing
for
groundwater
contamination, and (2) fact issues precluded summary
judgment
on
20
claim arising
from alleged deposit of
at
least 50,000 pounds of salt in pile within 200
feet of
two existing
water
supply
wells.
Affirmed
in part and reversed
in
part; cause remanded.
People vs. AMOCO OIL COMPANY and MOBIL CORPORATION,
Case no. 90-CH-79,
Tenth Judicial
Court, Tazewell
County, Illinois.
Subject:
Groundwater contamination resulting
from releases at above ground bulk petroleum storage terminals resulting in
violation of
Illinois’
Groundwater Quality Standards
Regulations (35 Illinois Administrative Code
620). I
served
as the principal Illinois EPA witness on this case.
The case was settled with a penalty
of $125,000 and the
requirement
of a comprehensive corrective action program.
People vs. STONEHEDGE INC. Case no. 94-CH-46, Circuit Court of the
19th
Judicial Circuit,
McHenry County.
Subject: This case involved
a violation ofthe potable well setback zone
provisions of Section 14.2 ofthe Illinois Environmental
Protection Act.
Stonehedge Inc.
placed a salt pile of greater than 50,000 pounds within the 200 foot setback of multiple private
drinking water supply wells.
I served as an Agency principal witness.
Stonehedge Inc.
was
found to be guilty ofviolating the setback prohibition in this case and was assessed a penalty of
$1,500 and attorneys fees of$4,500.
SALINE VALLEY CONSERVANCY DISTRICT vs. PEABODY COAL COMPANY. Case
No. 99-4074-JLF, United States District Court for the Central District ofIllinois.
Subject:
Groundwater contamination from the disposal of 12.8 million tons of coarse coal refuse, slurry
and gob.
Witness for the Illinois EPA.
This is
an on-going case.
XI.
Honors
Sigma Xi 4/81
Superior Performance
Award 1/86
Superior Performance
Award
11/87
Certificate ofCommendation for Groundwater Protection Programs 4/92
Certificate ofAppreciation for work on the Agency’s Cleanup Objectives Team 4/93
Certificate ofAppreciation for participation as an Agency TQM facilitator 4/93
Certificate ofAppreciation for participation on a total quality
action
team
4/93
Certificate ofAppreciation for participation in the Governors Environmental Youth Corps
Program 4/93
21
Director’s Commendation Award for participation in the development of the
City of Pekin,
Ii.
Groundwater Protection Program and commitment to the protection of Illinois
groundwater.
7/95
Certificate of Appreciation for outstanding contribution to the development ofthe Ground
Water Guidelines for the National
Water Quality Inventory
1996 Report to Congress from the
United States Environmental Protection Agency Office ofGround Water and Drinking Water.
8/96
Groundwater Science Achievement Award from the Illinois Groundwater Association for
outstanding leadership
and service in the application of groundwater science to groundwater
protection in
Illinois and in the development,of the wellhead protection program and pertinent
land-use regulations.
11/97
Certificate ofAppreciation from the Ground Water Protection Council for distinguished
service, remarkable dedication, valuable wisdom and outstanding contribution as a GWPC
member,
division co-chair and special committee member.
9/99
Drinking Water Hero Recognition by United States Environmental Protection Agency
Administrator Carol
Browner at
the
25th
Anniversary
oftheFederal
Safe Drinking
Water Act
Futures Forum in Washington D.C.
12/99.
Certificate ofRecognition from United States Environmental Protection Agency Region V
Adminstrator Fred Lyons
for outstanding achievements in protecting Illinois’ groundwater
resources.
12/99
XII.
PUBLICATIONS
A. Legislation and Legislative Development Documents
Co-Author
A Plan for Protecting Illinois Groundwater, Illinois Environmental Protection Agency, January
1986.
65
p.
Groundwater in Illinois: A Threatened Resource, A Briefing Paper Regarding the Need for
Groundwater Protection Legislation, Governors Office and Illinois Environmental
Protection
Agency, April
1987.
34 pp.
Illinois Groundwater Protection Act,
Public Act 85-0863, September,
1987.
68 pp.
~‘3
B. Regulations
Co-Author
Groundwater Quality Standards
(35
Ill. Adm.
Code 620), November,
1991. 79
pp.
Groundwater Protection: Regulations for Existing and New Activities within Setback Zones
and Regulated Recharge
Areas (35 Ill. Adm.
Code 601,
615,
616 and 617), December,
1991.
132 pp.
Principal Author
Maximum Setback Zone Rules For Community Water Supply Wells
(35
Ill.
Adm.
Code 671),
February 1988.
50 pp.
Minimal Hazard CertificationRules
(35
Ill. Adm.
Code 670), February,
1994. 21
pp.
Amendments
to
the Groundwater Quality Standards Regulation,
(35
Ill. Adm.
Code 620),
February 1994.
Regulated Recharge Area Regulation for Pleasant Valley Public Water District,
(35
Ill. Adm
Code 617), under development.
Maximum Setback Zone Regulation for Illinois American Water
Company-Peoria,
(35
Ill.
Adm.
Code 618), under development.
C. Groundwater Quality and
Hydrogeology
Principal Author
Cobb,
R.P., and Sinnott, C.L.,
1987. Organic Contaminants In Illinois Groundwater.
Proceedings ofthe American Water Resources Association, Illinois Section, Annual
Conference, Champaign, IL, April 28-29, p.
3 3-43.
Clarke, R.P., and Cobb, R.P.,
1988.
Winnebago
County
Groundwater Study.
Illinois
Environmental Protection Agency. 58 pp.
Cobb, R.P., etal, 1992.
Pilot Groundwater Protection Needs Assessment for the City ofPekin.
Illinois
Environmental Protection Agency.
111
pp.
23
D. Groundwater Protection Program Documents
Principal Author
Buscher, W.E., and Cobb, R.P.,
1990. Maximum
Setback Zone Workbook. Illinois
Environmental Protection Agency. 62 pp.
Cobb,
R.P.,
1990. Illinois Groundwater Protection Program: A Biennial Report. Interagency
Coordinating Committee on Groundwater.
53 pp.
Cobb, R.P., Buscher, W.E., and A.
Dulka,
1991. Illinois Approved Wellhead Protection
Program Submitted to the United States Environmental Protection Agency Pursuant to Section
1428 ofthe Safe Drinking Water Act. Illinois Environmental Protection Agency.
44 pp.
Cobb, R.P.,
1992. Illinois Groundwater Protection Program: A Biennial Report. Interagency
Coordinating
Committee on Groundwater.
118 pp.
Cobb, R.P.,
1994. Illinois Groundwater Protection Program: A Biennial Report. Interagency
Coordinating Committee on
Groundwater.
118 pp.
Cobb, R.P.,
1994. Briefmg Paper and Executive Summary on
the
Illinois Groundwater
Protection Act and Groundwater Protection Programs with Recommendations
from the Illinois
Environmental Protection Agency Regarding the Siting of a Low Level Radioactive Waste
Site. Presented to the Low Level Radioactive Waste Task Force on December 9, 1994 in
Champaign-Urbana.
Cobb, R.P., 1994.
Measuring Groundwater Protection Program Success. In the proceedings of
a national conference on Protecting
Ground Water: Promoting Understanding,
Accepting
Responsibility, and Taking Action. Sponsored by the Terrene Institute and the United States
Environmental Protection Agency in Washington D.C., December
12-13, 1994.
Cobb,
R.P.,
Wehrman, H.A.,
and R.C. Berg,
1994. Groundwater Protection Needs
Assessment Guidance Document. Illinois Environmental Protection Agency.
+94 pp.
Cobb, R.P., and Dulka,
W.A., 1995. Illinois Prevention Efforts: The Illinois Groundwater
Protection Act Provides a Unified Prevention-Oriented Process to Protect Groundwater
as a
Natural and Public Resource, The AQUIFER, Journal ofthe Groundwater Foundation, Volume
9, Number 4, March
1995.
3pp.
Cobb, R.P.,
1995. Integration ofSource Water Protection into a Targeted Watershed Program.
In the proceedings of the GROUND WATER PROTECTION COUNCIL’S Annual Ground
Water Protection Forum in Kansas City Missouri.
24
Cobb, R.P.,
1996.
A
Three
Dimensional
Watershed
Approach: Illinois
Source
Water
Protection Program.
In the proceedings of the GROUND
WATER PROTECTION
COUNCIL’S Annual Ground Water Protection Forum
in Minneapolis, Minnesota.
Cobb, R.P., and W.A. Dulka,
1996. Discussion Document on the Development ofa Regulated
Recharge Area for the Pleasant Valley Public Water District. Illinois Environmental Protection
Agency. pp 28.
Cobb,
R.P.,
1996.
Illinois Source Water Protection
Initiatives-Groundwater Perspective.
In the
proceedings ofthe American Water Works Association’s Annual Conference and Exposition in
Toronto Canada. pp 585- 594.
Cobb, R.P.,
1996.
Illinois’
Groundwater Protection
Program: A Biennial Report. Interagency
Coordinating Committee on Groundwater.
93 pp.
Cobb, R.P., and Dulka, W.A., 1996. Illinois Community Examines Aquifer Protection
Measures. American Water Works Association JournaL
plO.
Cobb, R.P., McMillan,
W.D., and K.E. Cook.
1996. Drinking and Groundwater Sections of
Illinois Water
Quality Report (Section 305(b) Report.
Cobb, R.P.,
1996.
Illinois’
Core Comprehensive State Groundwater Protection Program
Application. Illinois Environmental Protection Agency.
159 pp.
Cobb, R.P., 1998.
Illinois Source Water Assessment and Protection Program Application.
180
pp.
Cobb, R.P., et
al. October 1999, Ground
Water
Report to Congress, United States
Environmental Protection Agency.
Co-Author
Clarke, R.P., Cobb, R.P. and C.L. Smnnott,
1988.
A Primer Regarding Certain Provisions ofthe
Illinois
Groundwater Protection Act. Illinois Environmental
Protection Agency. 48 pp.
Kanerva, R.A.,Clarke, R.P. and
R.P.
Cobb
1988. An Issues /
Options Paper for
Comprehensive Water Quality Standards for Groundwater. Interagency Coordinating
Committee on Groundwater. 25 pp.
Kanerva, R.A., Clarke, R.P. and R.P Cobb
1989. Discussion Document for Comprehensive
Groundwater Quality Standards. Interagency Coordinating
Committee on Groundwater. 25
pp.
Dulka, W.A., and R.P. Cobb,
1995. Grassroots
Group Forges Groundwater Protection Law.
American
Water Works Association, Opflow, Vol.
21
No.
3.
2pp.
25
E. Geology
Principal Author
Cobb, R.P.,
1980. Petrography ofthe Houx Limestone
in Missouri. Transactions of the Illinois
Academy of Science Annual
Conference,
Illinois Wesleyan, Bloomington, IL..
26
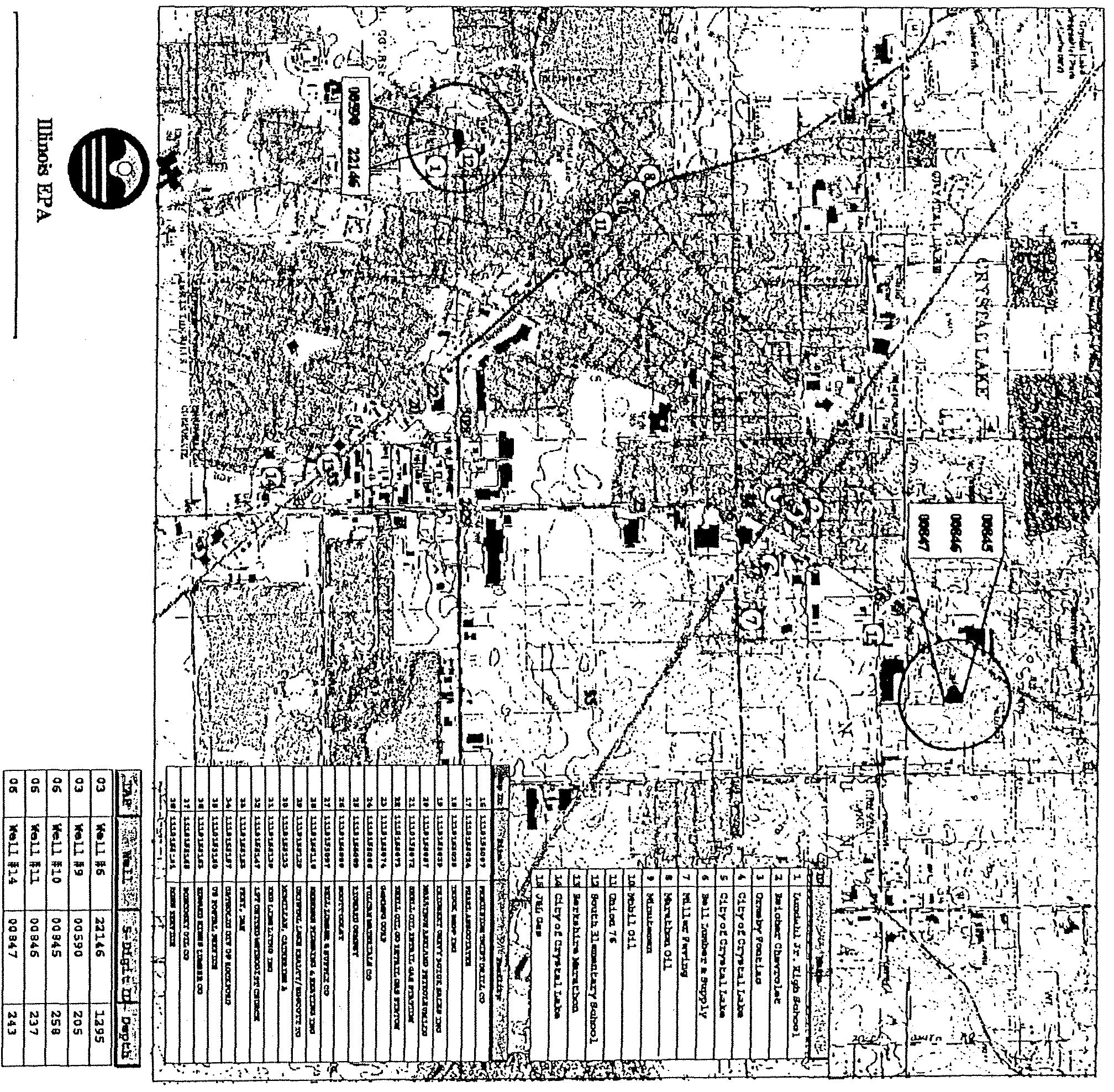
EXHIBIT Il—Maps of Community Water Supplies with MTBE Detections
27
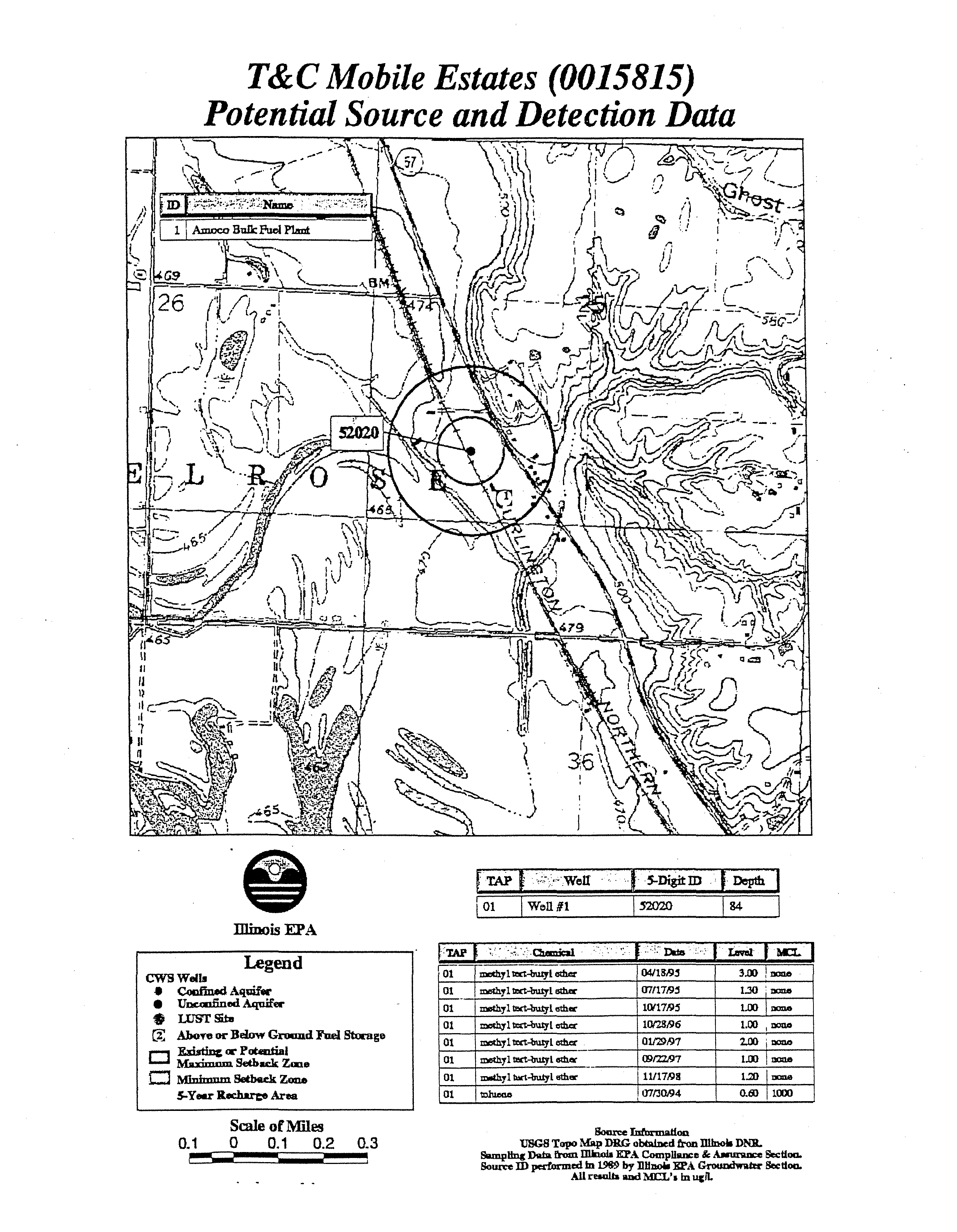
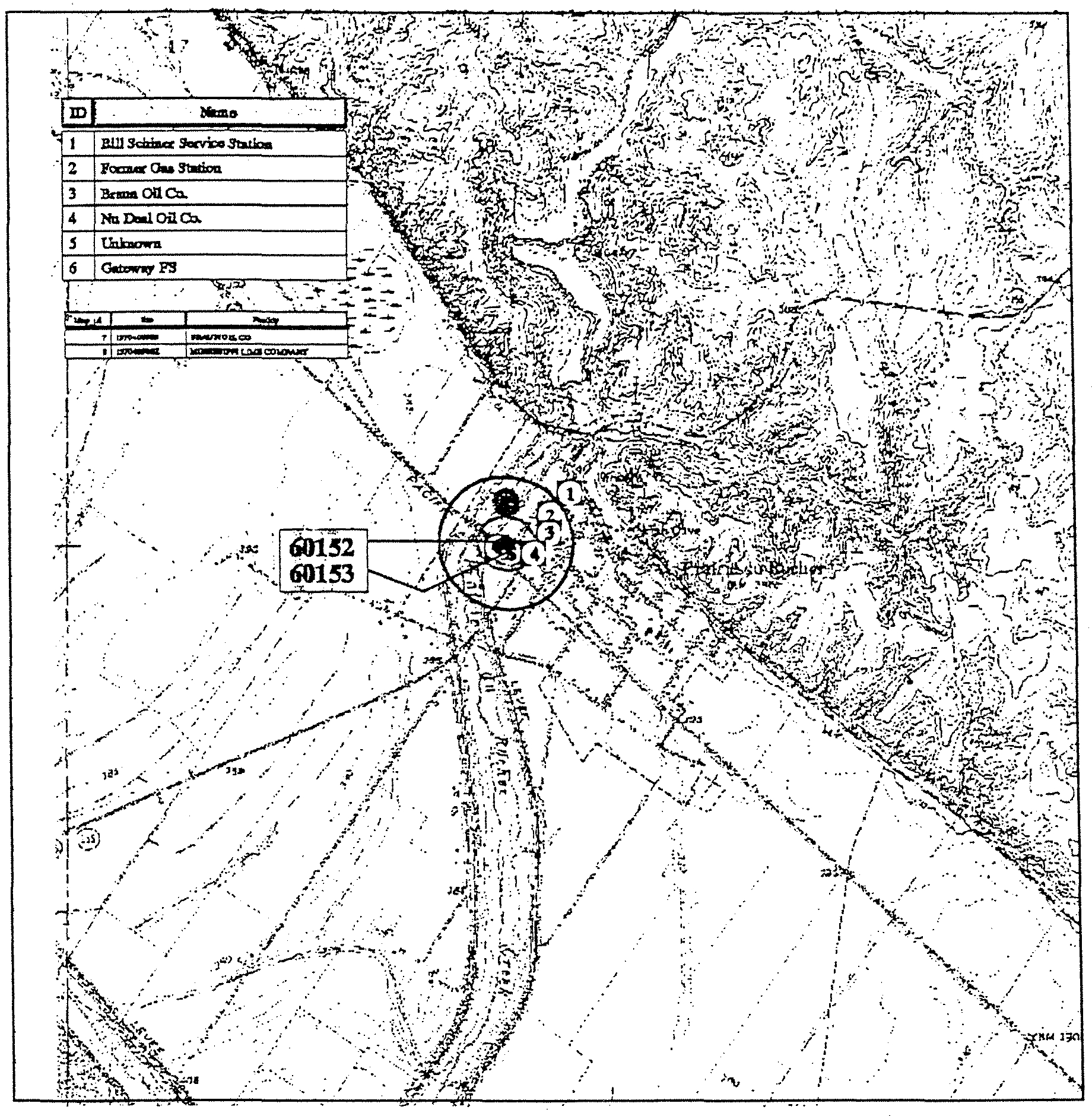
T&C Mobile Estates
(0015815)
Potential Source and Detection Data
iAi~
I
~‘:~WeU
~
5~-ñigitm
IJDpthi
roi
w~il#i
~52O2O
84
Illinois
EPA
Scale
ofMiles
0.1
0
0.1
0.2
0.3
~i~eth~
04/13,~.i
3.00
~L_
r~thy1~t-bu1y1
ath~
~I7fl7~9i
1.30
~J
~a
~.a
a__
~thy1
t~t.buLy1
ethu~
1G’17~95
LIX
oma
z~othyl~t-butyLcth~
10t2&96
1.00
~a
~hy1trct-u~y~eth~r
01J2~i97
2.00
mono
~h~1
t~ct-bu&yL
ath~
-______
(9/22i97
1.00
m~e
~hyltart.1~uiy1
ath~
11/17i9~
1.23
~a
~2L_
I
~ø1u&~o~
07t3(194
0.8)
1000
So~eInformaffon
~BG8
Topo Map
DRG ob~abcd
fronIllinois DNR.
~mplhig
Data from llb~1i
E?A
Comp1I~nc~
& A,suraj~r~ct1oo.
Sourve ID perf.ormt~d
In 19?~)
by
Illinois
XPA Growxlwater &cdoo.
AU rewlia sadMEL’s
knuglL
-
‘~‘
_\~___
.
--
~
‘~
-
-“~
.
..-
—
.~-
-
l~
I
::
~
)
‘—v
~
T
;~‘~
Aaa~Fue1P1~
it
\“\
~)
/1/ji
.‘ji
~G9
I11~
‘k~
~:—-.~
~
~
~/4.~/
~
r~:P~~
~
-~
-
-
..~
I
.
\
..
.
/
~
.~
.
~.
:4r’
~‘T~
.~
-~
~Lil
:
/
~‘
?LL\L.
o
..
\\
st~
~
~
/
‘~
\~
~
~/‘
/
/
~
SN
—
...
~
~
~
~
•1
.
/
..,
S.\
\
~
~_‘~\
~
‘::~
~
c~.
~
-.~
~
-
(S
,
a
~
_
~t
/
~L’~
~
~.
\‘~
)
~)/?tr~
~
-
~
~
(L~Y
r~
~\
)
~~~:-;‘
~
Legend
CWS Wells
•
Coufined
Aquifer
•
Uz~m~nrd
Aquifer
•
~
M,o~ooi~
Below Ground Fuel Sterago
r,
Ezisting
Potisitial
~‘
Maxiixmm Setbaok
Z4xie
M1y,Inm~~~
Setback
Zona
5-Ye.r
Rechar.
Area
~c.i
I
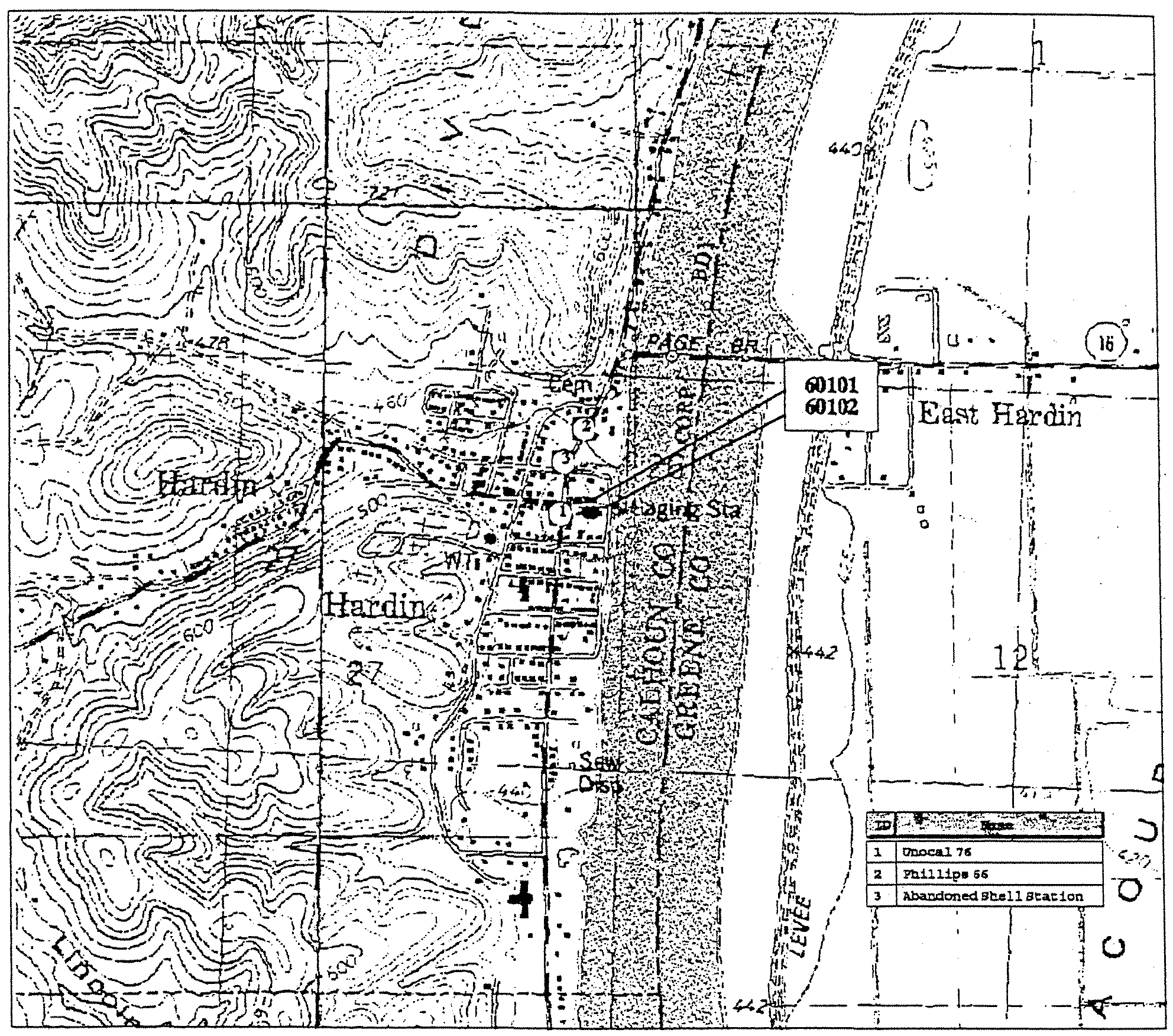
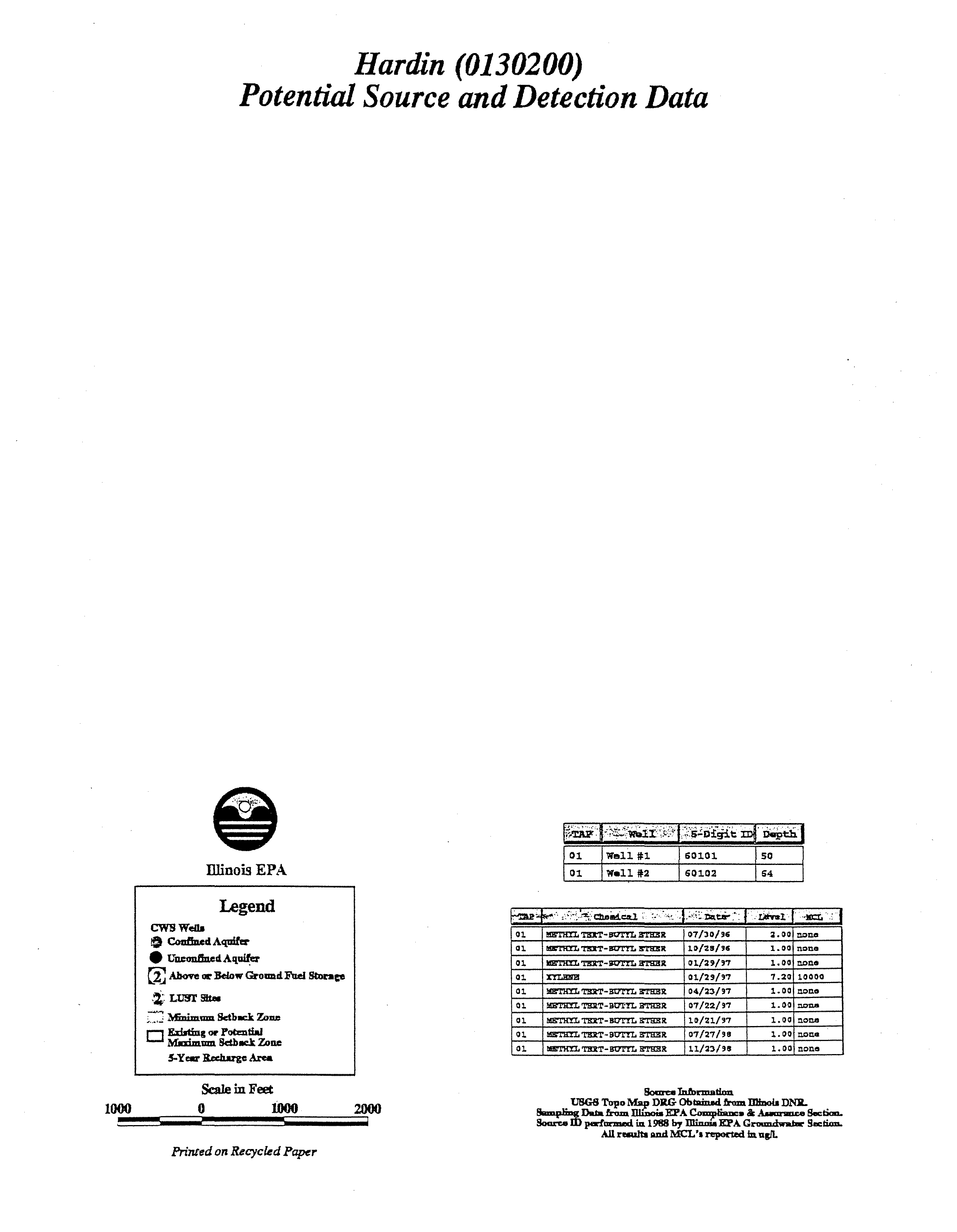
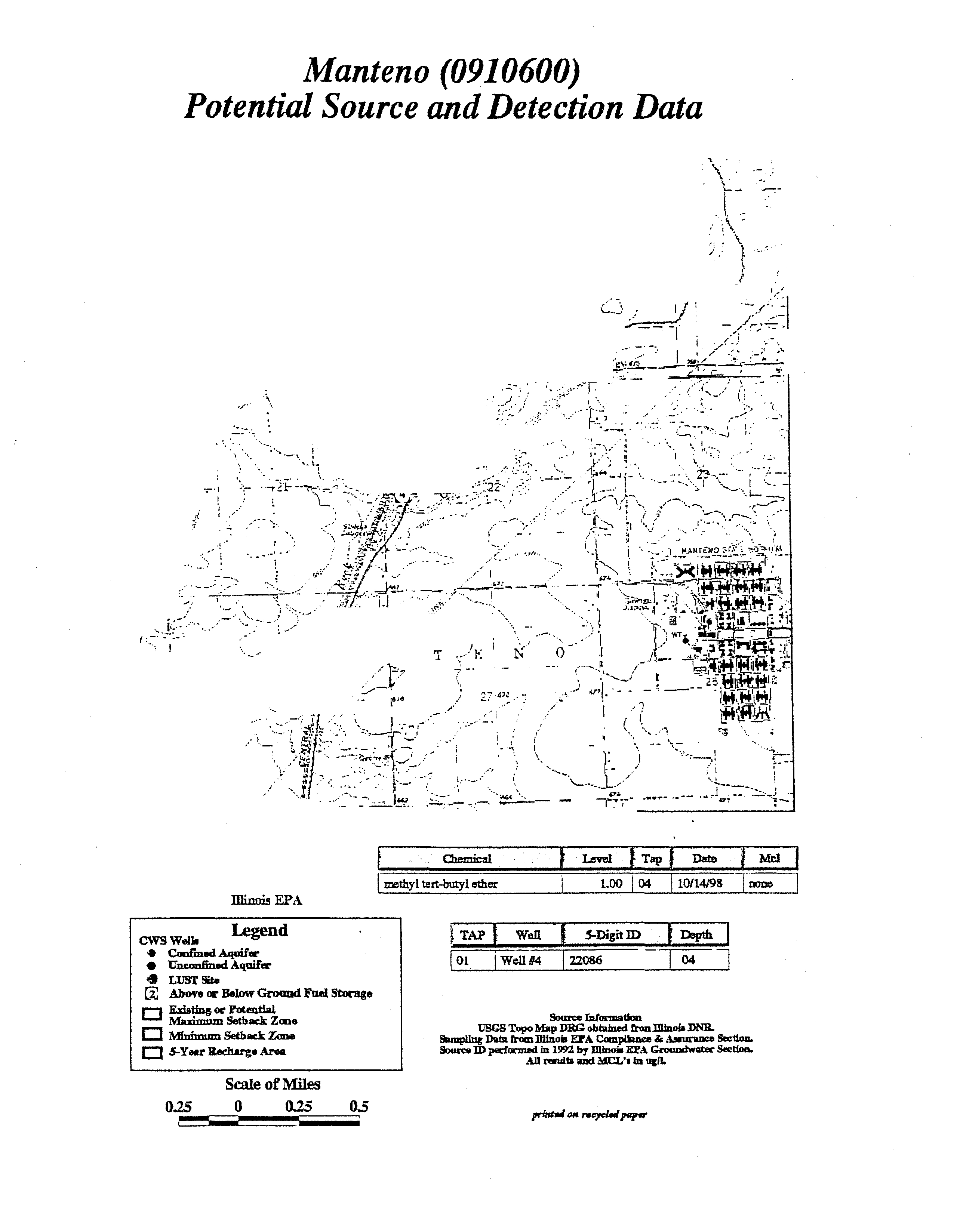
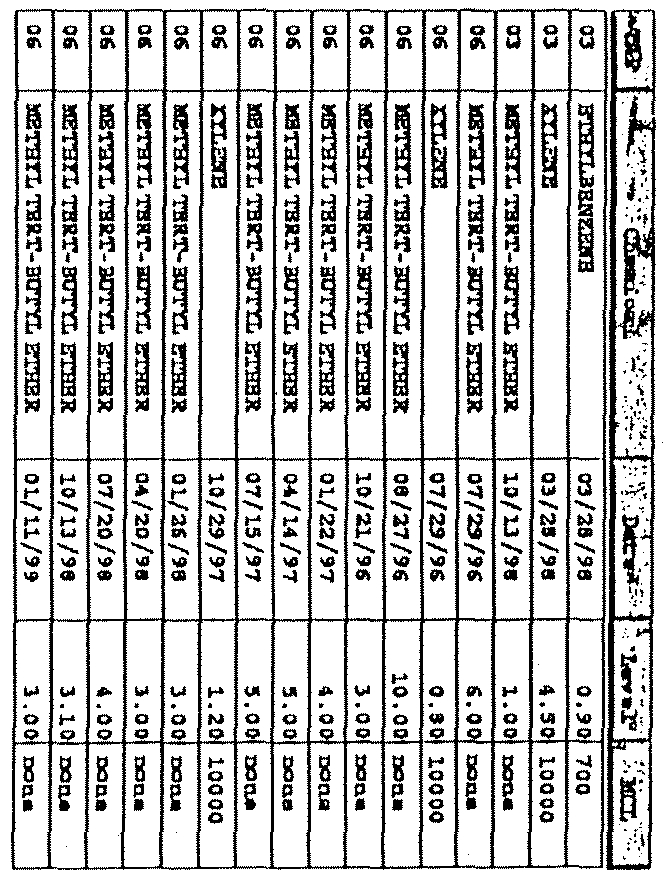
Hardin
(0130200)
Potential Source
and
Detection Data
flflnois
EPA
Scale in Feet
1000
0
1000
2000
01
Wall
l~1
60101
50
01
Wall
~2
60102
54
,
L
‘z~wait
~
~ii~
arrHI1TaxT-eurmrr~R
07/30/36
3.00
~ooo
err
1.~rarr-at7r77.
rrase
3.0/23/36
i.oo
rena
01.
err
L~1~r-~rrL
a~resa
o1./39/o7
1.03
rena
01.
X0L33B
0t/23f37
7.20
1.0000
01.
errxTh1~7-3~7yL3Tgu
04/23/37
1.00
rena
01.
MeTEILIUT-atY1.YX. 0T3~
07/22/31
1.00
rena
egrxo1~rezT-errrr.rr~a
10/21/07
1.00
nona
-
03.
07/27/33
1.00
rena
~.L_
eri,tU1aeT-3orT1~eaR
11/23/33
1.00
none
,Tnformadon
VSGS
Topo Map DRG Obtainid from IThnoleDN2
~mpling
Daia
from
IIIZDOI,EPA Co
han.
&
Aaaixrann. 8.cti~m.
Soarta 11) p~Iurmrd
Li~
19~8
by
IIliwna
EPA
Groundwidar ~ecthm.
All reaultsand MCL’e reported lnnç!L
Legend
CWS
Well,
~
Con~ncdAqulfor
•
UnemthoedA~uIfu
Above orBehow’ GimmdFuel Stnra~e
LTJ&T
SItes
~nimum
Sctb.ck Zone
Ma~momSetback Zone
Priiwed
on
Recycled Fczper
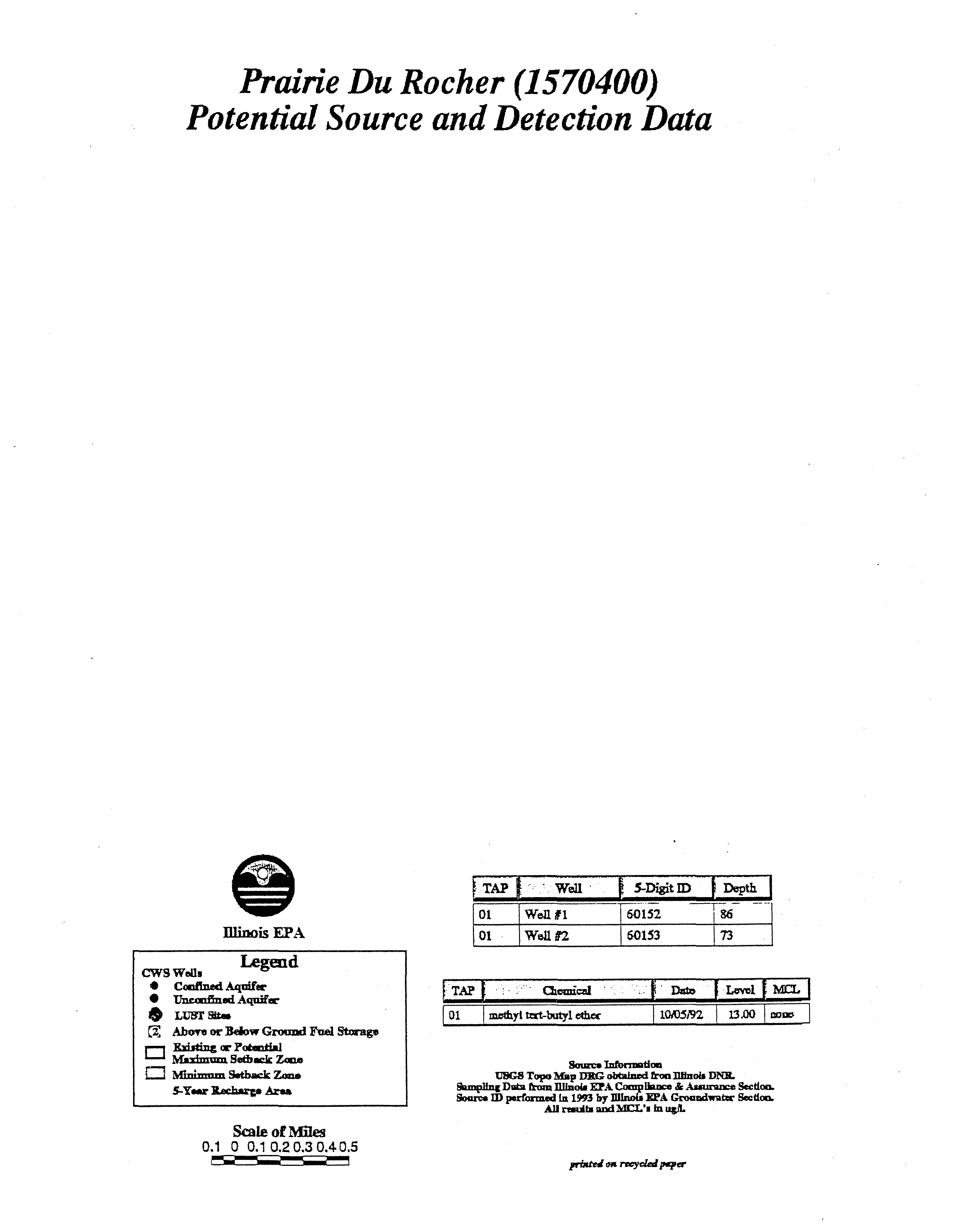
Germantown
(0270350)
Potential Source and Detection
Data
Scale in Feet
1000
0
1000
2001)
SourceIni~ormadon
tSGS
TopoMap
D~G
Obtained
from
Illinois IXhR.
Somplin~
Data fromIllinois EPA Coz~lisnce& Aauzr.uea Smtioa.
SourceID pi*lurmcd in19~8by IllinoisEPA Groundwater Section.
AU resultsand MCL’e reported inugll.
1flinoi~
EPA
Legend
CWSWell.
~
CaufinedAqotifur
•
UixnufincdAtp~ife’
AbovoerBelow Grojad Fuel St~sg.
“~
~‘
?~4.nhr,~n,.
Setback Zone
El
Fajating
ir
Petenliai
M~mmn
Setback Zone
5-Yesr bcbsrteAzca
oe~~I
~11~l.
60085
29
01
I~U~2
50005
29
01
t~l1
~3
50087
29
01
Wall
~4
60088
28
~.
erL7zIvr-rc~rmaTeBa
02/10/95
5.20
none
-i
01
0I~~T29r-5t7rYIWrB5Z
07/05/97
2.30
none
01
r1~2YLa~Z~2
12/14/93
1.90
700
01
X~L3N1
I
12/14/95
9.10
1000
liditaloa
~icyd.i
Papa.
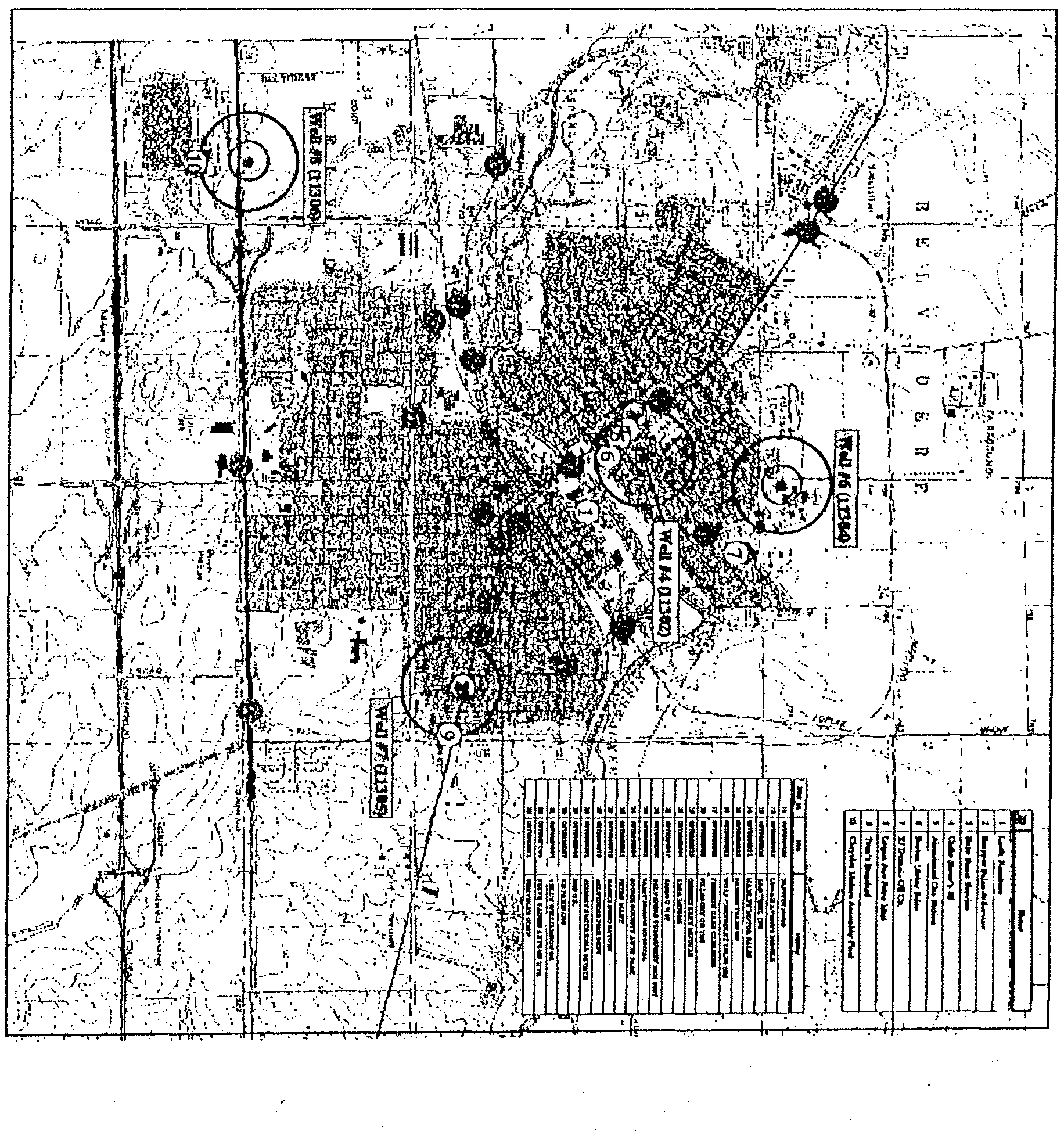
Grafton
(0830200)
Potential Source
and
Detection Data
-v.)4
-h..
,
~~:;
j
~
~
~
(
~i
~.
p
~1.
~
_~-..t
I
f
,
~.\_
~
..
.
~.
,
~3_
.
-
2
-~‘‘
~
~
.~.!
.
~ •
.
‘.
-
.
~
.-
j
‘
:~t
~
~
~
‘4
.
~
:
•.~~
-
..
~
-.
‘•
~
.
~--
‘~
.-~‘
~
~
.
-
-..
~
.....
..
S.
(
~
~
a.—.
~
~.,-
__S
~
•
.-
~
~
—
-‘:-~
~
—
_dt__.
~S•~
~
~
~
.-
.:
~•
~ .~.:
~
?
~
~
.
.
~_:
~~1~i~•::
—
—
.
..
~
.
•~
•~.r••~
1~i
~
~
~.
‘
1~
.-
.
.
r.
n~
•
.~.~
~3
_j~
u’.’-
\
—.
\
..
~
~
,
_
-‘
‘;•.~1.
~
•
1.—I
•.
..
-
.
-
..
-
,/
--
.,~-.-:
‘~‘
k
~
~
•
~
-
...
‘
‘
.
~
.
,
,
.
.t.-
,
.
.
.
.
~
. -
.
?~~
~
~
.~t
~
.
.
.
..
~:
.1
~°°~
60093
~ ~
~
01131
01132
.
.
~
. •~
.
~
..
.-
~
~
,
-
~
.
.
-.
.-
‘1
‘s.”
n
~
I
~
,
~
I
~
I
~
~
i_~._~t__e::.:~..~
‘I’
::
~
.
~
.
.
.~..
~.,
,.
.-
.
¼
~
r:-:i~!
.
~
.
.
..
~
.‘
~
,,
.
.
•
.,
..
.
~
.
.
I
~
~
.
—
‘
I.~
~
~
.~
~.‘
;~
-
..
.
-
11.
‘•f
•.:
;~1~
~
•~
,
•~
.
-
.
.
‘:-._.
~,II.s
,~
.
.—,
.__t.
,
~
.‘
.
‘~a
.-
~
—.
c
‘~
fi’i
~-_.
::
~
-.—
——..
1.~-
~
•
L
~.
\
..~
~
L
—.
1.3
—~
_
/
i.
..~.
—
S_
~
~
‘3~
l~’_
~
,
_—_t——_’
.____L.—
.—a..
~
-__.:.~..-;-_::;~-
...~...
P
_
I..
~
~
-
~
~
:~
~
-
-~
-~
‘i.
—
-.
.,.
-.
—~
-,-
-
-~
—
~
-..‘
.. -
~-
—
-
~
r
~
./i~
-..c
-.
~
~—e~
-
-‘
~
..~_.
-~
..-.
—
-.
-
-
3
‘~, ,-—
~L’~
~
~
ii
‘~--~2
~
-
6
-
~-
-
-
- -,
1
-
~-I
-~
~
0
-
~
.,~
-
/
~.‘
~
-
-.- -~
—
-.
-.
-?
—
—
—
-
-n
..-S~
~
//
(/~~
~o-~-~
~
..
-,.
-
..--.
-~
—
-
-.
.-
...
..
-~.
1-
~~‘:
—~~-‘
~
~
:~
~
--
-
~
\t5
T~
~
~
~
0530205006
rerrnrnos~rxoaa
.—
— ,~-/-.~—
-.
_______________________________
4
3530230005
2RA3~w~
c~oa
I
~-
‘
~
-
I
1
I
xectia.nc~
Inc
~‘,moco
3
0530220009
I5~OER3013IZQA9C0
~7’
—
-...~
1
2
~f.oii
Twin. Ri7ers
~evelnpment
~
/~‘
20
——
-
I
‘.-
—~
~
~
AI1pr~
~
.
-‘.
I
~
Illinois
EPA
F!i
101.
l~11*2
50092
55
101
0(e1153
60093
63
r~
wall 5.
03.133.
9
I
Well
52
03.132
0
L~ei
~‘~~‘chanxc3.~
~
~
f
0
01
slErHmTewr-BuTsI.
rrotut
03/11/97
-
i.oo
01
lerHYLT2aT-3~rLrrH~
04/16/97
1.00
none
0.
orrx?LTSaT-HQTIL
5~2~
10/19/95
2.00
none
Legend
cwS
wall
~
Ceaftsad âqalfar
•
~bq-yeer3eiswC~,eaI
Pusi Sloeeu
LV~T
SItes
Ml,Li.noS.th~kZ.os
El
Tx~5er
Set~deZa..
5-Tesr llc~ri.
~
Scale in Feet
1000
0
1000
2000
3000
US08
TopoMe~p
ORG Obtained
from Illinois
DNL
Sampling
Data from
Illinois
EPA
Comj~hiaiace
& Assurance Section.
bum.
IDpezloroned in
l9~
by
flhlnoss Rome!
WaterAaaoclstisii.
All resultsand MCL’. reportedin ugii.
ceo Rrc~yr1cdPeper
Illinois EPA.
Scale
in
Feet
1000
1000
200)
3000
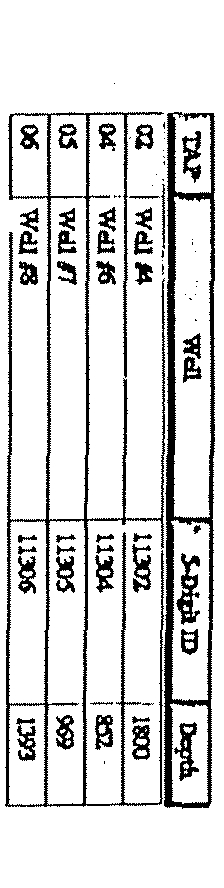
~T1~?
~-WeI1~
~J
r~~j
I
~
Wall
#5
20098
63
05
~el.*7
00901
1300
j~~j
~~ca1~
~:
Z4~D5~a~
-~
03
X~(L3NE
05/02/95
j
0.60 10000
03
h~TrLTl~~r—8t3TtLEThtRR
10/17/96
2.00
non.
Las
rti~sa
10/33/57
0.60 10000
tECh
Topo Map ORG
Obtained from
DEnnis
IP~L
Sampling Datafrom Illinois EPA Coao$ionwa & Assurance Sactine.
Source ID perfermed in
1997 by fllinoss
EPA Gioundwe~ Section.
All
results and MCL’i reported inugh.
South
Elgin
(890800)
Back to top
Potential Source and Detection Data
Legend
CWS
Wells
~
Cesitsid
ctqed~r
•
Uecneflaed Aa;u~r
Abcs.
orSelow Graa.d
Teat
Stesag.
-~
LUST Sitea
=
Miniu.m
SetbackZea.
El
~Istln5
ari’atesthl
Maxinix. Sattack Zoøs
S.T~arbebacg.Azac
Printed
on RecycledPaper
Manteno
(0910600)
Potential Source
and
Detection
Data
I-’
-
1
—
r
-
—~
22
—
~
~--
-
-
-
WAMts/0r~
~
M
--:‘~44
~
-
-
:~•
/.
~
I~
~
-- -
~--
~-
~
~
-
-.
•~
-.
—
-
-
-
/-------~-
/
-
-
1
_~IlbPi~~
)
27-~’~-~
-
-
—
—
-
—
-
- -~‘
~I
-
I
-
-‘
-.3
I-
-,
--
_~——~-———
--
.~
-
Scale
ofMiles
0.25
03
-
I
~thyl
~~tt-bi.i1y1
oilier
~-LeveI
L0O
JTap~
Data
04
j
10/14/98
JMCI
~~ne
1
~TAP
~
Wefl
5-DigitlD
-~
Depth
oi
1w
22086
04
1
Source
fmmatkon
USGSTopoM!ip
DBJ
obtainedfun
Illinois DNB.
Sançllng Pain fromUhinots
ErA Ccenpltsnce
& Assurance Sertlou.
Source IDperfir7ned
In
1992 by Illinois EPA
CrouomiweterSecUoo.
All results and
MUL’s In u~IL
/
‘-I-
-
—
Illinois
EPA.
CWSWeThe
Legend
•
Confined Aquifes~
•
Unconfined Aquifer
•
LUST
Site
~
Ahoy.
~
Below(h~oomd
Fuel Storage
D
Existbi
or PotentIal
Maxinwm Setback
Zone
D
r~en~n
set~
Zone
D
5-YearRscharge Area
025
0
printai~
Os racyclaJ7izpsr
Island
Lake
(0974540)
Potential Source
and
Detection
Data
~
~
I
~
-~
•1
H
?3(
(‘
~
..,
//
~
/~—~-~
‘~i
~.
~.
-
~
~
~
~
~
~—
—4-.
-,
-
~—
=-
—
—~_._2~~
;
~
~
~*~
~
/
I
~
-
~
~..
\.
/
y’.c~
~
/
*
~
-—~
~
~
c-~
~
-~
—
r
—~th”~
-\
~
~
:
~
~
-‘
i.\
~
~008U9..
.~‘-..~-~‘--
-.
4~\
~54
~,
-c,.
-
I
\
~
~-
-~
\
-
3
—
~‘
e-~
~-
~‘
:
itt,
~
—
-
I
—,
—
‘I
.---
-
~.._-‘
~t:-~’
~
~
;.~.,
~
-\~-~-
-
~.::
~
~
0
)
‘-
.••1
—
~
/
—
~
~
c
-~
~
~
~2
S
\
‘~
/
~
~
-~
~
r~
-
-~
-a’-
~
4
--
\~
~t~il1
I
.~-
çi~~
~
~
_2
0974545002
~LoIxLco
-
:‘~
~
~
~
‘~
~
~
-—
---—~—-—
-
..
.
I
~
—s-
-.a.-
1
A6~A.i!c*lenry
—
~
°~
/
-‘
~?
f
~
OP
1000
0
1000
2000
09
Wall j~7
toans
e5
09
wallM4-G
00625
146
09
W.ll 1~4-10
00614
146
09
W.11l~3
00921
992
09
Wall
415
01100
1310
j:TA~
t.~.T-1~.LaV~1.j
~l~T.t~
Os
wruyLT~-aurfLwr112a
12/09/93
50.00
nOne
09
t~T~tTh73X7-SUTTh37H3~
01/25/94
37.00
nOne
09
02/15/94
30.00
none
ros
rBThTBnT-BLflYt.BT~t3R
05/25/94
2000
none
illinois
EPA
Legend
CWE
Wa*a
~
t~oufls.d
MuIf~
•
UmsaaoiAq.~tsr
Alice.
or
lelow CmensA toutStnzeg.
Z
LUST
Sites
Mlutssom
Setback
los.
D
E~xtatsg
or Pitsotisi
~xInam
Suttack lea.
Scale in Feet
Saints
Information
USGS
Topo Map
ORG
Obtainedfrom
Illinois
DNL
Sampling
Data
frone
Illinois
EPA
Con~uIIance
&
AseumnmsSection.
Source
ID
pwfurmed In
19~
by flhincia
EPA
Groundwater Section.
All results and
MCL’s reportedin
ugh.
printed onRecyded Paper
LI
N~.__—
-
-
-
‘~a~~7~r-
~-
‘-~1~
~i~2*’-
17~T~f~
~
~-~a~-
~:
~
•
I
~
_*
‘-0~~
I
~
_.~~__J’..__
.-‘~!t
-.
—
a
•
•
q
~
-
~\.
\\
~
~
(~
~
1
~
-
7
~
~
~
~2
‘,
~
k~~
~
~
~
/
I
~.
/
~i’..—..
c~.—-—
-
~
~---~3--~-
1~i~
(y~~J721
I
~
~
___
(
~
___
110~3a74
0so~
~
-
--
__________
—
—~r
—~
—
:
~
~
——
/
I
I
t110t~51~ø
DC
POSSOX.
—
__._
..4_._
.L._.
~_
‘c.
i
1L13155t6~
,XanS
tO~CZ~O
CD
J
~-
J.
J
7
r
~—--~—-
~
F.
Wl1
5—Digit
~
52
Jweii
82
00280
1320
~
~‘-~
C2~eeicaL
Oat,
tv.i
MCL
02
~
~
~T0rL
TERT—6U31L
CTHER
MET9YL
7ERT0UTYL
0799R
MZrH’CL
TOR7-BUTtL
07M~R
03/06/95
05/16/95
02/25/97
4.00
I
12.05
8.00
non,
none
-
non,
Crystal Heights Association (1115100)
Potential Source and Detection Data
I
Illinois
EPA
Legend
CWS
Wells
•
Confined
•
Unconfined
•
LUST
Sites
Mininum
Setback Zone
J
Existing
or Potential
Maximum Setback
Zone
Scale in Feet
500
0
50O_~_
Source
Information
USGS
Topo Map
ORG
Obtained Uhinoi~
DNR.
Sampling
Darn
from lllinoia EPA Compliance
Aoauxanct Section.
Source ID performed in
1985 by lihinoi~
EPA
Groundwater
Section.
All
reaulta
and MCLs
reported in m~/1.
1000
Feet
Printed
on
Recycled
Papar
Li
I
j
E
U
flU,~ew~
Lj~.
II~
~4
I
a
a
I
McHenry
(1110600)
Potential Source and Detection
Data
-
~
1~1__.~~~!~‘~1~~~~
~
.:‘~
~~•—~‘.i’~
~
nn~.i~
2020721U08
~
~
x
~
~
“~,
‘~‘~
~
~
z~~1~_t__~
‘-~
~
1~-~:Ii_11
~
_~l
\
~
__
-
II
/
I
1
‘~
‘—‘
‘
~r
~
~
~
~r-’er~a
)I~~Ik
j~
~
~
~
-~~/_flI~”/~1
~
•t~
i-.
~
-
*4’
r
-
_
—
~‘
~
—
r—
L
..—
-
/
.-
/
~‘
/J~st~’
-
~
-.
~
~
.~“
~
~
~:~-_
~
~
~
~1-.
~ç~:
:/
~:-~
-
.i
~II.’~
-~
~
~
~L.!-
~
~
4Well~~j
~
~.Depth
I
01
We.1I~2
20207
60
Well
t~3
20208
185
01.
Illinois
EPA
Legend
CWS
Wells
~
Con~ned
Aquifer
•
ticon~ned
Aquifer
Mauve or Below
GroundFurlStornge
~::
Minimum
Selbeck
Zonc
~
Existing er Potential
Maximum
Setback Zou1e
5-Year Recharge Arts
Scalein
Feet
~at4
;..Lertsl
1
~--
I
Mgrtrrt.
Txa’r-ntrryr4
OnIBR
I0l/26/98
980125
j
1.00
nort~J
SourceInfo~nati~u
USGS TopoMap ORG Obtained from Illinois DNL
Sampling Data
from illinois
EPACompliance & Aamrmce Section.
Source IDperfumed in
190~
by Illinois EPA Groumdwa~Section.
All results anal
MCL’s
rrpoctrd lnugIL
1000
0
1000
F~rlmtrd
jjg
Recyded
Ptiper
Marengo
(1110650)
Potential Source
and
Detection
Data
Scale in
Feet
1000
I)
1000
Sauces
tofunattan
130GB Tepe Map D~G
O4ataiaad
fram
Ulinais DNR.
Saiuplia~
Th.ta
Pow-sn
Uliassis EiSA
Cnuylisnce
& Anuranc. S.ctiaaa.
SaucesII)
parferrmei
in L9~*
bylimo,
EPA
Gtnozoelwwb.r Seetian.
All
r..xlta
.ordMcli
raportad In ulL
1linois
ZPA
Legend
~--I.~-n-~~
oo
SIUCS
201*3
II
104
ww-Ll*1
00145
50
CWS
Wells
~
ConflnedAqulfer
•
TJncmafnedAquifer
Above or Below GruuaulFuei Storage
-~.
LWT
Sites
L.~
Minimum
Setback Zone
D
Existing or
Potential
Maximum Setback Zone
5-Year Recharge Area
~_
.~
St
~
-~-
n/mISC
g_
Ca,
S..
tL.L’
04/il/IS
1.51
1.5
a_.
L.inzn
!!—.—
~z
21/IC/IS
StIlls/CC
04/Ca/SC
2.SC
S.C
5.20.
S.C
l.a.
5.2
a
L~~5
IC/CS/CO
1.55
5.2
!!.__
I.f~5
1.11
S.C
•t
12/72/55
1.50
5.5
fl
1!!’fl
Sn/Cl/St
5.35
1.2
is
,~l
Ca/il/Ct
a.,,
S.C
01
anesn
-
2*/CS/Ct
3.42
S.C
02
l~fl
n/li/Ct
t.ss
a..
CS
I~S
P5/Cl/SI
l.a
,.a
~2
~
I*
0210170.
nw—7a
Ones
rn/nm
52/It/PC
,,,s~ s-a
3.55
55
SC
fltWTh?~3_yThCllfl
raeant.n~—nra0.xTtes
CC/mt/S.
55/IS/SO
1.15
—
5.oSIau.
a.
~
fl_~_
ct_yr.
tm—sown
awn
c,Thtm-isrT0.321fl
nttTtt..flttThSlCfl
ISIS,,,.
12/CS/CC
CC/la/SI
•.ssI—
a. sa$
fl__
~a12tL
,—xvrfl
Olin
CC/ma/SI
3.2*
wn
annxeSw-llflnT.
STIn
I
nTOThtStflt0.
liSfi
G4
nnyr.ssn—aowmnnsn
fl/wa/fl
Ia/SC/P.
51/iT/SC
a.
so no
-
saul no
-
m.
—
&bptd
~w,
2a.7dend Pope~
L
~
~
-
~
~
..
-~•
~-
~
I
-
1,.
~
t__.
-
-
Corr1
~
‘-~
I
-
~
~
—.
I
~
.
1._n’
l,•
-V
-.
-
~‘•~
/
~
—..
.
-~
-I
-.
•
~.
—
-
-—
~
..~
.
.
.
-
-
.
-—
-.
~-
——
.
—/~
-
I
—.
i
-
—
-.
0.
•.
I
-
.
~
-..
~1
\..
-
~
I.
-
-
ri~-
-
N
r’-~
s’•~L
~r~—.1t
..
.
.~“
9
-
I
—.
aI_
-
...
—
-
2’-
-
.
•‘~
-
-.
.
-
.
-.
~
I)
.
-
..
/
-
-.
-•
-
I.
—-_—-l
-~(~~‘
—
..
—
I.
•
.~
—,
-
•
---.-~:.~.
I..
.L~.
-
IJ.
-
-
~
-~-~
-.
•./‘•.
)_
-
.~?
.
.•.-.
•
...-~•
—-:.
.-~
‘-~~
-.
I’-
-
o~.
~
‘-~
—-:~~ -
1:
.--?
-•-
~‘
~•
—
1
-
-
—.
-
~.
-
.~
-
•~:
-
--.
-.
-4-
r—~
C
I
~-
I
I
I
i
‘
‘—~
I
I
.
.
j.
O,gr
—
-
——
‘
-
-
-
._Tr-....
-
-
-
r
-
I—
1
4
r.~1
..~.
~
~
-•~
~
I.~
--
•~
•i
‘---
~.
•1
t.
._
.1,
~
.
-—.5
.
.
.
S
‘
II
j
I
-
°‘O
j
I
/
—
~—
_.~-Z~
-~
~
~.)
-~
I
-
-
-~
—
I
—
IL)
r
-~
-
—
~
I
/
~
-
~
~
I
L
*
—
.1--
-
.
~
~-
.-
.~
-
~
-:
~
I
-
01
\V:ll
#1
I
47607
Dl
Wofl#2
1155
47608
101
IWdfl~
60
I4~09
E~
---_~-:-:.~-~(~~
~L4
01
~7t~
ms7ttut.brt3-t,th.c
Y26s96
10/21197
3131
4133
ama
ems
0’i~
besarso
.-.
1~
~0
b~e
-
9/19194
4131
6~
besamo
6/Z7193
LII)
10
01
d?~1bnzaio
9119194
2113)
119)
01
~thcazo,o
6/Z7l9~
0.11)
719)
0
oh~*eszma
6/3)196
5.11)
719)
01
.tpt~zmo
3/31190
0.5)
719)
t2kylbeszmo
6,23t98
3.5)
119)
01
sth1lbnsauss
9,29190
11*)
01
tthsano
9/19194
033
11910~
01
x~loss
~Etvimo
T
-
lAO
~XC0
9/19194
9.5)
11331)
01
9/31196
1.3)
119)13)
31
~isus
6/13/90
3.~
1~3)
Saybrook (1130950)
Potential Source and Detection Data
Illinois EFA
-
Legend
CWS Wells
•
Cesofined AqaMer
•
Uneeao~ned
Aqulfar
•
u~s’r
~ta
~
AJxn-.orBewGrotmdFmlStnnge
-
~
Existing ~
Potwtial
Mo.20S,TPnI
Setback
Zciia
~
MInimum Setb*ck Zum
5-Year
~acharge
Area
Scale
ofMiles
0.25
0
0.25
05
lousesInfuxoatlon
UBGSTopo
MapORGobtained frimlb~ois DINE.
Sampling
Data
frc*u flllnol*
EPA
Cotnpllance & Aunranc, Sectloo.
~
ITS
s~.4.assa.C
t..
1001
*.o
USIs.s.tp
WPA
(1.~....Cs.25.
n...,251.
~.
B,
*‘~
‘9”
If
I
•1
t~c~
L
~
~
~
~
01
1
C-C-
4201
‘4
52
I-
N
N
H
N
)4
Somca blformnton
USGS
Topo
Map ORG obtained froafllinoia ONE.
Sswnpllasg Data fromIllincin
EPA Cainpllance& A.eaar,ncs Seethan.
SoureslDpedorinsdin1~8by Ulinota
EPA
Grotmdwalor Station.
All
resultssadMCL’s in
sag/i.
~3~omPt_~
eso~Cos
6
tk*sx.~s/as5abt,~’
7
~s3q.It~~at42eOs.
I
“~csspss’sGa-.sa
w
11
12
‘3
~.oaca
nd~l5ssesy
,taU2*~
c~I
3
Nokomis
(1350450)
Potential Source andDetection Data
—
-.
-
—
~I
I
‘~
—.
.
-•
•I---—~
-
•
I
-
I
-
‘.
—..—.
1’
~‘
I
.I
II
---.
./
.
-~
---•
.
,_j~T~
~
/
—
i
~
~
-
-•
-
~
~
-,
~
..~.......
~
2CJ~
,
—--‘
1
‘-k,
-‘r
-
-7
3
-
-
I
--
~•••/~•.~‘
~
I
I
‘
I
-
-
-
-
II
‘
-
I,I
-
:
-
:-
-
~~L-~T-.
‘-1
__________
-
!_
~-~_~_:
4;~
~
~
______________
-~.:.
-
-
-~
I
•
~
___________
I
I
T
Mb~’
I’
________________________
•
,~-
-:.~.-
-~
:~~~!‘4i’
N-
_________________
-.-—~_.-o..__~_.~o.’-..~
-..
~
.
.
-
.•
,~
~.
—
~
~
P.—
t~lCC.
~-
-5.
40A~J~dTt~4~i-1
I
13’ss.SstDtt3ali.ap
-
•i
--‘—-i
-
-
11
-
•-~q’;11~’1~
;1-
~
-
a
n.aaeso
Iaa~2m.csa
I
.
‘
‘—
t~
1’
-.1
I
01
1254am
~240512112
~Tsa~
in
‘
~
.
--
-
“-.4-
-
‘~
-
I
-
-
.‘~
-,
~
-.-:,.~
•,.
~
,;s..
2
r
~/N7..I
-..
~
-~
1
-
-.
—
—
—
-.—
—
I~
—
—.
52113
‘~“‘,
~
—
I
‘
~
52i14~
i-
~
/
i__I
-—
-
I
52112
i
-
-~-.
I
I
I
-
-
I
•
01)305
_‘~
k—
I
r
2
—
/
‘
52108
.~,l
~
—~
-
---.--
~r—”~/~
I
—
01191
~
~
/..‘
—
...
.._~
1
II
1
~
I
4
I
J—.4
—.-~—1
—I
4I
~
-
-
-
-
~~~_L:’~
T
ln0~
~-
I~
I
~
~
~‘
C,aa1ta,
1
_~__~
~
F:
I
V
/
F~
-
—~-
—~
I
-
~-
L~.i
:
~
-~
1~2
r
-
4~i~r
I~t’
~
~
~
C
~
-
-
—
-
~
--~~
~
~
-~
.-.
-~
I
/
~
7.
0_
I
1
I
•1•-•~
~-s~’
~-‘
IHOL,,
-
-•
,‘.‘•
--1-
~
•/~..
I
_!
-~
—
__~
—
—~~5
~
~
_~~___~,
-
-
-.~
_‘•~•-__-_L:-~
~
-
~
c~’-.-~
In
j
A
,‘
1°—
,
I
—
I
‘~
I
I
I
~t.
—
—
illinois
EPA
Well
-.:
5.~L±~Pt~
I
Well#4
52108
140
01
Wcllfl
52112
40
.~.L_
W~11#9
52113
47
01
WeIl#10
52114
49
01
Well
111
00305
-
41.
—
Well#13
01191
36
Legend
CWS
Web
•
Con6ned Aquifer
•
Unionsfinpd Aquifer
4
LIJEE
Site
ci~
Abo~
or Below
Gri’critdFI~eIStorage
D
£xiitia~or
Potential
Maximum Setback Zzcs
Mlnimwn Setb~kZoos
-
5-Year Rschaxge
Scale
ofMiles
0.5
II
03
F~-~.~anuics1
om~y1trrt.bii1y1ether
3126i96
LO0
methyl
~et.buty1cthu
3/11,97
L00
2~_
6/20195
(1.60
5.0
beaszeuam
12/26195
1,00
5.0
~J
onrseyd.Jpaapar
Prairie
Dii Rocher (1570400)
Illinois
EPA
ScaleofMiles
0.1
0
0.1 0.20.30.40,5
-TAP
-.
-
-
We
-
-
j
S-Digit ID
Depth
01
Well #1
f~oi52
--
--
01
Well#2
60153
73
TAP
-
:‘-
-
aicu~
-
-
-
I
Le~J
~LJ
01
methyl
t~t-buty1
ctbcc
10~)5I92
j
13.00
Souse.
nfonouthmwm
USGS Pope
Map PEG ob(~IxscdfreeIllinois
DINE.
Sompling
Debt
from Illinois
EPA
Coampikoce &
Asgureen Section.
Source 1)
performed In 1993 by
flhlzwfi
EPA
Ctmmdwater
Section.
A.ll resoits andMCLe heu~!I.
Potential
Source and Detection Data
Leg~id
CWS
WeL1~
•
Cesoftued Aquifer
•
Th3w1ñn1d Aquifer
•
LUST ~
~
Above or
Below Ground Fuel Storage
~
Existing or Fot*anial
Maxlmuzn
Sed,ack Z~ce
o
~nm
Setback Zee.
S-Year
1ecbarr~.Area
,riatric*
rrcyckJpsp~
Scale
of Miles
TAP
Well
5-DigitID
L
I~Pth
I
T03
Well#
00247
62
L~i
-
c~~’
F
Dam
Level
f
~
03
methyl
tect.bulyl ether
OSA)7196
2.00
e.~ee
Soutte Inforusition
USGS Topo Map
ORG obtainedfron IflInola
ONE.
Sampling Data from Ulinois
EPA
Complienes & Ajaurnee Section.
Source ID perfonnedin
1990by Illinois
EPA Ground~ter
Section.
All results
and~dUL’.in sag0.
Rushville
(1690200)
Potential Source and Detection Data
Illinois EPA
Legend
CWSWr&li
•
Confined Aquifer
•
UncamifinedAquifer
•
~
Above or BtIow
GroundFuel Storage
D
Ezisthm
or
Maximinn Setback Zon.
D
z~mnmrm’
setiac~
Zosi.
5-Year
RechareArea
0.1
0
0.1
0.2
pr~et.d
eta
rwcyc1cdpqn~
Creve Coeur (1790100)
Potential Source andDetection
Data
0
Fr~-~
_____________
illinois EPA
____________________________
~-ra~ ~
--
I
--
-
o*~*-
-
(
-
-
i..va~
-
01
asthy).
0a.ie~bu0yl
.t~ae
1/29/so
0, OO36~~esaa
0~.
methyl
t.rt-.biityl
sth.r
3/26/90
-
0.00320
eons
0~.
matby1tset-bim~yla-t2~r
12/29/90
0.00370
eons
01
methyl
cart~bunylether
1/L6/9~.
0.00690
none
01
methyl
tsrt-bi~nyl
ether
2/13/91
0.01000
nnrma
2.~_.
methyl
e-art
-b~yl
sc~r
4/23/93
0, 00230
none
01
methyltaz~t-b-u~ylethsr
6/16/9
0.00260
none
01
methyl
tsrt.b~tyl
sthe~
9/24/9
0.00140
none
01
methyl tert—bunyl
ether
12/17/93
0.00120
con.
01
methyl tart—butyl
ether
1/14/94
0.001101 eon.
01
01
methyl
tent
-butyl
ethex
meyltert-.butylether
4/20/94
6/30/39
-
0.00110
non.
0.00100
none
01.
-
Woll#1.
-
-
50382
91
01.
W.ll *3
-
50383
78
01
Well*4
50384
81.
Legend
CWSWelII
~
Couthoed Aquifei
•
Uncesiflned Aquifer
~
Abov,
or Below
Grow*d Foal Storage
O
E~uabng
er
F*esuial
-
Maximwn Setback Zone
____Setback
Zone
5-Year Rcchar~e
Axes
Scale
of
MiLes
ILl
1)
0.1
0.2
0.3
SourceThfora*tion
USGSTopo Map ORGobtained
frost Illinois ONE.
Sampling data obtained from AMOCO.
Source IDpeelbeinedin
1959 byllllnels
EPA Growidwmster Section.
All results aisi?&~L’e
In
usJL
pr0u~mioa
r*~c/e*ipepw
Marquette
Heights (1790400)
Back to top
Potential Source and Detection Data
Illinois EPA
Scale
ofMiles
0.1
0
0.1
0.2
TAP
-~
Well
-
--
-
-
5-Digit ~D
~j
Depth
L~
We!1#4
50280
95
01
WeIl#5
50231
94
L?d
-
~
--J:~. P1~J~LI
01
I
n~hy1
tert-butyl ethee
i1fl)9~90
Lll)
I
01
nyltt..botylethee
07i07j90
L20
cone
SourceInforimetion
USGSTopoMapORG obtainedfoe illinoIsONE.
Sampling PainfromIllinoIs
EPA
Compliance
Se A.uwunceSection.
Source 0) performed
in
1990 by minois
EPA
Groundwater Section.
All resedts and ~L’s
In ugh.
CWSWell~
Legend
•
ConfinedAquifer
•
TJxzexaifinrdAquifer
•
u~r~t.
—
~
Above
or Below
Gruuzui
Fuel Storage
-
Existing orPetartiial
Maxio.mnn Setbeck
Zosie
Minimum Setback Zone
5-YearRechargeArea
pimtriam
rrcycivdpoprr
North Pekin
(1790550)
Potential Source and Detection Data
illinois
EPA
Scale
ofMiles
TAP
~
-
--
--
-
~1e1l
-
-
- -
- -
- ~
~
11)
01
Weu#2
50211
104~
02
WrilfI
50210
85
j
~T~I—-~
--~
I~(
~I
:01
nothyt
t
r9-bot~-I
ethac
07123I90
0.40
neon
01
mcthyl
t~t-bo~y1
eth~
07/23i90
0.90
neon
-
01
ne~y1~t-kotyl
tibcr
-
07i23i90
0.~
neon
02
methyl
t~t-buty1
ethec
I
07U9190
1.10
neon
Scums Informelicu
USGS
TopoMapORG obtainedfoe illinoIs ONE.
Semp5ng Oata fromillinoIs
EPA Compliance &
Aiwrmacs
Section.
Source 0) performed In
1990 by IllinoIs
EPA
GrowxheaterSection.
Allre.ults and~L’s in ngIL
°
-1f~’(.~
~1-~
~
/-
-•~t~-..i~
i
~
-
I
~
~
-
-
.-
2
I
MeucceDety ‘eo,p.er
.~
/
~.--
.:
:-
~-~-
~ / /
L~e.94in
3
et.~ato3eIn
-~
-
:~.. -ç
~
1
~
c&
~
-.
;S.
~
.:
-/
~
~
Ce6~y’IGe6~.~r5
I
~
__I
______
-~
~-
)_
-
‘‘r~~---
!
~
‘I
El
I
A/~”
~
I
.
/
~
—~
—-r---l~_
/
7
~
—
“.:~
~
/
‘-~
‘/
~“
-
6
~
9~,
w. ~
-
-
I
-
—
~
~
I~17anmeI1
I~cInz~Dc~,vu.LAo4ps~-
-~
~-:,‘;~::‘
____
u..
:
~tc-.
1/
~
..
~
r
~
.~tk~f
/
-~
-
~‘L_!~==~—
-,
~
-i-----.
-~
-
-
I;,
.
--.
-,
-,
~Gl,
-
-
,~\
~
-r
I
/
~
~,
7
~v~rt~t9
m
‘
~
~-
-
-
-
~
o~’~’~
I
—~ne,..
~
-
~
~J,,t’
‘
1~—~’
-.
~
~
--
~
/
-?
-
~
•:r
~r~
-
-
-,.~
f~•:..
-
-::
-
I
~I.1sii
.—,.
/‘
I
-
—
I
P
F
I
-
-
-
--.
-.
-
/~—.-.j
-t~-A.1’,
j
—
-~-
V~~-
~-~~---H-~
~
-.
-.
if
I
-
-~
‘~*~
~
~
-
-
~
-
-~
~
-
—
‘~J
~
CWS
Well&
Legend
•
Coefloed Aquifer
•
Uncoe~ned
Aquifer
•
LUST
Sltn
~
Above or Below
GroandFuel Storage
~
Existing or
Fetoutia
Maximum Setback Zone
~
~Th,ho~
Setback Zoca
5-Year
Recharge Area
0.5
0
0.5
R
N
c
--
TT~
-
-I-r/
-
-
-.
--
4
~
-
I-
-
-
-~‘
-
~
r.
.
4’O
I
.‘.‘I
-~
-
I—
-
,‘.~‘-/
~
1-
-‘
I
-
I
1
--
I~
-
—::~
~
-
-
---
.
-
—.
-
-
~,_
—
-;
-
~I.
--
I
I
-
-~---.~-.
-
I.
—
~
—
I
4
JOSCTII
41
n-r.~
I
-,
~
~
~--
-
-
I
-
-
-
-
~
~
4
~
-
~———-
-
Ft
I
I
L~~fl’
—
‘
—
——
I
—
I
I
E~.t
—
‘
—
——
-.
4
1
-
--
-‘
I..~
-
—
r
—
—
—
—
—
—
I
—
—
-
I,
£,_
—
r
—
I~’
-
—
-
-
.-
-
?4~
-~
--
-
-
-
~
~.r~s_
•~u
~
—
I
I
.I-~
~
~
i
-
____
-.
I
—
~Ii
~
~
1
-
4.
I
—
-
_~-7
‘~
~-~—--~
-
~-
r~3
~
-
-
--
_
~_L~
~
——
~&~
:-
-
-
-
r
1
I
-
I
—
I
illinois
EPA
-~
--
Well 12
11917
136
01
WdU~3
11918
70
01
WelIf4
11919
131
01
Well #5
00716
tJ
--
-
-~
-
-
--
01
—r
-
-
--
11120395
im~
4.EX
~!
an~o
01
-
01
me~’1t:bu)Ie1bw
myloe-bciylehcr
dllV5396
3.10
02t)IBS
L10
e~
01
17713)398
1.10
ume
01
~qr~m
-
(ll~VI,94
120
l~X)-
01
07(3195
-
0~
1t~0
Rock Falls (1950450)
Potential Source and
Detection
Data
II
Legend
CWS
Wells
•
Cncillnsd Aquifer
•
Tiieronfined Aquifer
•
LUST
~te
~
Above or
Below
Ground Fuel Storage
D
Existing or
Potec~tht
Maximum Setback Zoe~e
M~ii~~m
Setback Zon.
5-Year Recharge Area
Scale ofMiles
0.1
0
0.1
0.2
0.3
0.4
Source lnforuaedoa
USGS Topo
Map ORG
obtained fr-ouBllnea DNR.
Sampling
Data
fromIllinoIs
EPA
C1nnp8~ince& Assurance Section.
Sour-ce 10
pcri’ornad in
1989 by illinoIs EPA Groundwstec Section.
AllresultaandMCL’s
inugJI.
pr*et.dmi
reeyc~adpaper
C
0
I
~
~
‘I
~fii
0
I-I
*
0
0
I-a
a
t~J
a
I
C
t
Q
Q
a
43
a
Ii
N
C
a
3-,
0~
C
we
0
0
C
1-3
0
4-,
N
C
0~
~0
V
0
(fl
Q
-3
0
I—.
1-~
UI
a
(L~
U
H
N.’
t’~
~
0
C
Loves
Park (2010150)
Potential Source and Detection Data
~
__
-
~
____
-
~
—~~--
~
‘~_LJL~_L_~
~.
-
_____________________1•
~
ET
1 -~-L
-~
~
~.
LI
~
-~
-
~tY
.
.
.~‘.
~
~,
•.
-..
j~
~
~
i.
rov~.
p~ri~Tr&nsi~
L~M?’.~
...~
-~
41
((-
-
~I.
-
~:
-~
-~
-•~.
-
~
2
T~ve~
P~
Bu~Qer~ge
7~
~
11613
-
j~~•~___
_____________
•
/
-‘
~
?
~
~
T
—
--
-1’
.- -~..
q
-~
~4
~
~
C
~f.
\
_______
_________________________
I
.~
—
—•
~•
I
-
—
_____________________
___
______
—
-\
~
—
L~III
—
•~
~
--/j~c-~~~.
3L_
~
_______
-
-
—~
-
‘,.
-
:‘
r~-~
_______________
Cd
3~
-
•.:~:
________________
~
-~
,~
—~-
:.:
~~==
TAP
I
w~ii
S-Digit
ID~
Depth
-
~-
Data
~
Lav..~
~
Illinois
EPA
0.
jmethy1tert-buty1et21e~
06/23/98
1.OO~
none
~auxveIDformatiwl
VSGB
Topo
M~p
DRt
obtainedfrua 1Btnui~DNB.
S~xuplth~g
D~d*
from Uhlnolg EPA
Coapft~eSc A~.uxa~e
sectIon.
Sowt. ID pe~fucnedin
1391 by Illinol,
EPAG~oundw~ter
~Uou~
All
rciuHi and ~1X~L’~
In ugiL
Scale of Mi~
o~s
e
Legend
CWS
We4Ia
•
Covffnrd Aquif~
•
Unc~nod Aquif~
~
LUST~t.
~
Abovo or Be’ow GroundFuel St~agø
E~i~tin~
*
Potenilat
Maximum Setback Z~ie
Minizntim Si~tbaekZmu
S-Year Rrchirge
Area
pr~tad
ai~
rtcyeZmIpap~r
-
Roanoke
(2030550)
Potential
Source
and
Detection Data
flilnois
EPA
Scale in Feet
~~1’~9
-L~
-
~Q)3t
221
~fl
~.1L*7
ai13~
~47
I2~___
L~3
3~.432
!~2
L~!_
31434
50
~oux~nn
VSG8 Topo M2p DRGObt~rdfrom
BU~o1iDNL
S~mpli~
Did*
from
fllimi~
K1’A
Cou~limica&A~nc~
Sectiim.
U) p~f~xm~d
in 19~
by Iliinmii EPA
Crt~dw~L~
S~uction.
A1lr~iItai~ndMCL’srtportrd
lnut(L
-~
~
T
~~—?
-~
I
(
~
~
:~
-
~
-
~
~
~_I~i
~
-~
-
-
~j_~
~31434k4N~
~7~W
~
I
—
I
\\
?~3
J
—
--.
~
~i
--
~
r-.__
—
-
4
2~
‘~-
~
.J~
.~-:~-t
-‘~
____________
--
-
-. -
-
~.
-
-
—..
-—
-,
—
-,
J
2
~
~
-.
-
—
.
—
--
•
I
.
I
-
•
I
~
~
I
~
~
9tor~
•
~
~
~
/
43.J.P~x’~
—
p
-
:-
-
•
-.
-
•
-
‘.
.
\••___—‘
_l
-
.
-
5
I~’.
~
~rn
--
-
~7
112
2H~\
‘~2’
7~:2~5r
Legend
cwg w.a~
a
c~
~
•
iI~flzed
Aq~r
Aba,~
or~.~w
CrUDI.d
TaaL ~toc~.
2
LU~T~t~e
MIa~m~a
~
~*
l~&*z~n~
~.tb.ck
Zon,
5-Yui~r
R~ch.z~.
~
.
~
~t~:~~
--Lac.~i.--~th~
j
)~rr3~-aurm~r~
06129/93
~5OO
none
2i_
~HThTBRT-B~T~TH~
08/30/93
14..QO
ncfl.
02
~THTh
TBR~D-B~TYL
~
11/22/93
2~.
00
r~Ofle
02
iJ./2a/~3
4~.OO
r~e
02
r(LrB~T—3L~r~
O2/O5/~S
4~QO
nofl~
02
u/os/~s
39.00
rona
1000
0
1000
2000
Prinud
on
Recycled Paper
EXHIBIT ffl
—
MTBE Taste
and Odor Thresholds
28
EXHIBIT IV.
-
MTBE Health Advisory
29
Drinking Water
Advisory:
Consumer Acceptability
Athice
and Health
--
Effects Analysis
on
Methyl Tertiary~Buty1
Ether
(MtBE)
December 1997
p
U.S.
Environmental Protection Agency
Office of Water
EPA-822-F-97-008
TABLI~OF
CONTENTS
LIST
OF ABBREVIATIONS
.~
..~.
I
FOREWORD
.
EXECUTIVE SUMMARY
..
......
1
1.0.
INTRODUCTION
~
5
2.0.
MtBE n~r
TEE ENY~.ONMENT
5
3.0.
CHEMiCAL AND
PHYSICAL PROPERTES
6
4.0.
TOXICOKNETICS
7
4.1.
-
Dosimetry: Route-to~Route
Extrapolatloil.
7
4.2.
NSTC’s Extrapolation ofDose from
Inhalation
Exposure
9
5.0.
HL<H
EFFECTS
DATA.-
10
5.1.
Human Studies
10
5.2.
Animal Studies
ii
-
5.2.1.
NoncancerEffects
11
5.2.1.1.
Acute and Subchronic
Ii
5.2.1.2.
Reproductive and Developmental Studies
13
Reproductive Studies
13
-
Developmental Studies
14
5.2.1.3.
Neurotoxicity Studies
15
5.2.1.4.
Mutagenicity Studies
16
5.2.2.
Cancer Effects
16
-
5.2.2.1.
Studies of
the
Carcin.ogenicity
of
the
-
Parent
Compound (MtBE)
Gavage Study
-17
I~Iiaiation
Studies
17
5.2.2.2.
Studies ofthe Carcinogenicity of
MtBE Metabolites
21
terti
wy-B
utyl Alcohol
2~
Formaldehyde
21
6.0.
ORGANOLEPTIC PROPERTIES
7.0.
CHARACTERIZATION OF HAZARD AND DOSE RESPONSE
23
7.1.
Hazard Characterization
23
7.2.
Characterization of Organoleptic Effects
26
7.3.
Dose Response Characterization
26
7.4.
Comparison ofMargixis of E~pos~ure
with Potential
Environmental Concennations
and Guidance on Taste and ~
28
8.0.
REFERENCES
30
/
LIST OF
ABBREITJATIONS
DWEL
Drinking-Water-Equivalent-Level
HA
Health Advisory
kg
-
kilorain
L
liter
LOAEL
lowest-observed-adverse-effect level
MoE
margin
ofexposure
mg
milligram
MtBE
Methyl tertiw-y-butyl ether
MTD
Maximum Tolerated Dose
NOAEL
no-observed-adverse-effect level
OFW
odor free water
ppm
parts per million
microgram
TEA
tertiary-butyl.
alcohol
VOC
volatile organic compound
FOREWORD
EPA’s
Human
Health
and
Criteria Division
(HECD)of
the Office ofWater developed an
Advisbry document for methyl tertiary~butyI
ether
(MtBE).
This
document
is
a nca—regulatory
document that
analyses the currently available caiicer a~d
non-cancer data on
this contaminant,
as well as studies on its organo1ep~c
(taste and odor) effects.
The document
is
not a
mandatory
standard for action; however,
this
Advisory supersedes any previous drafts ofdrinking water
advisories for this chemical.
There are many uiacertainties and limitations associated with the toxicity data base for this
chemical.
The animal tests available to date (1997) were not conducted by exposing the animals
to
MtBE in drinking water, but rather by
inhalation.
expo~ure
or by in~oducing
MtBE in oil
directly to the stomach several times
a week.
Aithoughuseful for identifying potential hazards,
limitations of the reported studies do not allow confident estimates ofthe degree ofrisk MtBE
may pose to humans from low-level drin~ng
water contamination.
The toxicokinetic models are
also limited
in. helping to perfoim au
adequate extr2polatiou from the inhalation data to actual
oral exposure from drinking water intake.
Addi~ona1
research
is
needed to resolve these issues
before a more complete health advisory
ca~
be issued.
Therefore, given the needs of the States
and Regions for an Office ofWater (OW) positionon MtBE contamfn~tionofdrinldng Water,
EECD developed this “Drinking Water Advisory: Consumer Acceptability Advice and Health
Effects Analysis
on Methyl tertiary—Butyl Ether (MtBE)”.
MtBE is
generally unpleasant in taste and odor.
Studies have been conducted on the
concentrations ofMtBE in diinlcing water
at
which individuals can detect the odor or
taste
of
the
chemical.
This Advisory recommends that keeping levels of
contamination
in
the range of20 to
40
p.g/L or below to protect consumer acceptance of the water resource would also provide a
large margin ofexposure (safety) from toxic
effects.
The Advisory discusses the limitations ofthe current database for estimating a risk level for this
contaminant in drinking water azd characterizes the hazards associated with this route of
exposure.
This document has beenpeerreviewed both internally in
the Agency and externally
by
experts in the field before its release
to the public.
Note:
In this Advisory, we use a risk characterization method called “Margin of Exposi.ire (or
safety)” which is different from
traditional slope factors and reference doses (RfDs) as estimates
of response to defined exposures.
The “margin” is how far the environmental exposure of
interest
is
from the lower end ofthe exposures at which an~maisor humans have shown some
toxicity effect. The use ofthe margin ofexposure approach is
helpful in the following ways:
1.
It
allows for comparison ofexposures associated with carcinogenic potenfial to
those associated
with non cancer health effects;
2.
It provides
the risk manager with a quick check to
decide
Lf
the margin
of
exposure
(safety)
appears to be adequate even
when mathematical
extrapolation of
U
data from high to
low dose cannot be done; and
3.
it gives
a better understanding ofthe degree of
risk
1
-
associated with extrapolation ofexposure
data from animal studies to humans.
For example,
given the limited number ofanimals that usually can be used in experiments, they, at best, would
detect a one in ten response (1
x
1O~t).A
common procedure for carcinogens
is
to
mathematicalTy extrapolate from the exposure levels ofanimal tests to
estimate risk at lower,
environmental exposure levels.
Ifthe ex~apo1ation
is done as a s~aight
line, a risk estimate of
1.
x 1O~generally corresponds to
a margin of exposure of 100,000.
Lf the true, but unknown,
relationship
is downward sloping, not a s~aight
line,
the risk at a 100,000 margin ofexposure
would be less
than
1
x 1O~and might be zero.
Health and Ecological CriteriaDivision
OfEce of Science aiid Technology
Office ofWater
111
DRINKING
WATER
ADVISORY: CONSUMER ACCEPTABILITY ADVICE
AND
HEALTH EFFECTS ANALYSIS ON
METHYL TERTTARY-BUTYL
ETHER
(MtBE)
EXECUTIVE
SUMMARY
MtBE
MtBE is
a volatile, organic chemical.
Since the late
1970’s, MtBE
has been used as an octane
enhancer in gasoline.
MtBE promotes
more
complete burning ofgasoline, thereby reducing
carbon
monoxide and ozone levels.
Hence, MtBE
is commonly used as a gasoline additive in
localities that participate in the Winter Oxygenated Fuels pro~amandlor the Reformulated
Gasoline pro~amto achieve or maintain compliance with the National
Ambient Air Quality
Standards.
A limited number of
instances
ofsignificant contamination
of
drinking waler with
MtBE have occurred due to leaks from under~oundand above groundpetroleum storage tank
systems and pipelines. MtBE,
due to
its small molecular size a~.d
solubility in water, moves
rapidly into ~oundwater,
rasterthan other constituents of gasoline.
Public and private wells
have been contaminated in this manner.
Non-point sources, such as recreational watercraft, are
mast likely
to be the cause of small amounts ofcdntamination ofsurface waters.
Air deposi~on.
through precipitation ofindustiial orvehicular emissions may also contribute to surface
and.
~ouxid water contamin.ation.
The extent ofany potential for build-up in the environment from
such. deposition
is uncertain.
-
This
Advisory
The EPA Office of Water is issuing this
Advisory to
provide guidance for communities that may
be exposed to drinking water contaminated with MtBE. The Advisory provides an analysis of
cuirent health hazard information a~dan eva1ua~.on
ofcwrently available data on taste and odor
problems associated with MtBIE contamination ofwater, as the latter affect consi.imer acceptance
of the water resource. This Advisory does not recommend either a low-dose oral cancer risk
number or a reference dose (RID)’ due to certain limitations ofavailable d.atafor quan~fying
risk.
Guidance is
~.ven
on
the coucen~a~ons
at which taste and odor problems likely woajd be
averted,
and how far these are
from
MtBE coacen~ations
at which toxic effects have been seen
1Reference Dose is
defined as “an estimatc (with unc~rtainxyspanning approximately a~
ordcr of magnitude) of
a daily exposure
to the human population (including se~si&iesubgroups) that is likely to be without apprcctable
risk
of
deleterious effects over a life±ne”(U.S.
EPA,
1987).
December
1997
1
in test animals.
(The measure used is called a 9margin ofexposure” orMoE.
For instance, if a
measured concentration is
100,000
times 1e~than~
the range ofobservation of
effects
in test
animals,
the margin ofexposure is
100,000.
Conclusion
and Recommendation
This
Advisory recommends
that keeping levels ofcontamination in
the range of20
to
40
~g/L or
below to protect consumer acèeptance of the water resource would also provide a large margin of
exposure (safety) fl-am toxic
effects.
Taste and odor values
are presented as a range, since human responses vary depending upon the
sensitivities of the particular individual and the site-specifc water quality conditions.
These
values are provided as guidance reco~izing
that water suppliers determine the level oftreatment
required for aesthetics based
upon
the customers they serve and the particular site-specific water
quality conditions.
There are over four to
five orders ofma~itude
between the 20 to 40
Lg/L range and
concentrations associated with observed cancer and noncancer effects
iii
animals.
There is little
likelihood that an MtBE concen~ationof 20 to 40 ~g/L in drinking water ~vou1dcause adverse
health effects in humans, recogni7ing that some people may detect the chemical below this range.
It can be noted that at this range ofconcentrations, the margins ofexposure are about
10 to
100
times greater than would be provided by an EPA reference dose (RfD) for noncancer effects.
Additionally, they are in the
range
ofmargins of exposure typically provided by National
Primary Drinking Water Standards
under the Federal Safe
Drii~king
Water Act to protect people
from potential carcinogenic effects.
When adequate data become available, the Office ofWater will publish another Advisory that
includes quantitative estimates for health risks.
This Advisory gives practical guidelines for
addressing contamination problems and supersedes previou~
draft advisories.
An Advisory does
not mandate a standard for action.
Studies
of
MtBE
Effects
There are no studies ofeffects on humans of long-term exposure
to MtBE.
All ofthe studies
available for hazard assessment are laboratory animal studies~
Cancer effects.
There are studies in rodents ofthe carcinogenicity ofMtBE,
as well as its
metábolites,
tertiaiy-
butyl alcohol (TBA) and forma1dehyde~The only oral cancer exposure
study was conducted by Belpoggi and coworkers (1995).
They gave MtBE to Sprague-Dawley
rats (gavage in olive oil, at doses
up. to
1,000 mg/kg/day,
4
days per week for two years).
Exposure caused a dose-related increase in the incidence of~ombinedleukemia and lymphonias
ui
the female rats and an increase
in Leydig cell adenomas (benign testicular tumors) in
the high-
dose male rats.
Use ofthis study
to quantitatively assess risks
from
drinking water exposure has
limitations.
There
are
potential difference~’inboIus
versus thinking water exposures and
possible vehicle (olive oil) effects.
Moreover,
there
are few details
on the
actual
reported
tumor
response
data provided in the report.
The
lack
ofhistapathological diagnoses
and
of
individual
animal data were
reasons
that the National
Research
Council panel recommended not
using
these
tumor data in risk
estimation until
after a thorough
peer review
of
this
study.
There
are two
studies on the
potential carcinogenicity
of
MtBE after
inhalation
exposure.
Chun
et al.
(1992)
administered MtBE
to F344 rats at
concentrations
up to 8,000 ppm for 2
years.
Exposure to MtBE
caused an
increase
in the incidence ofcombined
renal tubular
adenomas
and
carcinomas,
as well as Leydig cell
adenomas
of the
testes in
the
male rats.
The
mild induction of~.-2u-globulin
by MtBE
suggested that this
protein may have played
a role in
male
~t
kidney
tumorigenesis.
The
increase in the
incidence ofLeydig cell
adenoinas
ofthe
male
rats in
this
study
was
not significantly
different
from the
histori~al
control value,
although
the difference from
the concurrent controls
was
significant.
Induction of Leydig cell
tumors was
also
observed
in Sprague-Dawley rats alter
oral
exposure by gavage
(Bel~oggi
et
aL,
1995)
and
lends support to the conclusion
that
the appearance ofthe
tumor
in both studies is treatment-
related.
In the other
inhalation
study,
Burleigh-Flayer
et
aL (1992) gave MtBE to CD-i
mice
at
concentrations up to 8,000 ppm for
18 months.
This exposure was
associated with a statistically
significant
increase in the
incidence of
hepatocellular
carcinomas
in male mice
and
of
hepatocellular
adenomas in
female mice. ,The Chun et al. (1992)
and
the Burleigh-Player et. al.
(1992) studies currently cannot be
used
to calculate adequate hazard
advisorj
values since we
have no well-developed pharmacokinetic model
for converting
a chronic inhalation exposure of
MtBE to an equivalent
oral
exposure.
On-going work may
support
route-to-route extrapolation
in the
future.
The potential carcinogemicity oftwo metabolites ofMtBE,
TBA
and
formaldehyde has
also
been
examined.
In
P344 rats,
TBA has provided
some evidence of carcinogenic activity
in
the
males
(but not in
the female rats).
In
B6C3PI
mice,
TEA
exposure
gave
equivocal
evidence of
carcinogenic
activity
in
male
mice based on
marginally
increased incidence of
thyroid tumors,
and some evidence of
carcinogenicity
in female mice,
based
on
an.
increased
incidence of
follicular cell
h.yperplasia and follicular
cell
adenomas
of
the thyroid gland.
Data
for
carcinogenic activity is
ambiguous
for
drinking
water
exposure
to
formaldehyde.
A
study
by
Soffritti
et al. (1939)
reported
a dose-related increase in the incidence of
leukemia and
intestinal
tumors
in Sprague-Dawley
rats.
However, the
experimental data presented
in
this
publication
was
limited,
Another drinking
water study on formaldehyde by Til
and
coworkers (1989),
using
Wistar rats, found no evidence of
carcinogenicity.
The carcinogeriicity data support
a conclusion that MtBE poses
a
potential
for
carcinogen city
to
humans
at high doses. The data do not
support
confident, quantitative estimation ofrisk
at low
exposure due to the limitations
described
above.
3
Noncancer toxicity.
The collective evaluation of
the reproductive and
developmental studies of
MtBE
in animals
indicate that inhalation
exposure
can,
result
in maternal toxicity
and adverse
effects on
the
developing fetus
(Bushy Run
Research Center,
1991,
l989a,
1989b; Conaway et
al.,
1985). The
fetal toxicity in the
mouse
developmental studies indicate
that
it may be ~nore
sensitive
to inhalation of
MtBE
vapors
than
the rat or rabbit during gestation.
However, it
is
possible to conclude that, at low
concentrations, MtBE does not cause a developmental or
reproductive
hazard
by inhalation
in three different animal species.
This
also suggests that
humans
may not be
at
risk when exposed
to very
low concentrations of
MtBE.
Effects
on the
kidney
were observed in
rats
after oral arid
inhalation exposure to MtBE. The most
pertinent noncancer toxicity data come from a 90-day oral exposure study in rats.
The authors
reported minimal effects on the kidneys at doses of300
mg/kg/day
and
above
(Robinson et al.,
1990).
In these
animals,
the
MtBE was given
once
a day, as a
bolus
dose in corn oil. A single
oral dose of
MtBE
in corn oil would not be
considered
representative ofan
intermittent exposure
to
MtBE
that one would
normally
obtain
from
drinking
water
containing MtBE. In a
longer
term
inhalation study, histopathological abnorinaiities
were apparent (Chun et
aL,
1992).
Uncertainties
exist in
quantifying risk from the oral data
in the
short-term
study because of
the,
bolus
gavage dosing regime
and
the
less-than-lifetime duration
of the study.
The
uncertainty
in
extrapolating
between routes
affects
the
interpretation
of
the
inhalation
data.
The
studies
support a conclusion that
MtBE can
pose
a,
hazard
ofnoncancer effects to humans
at
high doses.
The data do not support confident quantitative estimation of risk at low exposure.
Taste and Odor.
Studies were conducted on the concentrations of
MtBE in drinking
water at
which
individuals
respond
to the odor or
taste
ofthe
chemical.
Human
responses
vary widely in
this
respect. Some who
are sensitive
can
detect
very
low concentrations,
others
do
not taste or
smell
the chemical even at much
higher
concentrations. Moreover, the presence or absence of
other
natural
or water treatment chemicals can mask or reveal the
taste
or odor
effects.
Thus,
variable
preexisting water conditions
around
the
country
will increase
variability in the
acceptability of
MtBE’s
presence in
drinking
water.
The studies have not been
extensive enough
to completely describe the
extent
of
human
variability,
or to establish a population
threshold of
response.
Nevertheless, the
available studies
allow a conclusion that
keeping concentrations in the range
of20
to 40
micrograms per ht~r
(~ig1L)
of’
water or below will likely
avert
unpleasant
taste
and odor
effects, recognizing
that
some people may detect the chemical belo~
this range.
Characterization Summary
Section 7.0
on
hazard and
dose response
characterization summarizes
the
MtIBE
data.
In
this
section,
a table
(Table
1) presents
the margins
ofexposure comparing animal effects
and human
taste and
odor data.
4
December 1997
1.0
INTRODUCTION
The purpose of.this Advisory
is to support
immediate needs
for
information
by State
and
local
drinking
water facilities
and
public health
personnel
due to MtBE contamination ofpotable
water.
Ongoing research is anticipated to decrease some of the
uncertainties
in the
current
toxicity data
as applied
to the
drinking water
route of exposure.
A
Health
Hazard Advisory value
will be issued when the data base is improved to
allow
greater confidence in the toxicity
conclusions.
Nevertheless,
there are sufficient
data to give a
general
picture ofthe ranges of
exposure that may raise concerns for people.
In addition, the
taste
and odor of
MtBE affect
the
potability ofwater at levels that provide an
additional
basis for
assessment
of
quality and
usability ofwater resources.
2.0
MtBE IN THE
ENY~RONMENT
MtBE.
is used as
an octane
enhancer
to
replace
lead in
gasoline.
It also
promotes more
complete
burning
of
gasoline, thereby
reducingcarbon monoxide
and
ozone levels in localities which do
not meetNational
Ambient Air Quality Standards
(ATSDR, 1996;
USGS,
1996)~.
Almost
all of
the
MtBE
produced is
used
as
a
gasoline additive; small amounts
are usedby
laboratory
scientists (ATSDR, 1996).
When used as
a
gasoline additive, MtBE may constitute
up to
15
‘(v/v) ofthe
gasoline mixture.
MtBE
production
in. the United
States was estimated
at 6.2 billion
kilograms
in
1994
and
21
billion
kilograms
in 1995 (NSTC,
1997 and
1996).
In the Clean Air Act of 1990 (Act), Congress
mandated
the
use
ofreformulated gasoline (R.FG)
in those
areas
ofthe
country with
the worst ozone or smog problems.
RPG must
meet
certain
technical
specifications set forth in the Act, including
a specific oxygenate content.
Ethanol and
MtBE are the
primary
oxygenates used in the
RFG
program to meet the
oxygen
content
requirement.
MtBE
is used in about 84
of
RPG
supplies.
Currently,
32
areas
in a total of 18
states
are
participating in
the
RFG program, and RPG accounts
for
about 30
of the
gasoline
nationwide.
Studies have identified significant air quality and public health benefits that directly
result
from the
use
of
oxygenated fuels.
The
refiners’
199
5/96
fuel data submitted
to EPA
indicate
that the
national emissions benefitsexceeded
the required reductions.
The 1996 Air
Quality Trends Report
showed that toxic air
pollutants, such as benzene, a known carcinogen,
declined significantly between 1994 and
1995.
Early analysis indicates
this
progress ma~
be
attributable
to the
use
of
RFG.
Starting
in the
year 2000,
the required emission
reductions
are
substantially
greater, at about 27
for VOCs, 22
for toxics,
and
7
for NOX.
About 40
ofthe U~S.population live in
areas where
MtBE
is used (EJSGS, 1996).
MtBE
is
a
volatile chemical; therefore,
in most areas, the major
exposure
to
MtBE
is
from air.
In some
instances, drinking water sources
may
be
contaminated.
Leaking underground storage tank
systems
and pipelines
for gasoline products are the cause of
reported ground water
contamination.
According to the Toxic
Chemical
Release Inventory published in
1995,
December
1997
approximately 3
of the
MtBE released from industrial sources enters surface water
or publicly-
owned treatment plants (ATSDR,
1996).
Surface i~,aters
can also
become contaminated as
noncombusted MtBE in
gasoline
is released
into
air
and
precipitated by rain
and
suow.
Unlike
most
gasoline
components,
MtBE is
a
small,
highly
water-soluble molecule.
Therefore, it
it does not bind strongly
to soils,
but travels relatively rapidly to
and through surface and
underground water.
In
addition, MtBE appears to be resistant to chemical
and microbial
decomposition in
water
(ATSDR,
1996).
MtBE has
been reported in
ground
water and
drinking water derived
from
ground
water.
Based
on
monitoring
data collected by the U.S. Geological
Survey (USGS),
it
appears
that
wells most
susceptible
to
contamination
are shallow
ground water
wells
in urban
areas
(USGS,
1996).
There is
limited
MtBE drinking water occurrence in.foimation.
The information available
is
insufficient
to
characterize
the extent of
drinking water contamination
on
a
nationwide
basis,
because the samples collected are
generally
from locations with
known
or
suspected
contamination
(NSTC,
1996).
In air, MtBE
may represent
5-10
ofthe volatile
organic compounds that are emitted
from
gasoline-burning
vehicles,
particularly
in areas where
MtBE
is added
to fuels as
part
ofan
oxygenated
fuel program
(ARCO,
1995).
There
are no reliable
data
on
MtBE levels
in food, but
food should not be
a
significant
source of
exposure
to
MtBE.
Limited data
suggest that
MtBE
will
not bioaccumulate in fish or food
chains (ATSDR,
1996).
The recent report ofthe
National Science and Technology Council
(NSTC,
1997) provides
extensive occurrence data for
MtBE
and
other fuel oxygenates, as well as information
on
applicable treatment technologies.
For additional
information concerning MtBE
in the
environment, this report can
be accessed
through
the NSTC Home Page
via
link from the Office
of Science
and Technical
Policy (OSTP)
at the
following address:
Home Page at:
http ://www.whitehouse.govIWHJEQP/OSPIhtmIIOSTP_Home.htrnl.
Information
on analytical
methods
for determining MtBE
in
environmental media are
compiled
in
the
ATSDR Toxicological Profile (1996) for
this
chemical.
3.0
CHEMICAL AND PEYSICAL PROPERTIES
MtBE
is
an
aliphatic ether.
It is
a colorless
liquid
with a
characteristic
odor.
It
has
a low
molecular weight (88.15 g/thole), high volatility (vapor pressure 245 mm Hg at 25°C), and high
water solubility (40-5 0 g/L; ATSDR,
1996).
In
its
liquid or gaseous
state, it
is expected to be
readily absorbed
into the blood
stream.
It is moderately
lipophilic
with a log K0~of 1.24
(ATSDR, 1996),which will
facilitate
its
absorption across the lipid matrix ofcell membranes.
6
December
1997
4.0
TOXICOK1NETICS
There are no
data on the absorption of
MtBE
in humans after ingestion; the uptake
of
MtBE via
inhalation
has
been
reported
to be rapid
(Cain et
aL,
1994; Prah et al.,
1994;
Johanson
et al.,
1995).
In
animals, absorption ofMtBE
administered
by
oral, intraperitoneal,
or inhalation routes
is
rapid
and extensive
(Industrial
Bio-Test Laboratories Inc.,
l972a,b; Bio/dynamics,
1984;
Savolainen et al.,
1985;
Bio-Res
Lab.,. l990a,b,c,d;
Miller
et al.,
1997). The extent of
dermal
absorption in rats is
slow and
limited,
but increases
with
increasing dose levels (Bio-Res Lab.,
1 990a,b).
The metab~lismand
elimination ofMtBE and its metabolites
also proceed rapidly regardless of
the
route
of
administration.
After absorption,
MtBE
is
demethylated
to form
TBA and
formaldehyde
by the Q-demethylase ofthe
microsomal cytochrqme
P-450
system (Brady
et al.,
1990).
TBA
is
furthex metabolized
to formaldehyde (in
rodents)
or conjugated
with glucuronic
acid
to
form TBA-glucuronide,
which is excreted in
urine (Cederbaum and
Cohen,
1980;
Williams,
1959).
Other oxidative
metaholites
of
TBA include 2-methyl-1,2-propanediol and
aipha-hydroxy
isobutyric acid (Bio-Res Lab.,
1990b; Miller et al.,
1997).
Formaldehyde may be
reduced to
methanol
or oxidized to formic acid, which is
further biotransformed
to carbon
dioxide.
Since
MtBE
is rapidly absorbed into the circulation from
inhalation and ingestion exposures,
it is
expected that MtBE is distributed to all major tissues.
A large
fraction
ofthe
MtBE
in.
blood
has
a very short half-life of 10-30
minutes.
The minor long-term exponential decay component in
humans
exposed to MtBE
via inhalation
suggests
that a
small amount of
MtBE can
deposit in the
tissues (Prah et al.,
1994;
Johanson
et al.,
1995).
Animal studies showed that24-96 hours after
single short-term exposures, the total residual levels in
various tissues (brain, muscle, skin, fat,
liver,
and
kidney) were,
in
general,
low r~gardlessofroute of
exposure (Industrial
Bio-Test
Laboratories Inc.,
1972a,
1972b;
Bio/dynamics, 1984; Savolainen et al.,
1985; Bio-Res Lab.,
1990a,b,c,d;
Miller
et al.,
1997).
Several
investigators (Borghoffet
aL,
1996; Rao
and Ginsberg,
in press)
are developing
toxicokinetic
models
to derive concentrations in blood
and
brain
for
rodents
and humans after
short-term
exposure.
These
models will be
reviewed
before being used
for route-to-route extrapolation,
especially
when
exposures
are repeated or continuous.
4.1
Dosimetry: Route-to-Route Extrapolation
While there are
few reports available on the
effect
of
MtBE via ingestion, there
are many on
inhalation exposure.
Attempts have been made to crudely extrapolate inhalation dose-response
to an
equivalent oral dose-response
to offer a
perspective
on the possible
oral hazardlrisk
suggested by the
inhalation data
given that the available
dire~t
oral data are
so
limited.
In
so
doing, one must convert the
inhalation
dose to units ofmg&g-day, determine what assumptions
are
reasonable for extrapolating
this to
an equivalent oral exposure in
mg./lcg-day, and
then
7
December
1997
calculate a related oral potency (slope factor) using the calculated oral dose and the inhalation
response.
There
aie
several inherent
uncertainties
or
limitations
involved in the estimation of
human
equivalent oral dose from animal inhalation
data.
Factors
that impact absorption from the lungs
and
thus dose include:
1) the physical properties ofthe chemical (e.g., aerosol
or gas, including
the particle size), 2)
respiration
rate
and minute volume ofthe experimental animal, and
3)
exposure conditions (continuous vs.
intermittent
exposures).
Factors
that impact the interspecies
aspects ofthe conversion are:
1) allometric scaling
between species to compensate for different
body sizes, 2) differences in
respiratory system structure
and physiology,
and
3) the qualitative
and
quantitative
differences
in absorption and
biotransformation between
species.
Another important uncertainty
in the extrapolation is in
establishing
whether the
parent toxicant
or
its
metabolite(s) is responsible for the biological
activity.
The
absorbed
dose
via inhalation
exposure does not go
through
the same liver
metabolism
(the first-pass
effect)
as that via
ingestion.
Many chemicals (e.g. formaldehyde) produce
different
toxic
arid
carcinogenic effects
via
different routes of exposure.
This means
that it is importanyo
determine whether it is the
parent compound
or a metabolite that is responsible
for the observed
effects.
Specific
uncertainties
and limitations
in the toxicokin.etic
data
for MtBE
are discussed
below.
Most ofthe absorption data on
MtBE
were collected following short-term
inhalation
exposure.
Duration of exposure
and
the rate of
respiration
are
two
very important parameters which control
the absorption of
MtBE.
During
the
exposure period,
a state of
equilibrium
is established
between the inhaled
and
exhaled air; therefore, the percent
absorbed
dose by inhalation is
influenced by the pharmacological properties ofthe
toxicant.
For example, substances
like
MtBE
with
an anesthetic
effect at higher dose will slow down the respiratoryrate
and,
thereby,
slow down the rate ofabsorption
via
the lungs into the blood.
Accordingly, overall absorption. of
MtBE
would be anticipated to be
lower
at a higher dose because of
its
effect onthe
central
nervous system.
There
is not
enough
information to estimate the exact absorbed dose in long-•
term
inhalation
or
oral,
exposure.
As
already mentioned, via
the inhalation route, MtBE enters
the blood without passing through
the gastrointestinal
tract
and the liver which is responsible for
most
of
MtBE
metabolism by way
of the
hepatic
cytochrome P.450 system.
To
what
extent
MtBE metabolism
is influencedby
the
gastrointestinal tract is
not
known.
It is likely that differences
in the metabolism between
exposure routes do occur
and affect
toxicity.
Using inhalation exposure
to
estimate the oral
dose
ignores potential first-pass effects in
the liver.
However, the
uncertainties in
the route-to-route
extrapolation of dose for
MtBE are mitigated by the fact that
the
metabolites
qualitatively
appear
to be the
same
by differing routes, the distribution and excretion patterns are the
same and
the
tissues in which toxicity, including carcinogenicity, have been reported overlap between routes.
S
December 1997
4.2
NSTC’s Extrapolatioa ofDose from Inhalation Exposure
A
number
of the
studies utilized for this
Advisory involved the inhalation
route of
exposre.
At
present, there is no
appropriate toxicokinetic model
to convert an applied
inFl2iation exposure
concentration to
a dose in the
target organ, although
models
are under
development at
CUT
çBorghoffet al.,
1996)
and the University
ofConnecticut
(Rao
and Ginsberg, in press).
In the
absence of a well-developed toxicokinetic model,
the
inhalation
exposure
concentrations
were
converted to dose values following the method used by the interagency
task force
on
MtBE
(NSTC,
1996;
1997).
The NSTC (1996) conversionmethod
assumes that
for
a given exposure
concentration
ofMtBE,
the adjusted
external human
equivalent dose would be
the
same
from
studies of
any kind
ofanimals,
regardless ofthe species used.
The calculation also
assumes
100
absorption of
MtBE, and appears
tobe a default value in the absence ofreliable inhalation
and
absorption data.
The equation
used for
the dose conversion by
the NSTC (1997) is presented as follows:
Human
Equivalent Dose (RED)
=
C~ppm
x l0~
ppm” x MM x RR xEC
MVxBW
‘Where:
C
=
Atmospheric concentration
Mlvi
=
Molar
mass expressed in milligrams
(88,150
mg for
MtBE)
MV
=
Molar volume at 20°C(24.04 L)
RR
=
Human respiration rate
(20,000 LIday)
EC
=
Exposure condition. (# hrsJ24 hr) x (# dayslweek)
BW
=
Average human
body weight (70 kg)
The value ofl0~
ppm”
in the equation is
a
unit adjustment factor that
expresses the
amount
of
the
contaminant that
is present in each
unit
ofinspired
air.
When
the concentration of
MtBE
is
1
ppm, the exposure condition is continuous
(24
bra/thy and
7 days
per
week), the EC is
I
and
the
RED
is calculated as
1.05
mg/kg-day as follows:
RED
=
1
ppm x t0~
ppm” x
88.150 mg x 20.000 L/dav
=
1.05
mg/kg/day
24.O4Lx
70kg
In cases
where exposures are conducted for
6 hrslday
and
5
days per
week,
the EC is equal
to
(6/24)
(5/7) or 0.1786.
Consequently,
1
ppm ofMtBE
is equivalent to 0.1875
mg/kg-day.
9
December 1997
The Office of Water
has
presented the NST~(1997) methodology for extrapolation of the
inhalation
exposure doses
to oral doses
in
studies
with MtBE
in
order to be
consistent with
the
risk
assessment values of
those provided
in the NSTC
(1997) report.
The
limitations ofthe
methodology generate significant uncertainties.
5.0
HEALTH E~CTS
DATA
5.1
Human
Studies
There
are very
limited
data on the effects of
MtBE
in
humans
by any route of
exposure arid’
no
data
are
available for the oral
route.
In
cases where
37 or 43
human
volunteers were exposed to
low levels ofMtBE in air (1.39 or
1.7 ppm) for
1
hour (Cain et al.,
1994; Prali et al., 1994); there
was
no significant increase in symptoms ofeye,
nasal,
or pulmonary
irritation when
the results
for
periods
of exposure to MtBE
we~e
compared
to
results
from exposure to
ambient
air.
There
were
also
no
significant
effects on mood (determined by the
Profile
ofMood
States test)
or in the
results from several performance-based neurobehavioral tests.
In both studies, the females
ranked the
quality
ofthe air containing MtBE lower
than
the control atmosphere.
However, in
the study by
Cain
et al. (1994), where the
subjects
were
also exposed to an
atmosphere
contair~ing
a
7.1 ppm
mixture
of 17
volatile organic compounds (VOCs) that
are
frequent air
contaminants
in areas
around
gasoline
stations,
the
air quality ofthe
MtBE-containing
atmosphere ranked
higher than
that with the VOC
mixture.
The results
from
studies of neurological
effects
(headache, dirziness,
disorientation,
fatigue,
emotional distress, etc.),
gastrointestinal problems (nausea, diarrhea),
and
symptoms of
respiratory irritation in individuals exposed to
MtBE
vapors
through MTBE-containing
fuels
are
inconclusive (Hakkola et
al.,
1996; Moolenaar et al., 1994; White
et al.,
1995).
The
three
studies
cited were different in
their
design and
utilized
slightly different parameters for monitoring
effects.
All
studies evaluated exposure to
a
MtBE-gasoline mixture and
not MtBE.
The studies by Hakkola
et al.
(1996) and White et al. (1995) compared the effects in two groups
exposed to different concentrations of
MtBE
from
treated
gasoline because oftheir
lifestyles.
The moderately-exposed
individuals
either drove a gasoline delivery
truck, worked
in a gasoline
station
or worked on
car repairs.
The minimally-exposed
individuals
merely used a gasoline-
powered vehicle to go to
and from
work or as
part
oftheir job.
Hakkola et al. (1996) found
that
there were no statistically-significant differences between
the
signs
and
symptoms reported by
101
drivers
oftanker
trucks in Finland (where the gasoline contains
10
MtBE)
and 100 milk
truck drivers.
Blood conc~ntrations
of
MtBE
or
its
metabolites were not monitored.
In
the
study
by
White
et al.
(1995),
the
odds
ratio was
8.9 (95
CI
=
1.2-75.6) for the reporting of one or
more symptoms when
11
individuals with blood
MtBE
levels of 2.4
~g/L
were compared
with
33
individuals
with lower levels.
The odds
ratio increased
to
21
(95
CI
=
1.8-539) when
commuters were excluded from the population studied and
8 workers with blood levels of 3.8
10
December
1997
j.~g1Lwere compared to 22 individuals ~th
lower
blood
MtBE levels.
All individuals
lived and
worked in the area
around
Stamford, Connecticut.
A study in Alaska (Moo
lenaar
et aL, 1994) compared
effects and
blood levels of
MtBE: from a
time period
when oxygenated fuels were in
use
(Phase I)
to those
after the
oxygenated fuels use
had stopped
(‘Phase U).
The subjects were volunteers who were occupationally exposed to motor
vehicle exhaust or
gasoline
flurnes.
Eighteen workers participated in Phase I and
22 in
Phase
U.
Twelve of those that paiticipated in
Phase I ofthe sttidy also participated in Phase El.
A
questionnaire was
used to gather
information
on
signs
and
symptoms arid
blood
samples were
collected for measurement of
MtBE
at the beginning
and
end ofa
typical
work day.
In
Phase I,
the
median post-shift MtBE level
was higher than
the pre-shift value (1.80 vs. 1.15 ~gfL).
DuringPhase U, the values were more comparable
(0.25
vs. 0~21j~g/L).
Median post-shift blood
measurements
of
TBA
were higher
duri~g
Phase I than in Phase
11(5.6
vs..
3.9~g/L).
Signs
and symptoms that could be associated
with MtBE exposure were reported
more
frequently
during Phase I than Phase U (Moolenaar
et
aL, 1994).
During PhaseI,
50
or more
ofthe
participants reported headaches,
eye
initations and
nose
and throat irritations.
Reporting
of
these symptoms occurred in less
than 10
ofthe
participants during Phase
U.
However, it is
difficult
to evaluate if psychosomatic
factors and individual sensitivity had influenced
these
results.
The
volunteers
may have chosen to
participate because oftheir
sensitivity
to
contaminants in the atmosphere.
Perfusion
of
MtBE through
the bile duct
and gallbladder was
once used as a
medical
treatment
for gallstones.
During this
procedure, some ofthe
MtBE enters
the blood stream and is
distributed systemically.
Effects reported in patients treated by
this
procedure
included sedation,
perspiration, bradycardia
(slow heart beat) and elevation of liver enzymes
(Allen
et al.,
1985;
Ju.liani
et
al.,
1985,
and Wyngaarden,
1986).
These signs cannot be
attributed
totally to MtBE
because ofthe confounding effects of
anesthesia and
the infusion
process
itself.
5.2
Animal
Studies
.
-
5.2.1
Noncancer Effects
5.2.1.1
Acute
and
Subchronic
Studies of the systemic effects of Mt.BE have
been
conducted in animals, but the majority
involve
inhalation
exposure.
Since
this
Provisional HA is
mainly
concerned
with
the
effects
of
MtBE in
drinking
water, it will focus on
oral toxicity
studies.
From an
acute
standpoint, MtBE
is
not
very
toxic.
The oral LD50 in
rats
is.3.9
g/kg (3,900mg/kg).
Treated
animals
exhibit
central
nervous system depression,
ataxia
and
labored breathing (ARCO,
1980).
11
December
1997
In a two-week study,
Sprague-Dawley rats (lO/se~/dose)
were dosed daily with MtBE
in corn oil
by gavage
at 0, 537,
714,
1,071
or
1,428 mg/kg/day.
At the highest dose; anesthesia
was
immediate, but
recovery was complete within
two hours.
Although
there
was a dose-related
decrease
in body weight gain, it was
significant only in females at the highest treatuient regimen.
Increases
in
relative
kidney
weights were rioted in the males at 1,071
and at
1,428 mg/kg/d~ay
and
in females
at the 1,428
mg/kg/day
dose.
There
were no
gross lesions
seen at any treatment level.
Based on the increases
in relative
kidney
weight,
a No-Observed-Adverse-Effect-Level
(NOAEL) of 714 mg/kg/day
and
a Lowest-Observed-Adverse-Effect-Level (LOAEL) of 1,071
mg/kg/day
are established by these
experiments
(Robinson et al.,
1990).
Sprague-Dawley
rats
(10/sex/dose) were
treated
orally
with MtBE
in corn oil for 90-days at 0,
100, 300,
900 or
1,200 mg/kg/day.
Anesthesia was
evident at the highest dose,
but as in the 14-
day study, full recovery occurred in two
hours.
There was a significant
decrease in
final
body
weight offemales
only
at the highest
level of treatment.
The diarrhea seen in the treated
animals
was considered to be the consequence ofthe bolus dosing
regime.
In females,
there
were
increases in
relative
kidney weights
at 300,
900
and
1,200
mgIlçg/day, while in males, increases
were noted only at the two
highest treatment levels.
Reductions in blood urea nitrogen, serum
calcium
and creatinine were observed in
males and a
reduction in cholesterol in females was
reported, but there were no clear dose-dependent results.
Based
on the
alterations
in
kidney
weights,
a
NOAEL and
LOAEL of 100
and
300
mg/kg/day, respectively,
are
identified by
this
study
(Robinson
et al.,
1990).
Sprague-Dawley rats (60
animals
per sex, per dose
group) were
given
0,250
or 1,000
mg/kg/day.
MtBE in olive oil via gavage, 4 days per week, for 104 weeks.
This dosing regimen gives a
7-
day time-weighted average daily dose of0,
143
and 571
mg/kg/day.
Survival
appeared to be
decreased in
female rats after
16 weeks, but no
statistical treatments
on
data were reported.
There
was
no reporting ofhematological,
clinical
chemistry or urinalysis
parameters, or any
indication as to whether or not these endpoints were evaluated.
The authors did not observe any
differences in food consumption
or final body weights in the various
groups.
In addition, they
did not report any noncancer histopathological changes (Belpoggi et al.,
1995).
Due to the
limited scope, intermittent treatment schedule
and
scant data reporting in
this
study,
it is not
possible to set a NOAEL or LOAEL.
The
subchronic data
from
the
study by Robinson et al.
(1990)
were
used to develop
a DWEL
for
kidney effects from
MtBE.
The increase in kidney weights at doses of 300 mg/kg/day
and higher
was considered
to be an
adverse effect,
since increases in organ weights are a marker
for adverse
organ effects
(Weil, 1970).
The diarrhea observed
was
considered to be a gastrointestinal
complication ofthe gavage dosing.
Based on the NOAEL of 100 mg/kg/day,
a
DWEL for
kidney
effects of3,500
~g’L
ca~
be derived
for a70
kg
adult drinking
2
L ofwater per day,
using
an
uncertainty
factor
of
1,000.
The
uncertainty factor reflects a
10 for the less-than-
12
December 1997
lifetime duration of
the
study,
a
10 for interspecies variability
and
a
10 for intraspecies
variability.
Kidney
toxicity
was
also
observed
in
both
males and females
in the 2-year inhalation study in
F344 rats by Chun et al. (1992)
discussed in the section on. cancer effects.
In fact,
EPA derived a
Reference
Concentration of 3
mg/rn3,
based
on the kidney
and
liver
effects ofMtBE
(U.S. EPA,
1993).
These
data support the conclusion that,
after MtBE
exposure, kidney toxicity is of
concern.
However, the use ofthe Robinson et al. (1990) study for evaluation ofkidney effects
has two significant uncertainties.
One is that
the study
was
for 90 days
and
not
for a
lifetime,
a~id
the second is the extrapolation ofdose
from a single daily
bolus
dose in
corn
oil to the
continuous small
doses from
drinking
water exposure.
In
general,
it would be anticipated that a
90-day exposure
period
would tend to
underestimate
the toxicity, while the bolus dose would be
more likely to overestimate
the toxic response.
However, the relative
effects
of these
two
factors
are uncertain.
5.2.1.2 Reproductive and Developmental Studies
Reproductive Studies
Two inhalation studies in
rats
were available on the reproductive
effects
of
MtBE.
A two-
generation reproduction
study, was
conducted in Sprague-Dawley CD rats using target
concentrations of 0, 400, 3,000 or 8,000 ppm of
MtBE
for
6 hours/day,
5
days/week for 10 weeks
before mating,
during mating, gestation
and
lactation days 5-21
(Bushy
Run
Research Center,
1991; Bevan et al.,
199Th).
Statistically-significant reductions in body weight
and
body weight
gains
in male and
female
F1
and F2
pups were noted
with
the 3,000 ppm
and
8,000 ppm
exposures during the
latter periods
of
lactation.
At 3,000’ppm, only transient
body
weight
reductions were noted in F1 males
and females
during
their premating period.
At 8,000 ppm, pup
survival
was
significantly reduced (p 0.01)
in
the F1
litters
on lactation
days 0-4
and in F2
litters
on
postnatal
day 4.
Clinical
signs of toxicity
~werenoted
in both
generations
at 3,000 and
8,000
ppm;
this
included
hypoactivity
and lack of
startle
reflex.
Ataxia and blepharospasm
(eyelid’
twitching) were observed at 8,000
ppm.
At necropsy,
increased
liver
weights
were reported in
the F1 generation
at 3,000
and
8,000 ppm in both sexes, although no
bistopathological
effects
were noted.
The NOAEL
and LOAEL for both
parental
and
pup
toxicitywere
400
and 3,000
ppm, respectively.
A one-generation
study
(Biles
et al.,
1987) in
Charles River CD rats was carried
out with
two
matings, using
target concentrations of
0, 300,
1,300 or
3,400
ppm of
MtBE
vapor for 6
hours/day,
5
days/week, prior to
and during mating.
Exposure was continued
during 5-day
mating intervals.
In males,
exposure
continued
until
the
end
of the second
mating
to produce the
Fib
litters.
In females,
exposure continued du~ing
the gestation period
and
lactation days
5
to 21,
but not during the
first
4 days ofthe
lactation
period.
A NOA.EL
and
a LOAEL may be
13
December 1997
identified
at 300 ppm
and
1,300 ppm, respecti~iel~,
based
on pup
viability in the Fib
litters.
However, this
study has
limited
usefulness
in
the evaluation ofreproductive toxicity because of
some noted flaws (e.g.,
the loss ofone
entire
litter of 12 pups at
birth
in the mid-dose group
remains unexplained).
Developmental Studies
Four inhalation studies
were evaluated: one
in
rats (Conaway et al.,
1985),
two
in mice
(Conaway et al.,
1985;
Bushy Run
Research
Center,
1989a; Bevan
et al.,
1997a)
and
one in
rabbits (Bushy Run Research Center,
1989b; Bevan et al.,
1997a).
The Conaway et al.
studies in
the
rat and
mouse were performed at target concentrations of 0, 250, 1,000
or 2,500 ppm. of
MtBE for
6
hrs per day on days
6
to
15 of gestation.
Dams were ‘sacrificed
at gestation day 20
for
rats
and gestation
day
18
for
mice.
The concentrations for the Bushy
Run studies in
mice
and
rabbits were 0,
1,000, 4,000 ppm or 8,000 ppm.
Mice were exposed on days 6. to
15 of
gestation
and rabbits were exposed on days
6
to
18 ofgestation.
Mice dams were sacrificed on gestation
day 18
and rabbits
on gestation day 28.
In the rat study (Conaway et al.,
1985),
no effects were noted in
rats
at the highest dose tested,
2,500 ppm.
Also, in the rabbit study
(Bushy
Run
Research
Center,
1989b; Bevan
et al.,
1997a),
no developmental toxicity
was
noted at the highest dose tested, 8,000 ppm, but maternal toxicity
was
noted at 4,000 ppm
and
above.~
For mice, in the Bushy
Run
study, maternal toxicity was noted at th~
two higher concentrations,
(4,000 ppm
and
8,000 ppm).
Also,
fetal skeletal
variations and
reduction in fetal weightwere
noted at the higher doses.
In the Conaway et al.
(1985)
mouse
study, the most noted
developmental effect was a dose-related increase in the incidence ofskeletal
malformations
per
litter
with incidence of
7.4 percent in the control
group compared
to 11.5
percent,
‘16 percent and
22.2 percent in the 250,
1,000
and 2,500 ppm groups, respectively.
These
malformations
included cleft palate, scrambled
and
fused sternebra
and angulated ribs.
Cleft palate occurred in
two
fetuses
‘of
one litter in the control group; one fetus in the 1,000
pp’in
group;
two fetuses, each
in
a different litter ofthe 2,500 ppm group;
and
none in the 250
ppm
group.
There
were
also
17,
11
and
17.3
percent
resorptions
in the 250,
1,000 and 2,500 ppm
groups,
respectively,
compared
to
9
percent
in control.
Based on the incidence ofskeletal malformations in
these two mite
studies,
a developmental NOAEL in mice
can
be
projected
in the range of250
ppm
to
1,000
ppm.
The collective evaluation ofthe
two
developmental mouse
studies discussed above reflects
a
MOAEL
in the range of250 to
1,000
ppm for developmental toxicity.
The NOAEL of400 ‘ppm
for parental toxicity in the rat two-generation reproductive study falls within
the NOAEL
range
for developmental effects.
These ~a1ues
are
projected
as equivalent to
doses of 65.6 mg/kg/day
to 262..5
mg/kg/day, respectively.
Using
these
two
values, the proj
ected,
no-effect-concentration
14
December 1997
in
drinking
water for
humans
is
in
the range,of
2.3
to 9.2
mg/L
(2,300 to 9,200 j~g/L).Since the
NOAEL
in the reproductive study
is
also
400
ppm,
exposure to
MtBE
in drinking
water within
this
concentration range should not cause
reproductive
or
developmental toxicity in humans.
This
health range
assumes
that
a 70 kg adult
consumes 2 L
of
water per
day.
An
uncertainty
factor of 1,000
was applied to the NOAEL.
This
factor includes a 10-fold factor for
interspecies
variability,
10
for intraspecies variability,
and
10
to account for
acute exposure
and
the
limitation
associated with the conversion ofthe
inhaled
dose to an
oral
dose in the absence of
adequate
pharinacokinetic models.
The conservative
use
ofthe 10-fold factor for acute exposure should
provide an additional margin ofprotection for potential effects on
the
developing fetus.
5.2.1.3
Neurotoxicity Studies
Inhalation
exposure of
animals
to high levels ofMtBE is
associated with depression of the
central nervous
system
in the period immediately after exposure (Daughtrey et
al., 1997).
Symptoms observed
in groups of22
male
and 22
female
F344 ~atsin the hour
after a 6-hour
exposure
to
an.
atmosphere
containing
4,000 or
8,000 ppm MtBE included labored respiration,
ataxia, decreased
muscle tone,
abnormal
gait, impaired treadmill performance~
and decreased
hind-limb grip
strength.
These effects were not noted 6
and
24
hours
after the cessation of
exposure.
There were no
apparent effects
from
a single 6-hour exposure to
800
ppm
MtBE.
Subchronic
exposures
of~oupsof 15 male
and
15
female rats
under the same daily exposure
conditions used for the acute study gave no indication
that
the
repetition
of
exposure exacerbated
the acute
central nervous
system response
(Daughtrey
et al., 1997). ‘There
was a significant
decrease in the absolute, but not the relative,
brain
weight in the high-dose group at the end of the
13-week exposure period.
However, there
were
no significant changes in
brain
or
peripheral
nervous
system
histopathology that could be related to MtBE.
These
studies identified
800 ppm
as
a NOAEL and 4,000 ppm as a LOAEL for acute effects of
MtBE
on the
central nervous
system.
The
800 ppm
NOAEL
for acute neurotoxic
effects is projected to be
equivalent
to a dose of 210
mg/kg/day.
Using
this
value,
the projected no-effect concentrations in
humans is
7.35
mg/L
(7,350 ~gfL)
for
a 70 kg
adult drinking
2
IJday water.
An
uncertainty
factor
of
1,000 wa.cused
for
this
calculation.
The
uncertainty
factor includes
a
10
for
use
of a frank effect, 10 for
interspecies
variability and
10
for
intraspecies
variability.
The
uncertainty
factor does not
include an adjustment for the
short-term duration,
because the daily repetition of
exposure
had no
influence on the effects
observed.
15
December 1997
5.2.1.4 Mutagenicity Studies
Several studies were available
to
assess
the
mutagenicity ofMtBE.
With
one
exception,
this
chemical
has not exhibited genetic toxicity in
a variety of
in
vitro
and
in
vivo
mammalian
and
non-mammalian
test systems.
Positive results were noted in
a mouse lymphoma assay
in the
presence of microsornal enzymes (ARCO,
1980).
The
only
positive response
is due
to the
formaldehyde produced from
in vitro
metabolism
(Stoneybrook Laboratories Inc.,
1993).
The
objective ofthe mutagenicity studies is to determine whether MtBE’s carcinogenic
activity
is
associated
with
positive
in
vivo
genetic
activity (Mckee et al.,
1997).
The
weight of
evidence
from the mutagenicity data summarized below indicated that MtBE is not mutageriic.
MtBE was
negative in
sex-linked recessive lethal
test
in the
Drosophila
melanogaster
(Hazelton,
1989).
It was
also negative’in the
Ames’assays using
Salmonella,
both with
and without
metabolic activation (ARCO,
1980; Life Science
Research,
1989a).
Chromosome
aberrations
(ABS) or
sister chromatid exchange (SCE)
induction tests in Chinese
hamster ovary cells were negative with orwithout activation (ARCO,
1980).
MtBE
did not
cause
mutations in cultured Chinese hamster
V79 cells
(Life
Science
Research,
1989b).
Inhalation
of
MtBE
at dose levels up to 8,000 ppm
did
not
cause chromosomal aberrations in
bone
marrow
cells of
P344
rats exposed 6
hours/day
for
5
days
(Bushy Run Research Center,
1 989c)
or inicronuclei
in. bone marrow cells of CD-i mice exposed’ for
6
hours/day
for
2 days
(Bushy Run
Research Center,
1993).
IvftBE was also
negative for mutations
at the
hprt
locus in
lymphocytes
of CD-i mice (Ward et al.,
1995).
No increase in unscheduled DNA synthesis
was
observed in the hepatocytes of
CD-i
mice that’
were exposed to
MtBE
vapor concentrations ofup to 8,000
ppm for
6 hours /day
for
two
consecutive days
(Bushy Run
Research Center,
1994).
It
did
not cause DNA damage’in the
primary’ rat hepatocyte culture
test
(Life Science Research,
1989c), nor
was
it clastogeuic in
a rat
in vivo
cytogenetic
assay
(A.RCO,
1980).
5.2.2
Cancer Effects
5.2.2.1
Studies of the Carcinogenicity of
the
Parent Compound
(MtBE)
There
are
three chronic/cancer
studies
of
MtBE
in
two
rodent species (two inhalation studies,
one
in mice
and
one
in rats, and one gavage
study in rats).
High
doses of
MtIBE were
used in
all of
the carcinogenicity studies
and
in some cases they have exceeded the
Maximum
Tolerated Dose
(MTD).
16
December
1997
Gavage Study
When MtBE (99
pure) was
a~rninistered
oralJyto
Sprague-Dawley rats (gavage
in olive oil, at
doses of0,
250 or
1,000
mg/kg-day,
4 days/week for two years), no
significant
differences
in
foodlwater consumption or body weight
gain
were observed.
The
chemical caused
a dose-related
increase in the incidence of
leukemia and lymphomas in fem.ales (2158 in thecontrols,
6/51
in the
low-dose group and
12147
in the
high-dose group) and an
increase in
the testicular
interstitial
Leydig cell
adenomas in
the
high-dose
males (18.3
vs. 3.3
in the controls andlor low-dose
animals).
Survival was
decreased
15
and 20
in the low- and
high-dose
females, respectively
after 9
to
12 months of treatment (Belpoggi
et al.,
1995).
There are some
limitations
in the
reporting
of
the
data
as discussed below (quoted from NSTC,
1997):
The Belpoggi et
al. study
was
published in the peer-reviewed
literature.
However,
no detailed technical report ofthe bioas~ay.
is available.
Lacking
a
detailed report
about the bioassay, the
NRC
panel (NRC, 1996)
identified a number
ofissues
and
questions which
reflects upon the risk assessment
use o~thesedata.
The
NRC
noted that
the
morphological criteria used
to
classify histcpathological findings
for both the
lymphoma-leukemia and interstitial
cell
tumor responses were
not
adequately
described and that the study
did
not adequately
address tht impact
on
tumor outcomes or differences
in
survival between
controls
and dosed groups.
NRC
went on to say
that ‘because
ofthe
importance
of this
study
for
eventual use
in risk assessment, the superficial reporting
ofthe data and the
nature
of
the
observed lesions, the committee felt strongly that an independent
in-depth review
ofthe data,
especially
the pathology
(microscopic
slides) ofthe
critical lesions
is
warranted (as was
done
with
the inhalation studies) before the
data are used
for
risk
assessment’.
While the
NRC raised questions about survival differences and
the tumor outcome, it should be
hoted
that Belpoggi et al.
included
statistical
analyses that adjusted
for intercurrent mortality.
Several
attempts
by the
Interagency Oxygenated Fuels Assessment Steering
Committee
to arrange for a
pathology review of the Belpoggi et al. study have not been successful, hence, the
underlying concerns
raised
by
NRC
review
cannot
yet be resolved.
Inhalation Studies
In a report by
Chun et al.
(1992),
P344
rats were exposed to 0,400,
3,000; or
8,000
ppm
MtBE
by inhalation, 6
bra/day,
5
days/week for 2 years.
This
study
was
recently published as Bird et
al.
(1997).
Survival time was
statistically
and sigri&antly reduced in the
exposed male rats in a
dose-related
manner.
The mean body weights of the
8,000 ppm group (both sexes) were
reduced
throughout the experiment. (The mean body weight was de~reased
L9
in
the
males
at week 82.
and 13
in the females at the end ‘of the
experiment).
An increase in chronic,
progressive
nephropathy was
observed in the exposed male
and female rats.
The combined
incidence of
17
December 1997
renal tubular adenomas
and carcinoma.s2
was increased significantly in the male
rats exposed to
the mid-dose (controls,
1/35;
low-dose, 0/32; mid-dose, 8/31; high-dose,.3/20).
The reduced
survival rate of the high-do~e
group may have
decreased
the
sensitivity
ofthe
test
to produce
a
dose-related
increase
in
tumors.
A study by CUT (Prescott-Mathews et al.,
1997) shows
that
MtBE
caused
a mild induction ofa-
2u-globulin nephropathy and enhanced
renal cell proliferation
in
P344
male rats, suggesting that
a-2u-globulin nephropathy may potentially play
a role in
male rat kidney tumorigenesis.
EPA
(tJ. S. EPA,
1991) published
three
criteria for
establishing
whether a-2u-globulin is
responsible for the
kidney tumor
in male rats:
1) increased
number and size ofhyalinedroplets in
renal proximal tubule cells oftreated rats, 2)
accumulating protein in
the hyaline droplets
is a-
2u-globulin, and 3) additional
aspects
ofthe pathological sequence oflesions associated
with
a-
2u-globulin nephropathy
are present
EPA’s policy
states that if experimental
‘data do
not meet
the
criteria
in
any
one of the three categories, the a-2u-globulin alone is not considered
responsible for
the renal tumor formation and
the renal
tumOr may
be used for
risk
assessment,
both qualitatively
and quantitatively.
Based on the available data, EPA concludes
that
the
first
criteria,has
been met, but the second
and third criteria
have not
been adequately satisfied.
The
mechanism
ofaction of
MtBE kidney carcinogenesis in male rats
is not fully understood at
the present time.
In
this case,
the identification of the
full spectrum
of a-2u-globulin-specific
‘nephropathy
is complicated by a background ofchronic progressivenephropathy(CPN)
in
both
male and female rats
and
the
apparent absence ofone or more key
a-2u-globulin
pathological
factors.
The
apparent absencemay be
a
true
non a-2u-globulin consequence, it maybe masked,
by CPN, or it may be that the mild induction is
insufficient
to elicit the full
a-2u-globuJin
response.
It is possible that other proteins related to a-2u’-globulin may also be involved (EEl,
1996).
Ongoing research o~i
the potential role of
a-2u-globulin
accumulation in male rat
kidn~y
2Ren~1
Tumorincidence of
P344 Male Ra~
A~ftcr
1~h~1~tion
Exposure to MtBE (Chun et aL,
1992)
Administered exposure
Human equiv.
Dose*
Tumor
Survival-adjusted
(ppm)
(mg/1co~.day)
incidcnce+
Tumor incidence
0
‘0
1/50
1/35
400
75
0/50
0/32
3000
562.5
3150
8/31
8000
1500
3/50
3/20
4-tumor type:
combined renal tubular
cell
adenomas and carcinomas
See
section
4.2 NSTC’s Ex~npo1ationofDose
from Tnhalatiou Exposure
iS
December 1997
may improve
our understanding
ofthe carcinogenesis of
MtBE and
its metabolite, TEA, in the
kidney.
A statistically’significant increased incidence ofthe
interstitial
testicular Leydig cell
adenomas
of
the treated rats
was detected in the C’hun
et aL (1992) study
(32 in the controls, 35
in the low-
dose, 41
in
the
mid-dose, and 47 in the high-dose).
The increase in the incidence ofLeydig cell
adenornas of the male rats in
this study
(Chun
et aL,
1992; Bird et al.,
1997) was not significantly
different from the
historical
control
value, although the
difference
from the
concurrent controls
was significant.
The concurrent control
incidencewas
64
and
the historical control values
ranged from
64 to 93
in the
same laboratory (Bird
et al.,
1997). (Leydig cell adenomas occur at
a high spontaneous rate in the F344 strain ofrats.)
However,
this type
of
tumorwas
also
observed in another
strain
of rats, the Sprague-Dawley, upon
oral exposure
by gavage (Belpoggi
•et
al., 1995).
Since the Sprague-Dawley rat does not
have a signifcant spontaneous
background
incidence for
this type
of tumor, the conclusion
that
the
appearance
of the
tumor in
both
studies
is MtBE treattnent-related
is more
confident.
In a report by Burleigh-Flayer
‘et al.
(1992),
CD-i mice were exposed to 0, 400, 3,000 or 8,000
ppm MtBE by
inhalation,
6 hrs/day,
5
dayslweek
for 18 months.
Mortality was
increased and the
mean
survival
time was decreased in
the high-dose mice compared to
controls.
The body weight
gainwas
also decreased in the 8,000 p~m
group compared to the controls (a
decrease
of 16
and
24
for male and female mice, respectively), indicating
that
the high dose exceeded the M’ID.
A
statistically-significant increase was
found in the incidence of
hepatoceilular carcinomas
in
male mice
and
ofhepatocellular
adenornas
in female mice exposed to 8,000 ppm of
MtBE3.
The
hepatic
tumors
were
only
evidenced at the high dose.
Since MtBE is generally negative in
mutagenicity tests,
and the
hepatoceliular tumors induced
by
MtBE
in CD-I mice were detected
only
in the
high-dose animals
where the dose exceeded
the MTD,~
the authors ofthe study
(Burleigh-Flayer et
al.,
1992; Bird et al.,
1997)
considered
the mouse liver tumor
finding
not
likely
to be du~
to
a direct-DNA
acting
phenomenon.
The
NAS panel (NRC,
1996) also
3He-patoceilular Tumors in Female Mice After
T~Th~1adon
Exposure to MtBE (‘Burleigh-Flayer
et
aL,
1992)
Administered exposure
Human
equiv. Dose
Tumor
incidence
(ppm)
(mg&g-day)
Adenoma Carcinoma
combined
0
0
2150
0/50
2150
400
75
1/50
1/50
2/50
3000
,
562.5
2150
0/50
2150
8000
-
1500
10/50
1/50
11150
In
the
male mice,
the combined
hepatocdlluiar tumor incidence
for
the con~o1,low-,
mid-, and high-dose
groups
are
12147,
12/47,
12146 and
16137,
respectively.
-
19
December 1997
suggested that the non genotoxic, hormonaliy-reiated
mechanisms
are the most plausible
explanation for the development of
mouse liver tumors”
Based on
short-term
studies in
mice
at CUT,
Moser
et al. (1996) speculated
that endocrine
modulations may play
a role in the
hepatacarcinogenic
effect ofMtBE.
The
CUT studies
include:
a)
inhalation exposure (approximately
8,000 ppm,
6
bra per day, 5
days per
week) of
female B6C3F1• mice to MtBE for
3
or 21
days, resulting in an increased relative
liver
weight,
increased
P450
content
and
its
activity, as well as a decreased
relative uterus weight; b)
gavage
treatment ofB6C3F1 mice with MtBE (1,800 mg
MtBE/kg
body weightlday
for 3
days)
resulting in increased estrogen
metabolism
in isolated
mouse hepa~tocytes
(Moser
et al.
1996).
EPA has calculated three slope
factors
from the cancer studies
which
appeared in the NSTC
(1997) document
These estimates ofslope factors are
not likely
to underestimate
risk
for the
general population.
The ability to calculate such an estimate does not imply
gr~a.ter
confidence
in potential cancer
hazard. True risk
for most
individuals in
the population is likely to be lower
and
for some may even be nearly zero. Because there
are uncertainties inherent
in these values,
they should be used
cautiously.
The
first
slope factor is based on the Belpoggi et al. (1995) gavage study. Using the combined
tumor
incidence oflymphoma
and leukemia
in the female rats
and a scaling factor
ofbody
weight raised to the
213
power,
a slope
factor of4 x iO~
(mg/kg/day~’can
be calculated by the
linearized, multistage model4.
The second slope factor is based on the Chun et al. (1992) data.
Based onthe combined renal
tubular cell adenomas
and carcinomas in the
male
F344 rats,
using a scaling factor
ofbody
weight raised to the 2/3
power, a slope factor of 6
x
I
o~
per ppm
can
be calculatedby the
linearized,
multistage model.
Additional
understanding
ofthe mode ofaction of
this
response
could substantially alter these estimates or
make them irrelevant.
The
third
slope factor is
based on the Burleigh-Flayer et al. (1992)
data..
Based on the liver
tumor incidence in the female CD-I mice,
using a scaling factor
ofbody weight
raised
to
the 213
power, a slope factor of 3
x lO~
per ppm
was
calculated by the
linearized,
multistage model.
4Based
on
the Proposed Guidelines for Carcinogen Risk Assessment (FR
~L
17960,
April
23,
1996),
with
the
same
tumor
data,
using
a scaling factor of body weight raised to the 3/4 power,
an LED10
of
35.6
mg/kg-day
and a
slope factor of 2.8 x
1O~
(mg/kg-day)~are
obtained.
The
drin~ng
water concentation wifl be
12
~g/L
for a
risk
of
one
Lu
a
million using
this slope factor.
20
December 1997
5.2.2.2 Studies of the Carcinogenicity ofMtBE
Metabolités
tertiazy-Butyl
Alcohol
F344
rats were exposed to
TBA via drinking
water at concentrations of 0,
1.25, 2.5
or
5
mg/mL
for 2
years (the average delivered,
daily doses of
TBA
were approximately 0,
85,
195,
and 420
mg/kg-day for males
and
0,
175,
330 and
650
mg/kg-day
for females).
There was
some evidence’
of carcinogenic
activity
in male
rats
based on an
increased
incidence ofrenal tubular hyperplasia
and
renal tubular
adenomas
or
carcinomas,
and
no
evidence
of
carcinogenic activity in female
rats
(Cirvello
et al.,
1995;
NT?,
1995).
Compared
to controls, the
survival was significantly
lower for the high-dose animals, especially in the males.
Increased nephropathy was also
noted
in all treated
animals.
B6C3FI mice were expdsed
to
TEA
in
drinkingwater
at
concentrations
of0,5,
10 or 20
mg/mL
for 130 weeks
(the average
daily
delivered doses were 0,
535,
1035 or 2065
mg/kg-day
for
males
and
0, 510,
1015 or 2105
mg/kg-thy
for females).
There was e~uivoca1
evidence
of
carcinogenic
activity in
male mice,’ based
on
marginally increased incidence
of
thyroid tumors
and
some
evidence of
carcinogenicity in female
mice,
based
on an increased
incidence
of
follicular
cell
hyperplasia and
follicular cell adenomas ofthe thyroid gland.
Survival of
males in
the high-dose
~oup’ was significantly
lower
than
that ofthe control ~oup.
Thus,
the
National Toxicology
Program (NT?) studies of TIBA
show no clear evidence of
carcinogenicity
in either species.
Formaldehyde
There is
sufficient
evidence
of
carcinogenicity
in animals by
the inhalation route (IARC, 1995).
Inhalation exposure
of
F344 rats
to formaldehyde for 2 years at
14.3
ppm induced squamous cell
carcinomas ofthe
nasal cavity
in both
male
and female F344 rats (the doses were: 0,
2,
5.6
or
14.3 ppm, 6
hours per
day,
5
days per week), but not in female B6C3~E1mice
(same
doses
and
exposure conditions)
(Kerns
et
al.,
1983).
Lifetime inhalation studiesof for~.aldehyde
in
Sprague-Dawley
rats
at 14 ppm
(Sellakumar et al.,
1985),
and Wistar rats
at 10 ppm (Woutersen
et al.,
1989) also produced
nasal tumors.
By the
drinking
water route of
exposure, the evidence ofcarcinogenic activity
for formaldehyde
is somewhat
ambiguous.
One
lifetime
drinking
water study of formaldehyde in
Sprague-Dawley
rats
at concentrations of 0,
10,
50,
100, 1,000
or 1,500
ppm showed a dose-related increase in the
incidence of
leukemia
and intestinal
tumors
(Sofflitti et
al.,
1989).
Similar
to the Belpoggi
et al.
(1995)
study of
MtBE
(which
was
conductedby the
same laboratory),
the
reporting
ofthe study
is somewhat limited
and
the
pathology also lacks
an
independent review.
Another
2-year
drinking
water study of formaldehyde
using
Wistar rats
at doses
ranging
from 0,
1.2,’ 15
to 82
mg/kg/day
for males
and
0,
1.8, 21,
to
109 mg/kg/day for
females showed no evidence of
carcinogenicity (Til et
al.,
1989).
21
December
1997
6.0
ORGANOLEPTIC PROPERTIES
Water contaminated
with MtBE may have an unpleasant
taste or odor.
These
characteristics,
often referred to
as “organoleptic
properties,” cannot be used by EPA for developing
primary
drinking
water
standards,
but
are
ofconcern
and
do play
a role in
the
production of
finished
drinking water, as most
U.S.
citizens would not drink “unpleasing” water. Taste
and
odor may
also
alert consumers to the fact
that the water is contaminatedwith MtBE and, therefore, were
considered in the de~
elopment of
this
Advisory.
Not all individuals respond equally to
taste and
odor because of
differences in
individual
sensitivity. The
taste and
odor responses
reported
in observed individuals for MtBE
are
in
the
15
to
180 ~g/L
range for odor
and
the 24 to
135 j.~g/L
range for
taste
(NSTC,
1997, Young
et al.,
1996;
API,
1993; Prah et al.,
1994; Dale
et al., 1997). The ranges
are
indicative ofthe variability
in individual response.
The lower ends ofthe range for both taste
and
odor
are
the lowest
concentrations eliciting a response
among 7 of
9
participants in a’study
by Young
et al. (1996).
In
this
study, the
geometric
mean for
taste was
48 ~ug/L
and
that
for odor
was
34 ug/L.
Participants in
this study
were selected for
their
above
average
sensitivity
to
basic tastes and
odors.
In
fact,
3 ofthe 7 participants detected the lowest odor
concentration, while
4 of9
participants
detectedthe lowest
taste concentration.
The homogeneity in
the response among the
small group offemale
subjects,
along
with the geometric mean values support classification of
the subjects
as sensitive.
A study commissioned by the
American
Petroleum Institute
(API, 1993) and conducted by
TRC
Environmental Corp.
used
6-7
individuals
“chosen to represent a
normal distribution
of
olfactory
sensitivity”
to measure
taste
and odor thresholds of97
MtBE in distilled water.
Calculated
threshold values were 39
~ig/Lfor taste, 45
jig/L for odor detection,
and 55
p.g/L
for odor
recognition.
The
intensity
of the odor of
MtBE was
also reported to be greater in
water than
in
air.
The.subjects described the taste of
MtBE
in water as “nasty”, bitter, na~eating,
and similar
to rubbing alcohol.
ma
study by
Pi~ah
et al. (1994), the concentration of
MtBE
in distilled water
that was identified
as
having
an odor by 50
of the
study participants (19
males and
18
females)
was
180 p.g/L
This value is regarded as the
high end ofthe odor range
even though it is
a median
response
concentration.
There
were undoubtedly
individuals
who
could only detect the odor of
MtBE
at
even higher concentrations.
The Metropolitan Water District ofSouthern
California recent1~conducted a study on the taste
and
odor thresholds and
other characteristics of
MtBE
(Dale et al.,
1997).
They
found
that the
range for the 60
probability (±
1
SD) ofcorrect
taste
detection of
MtBE
ii~
odor-free water
(OFW)
and
untreated Colorado
Ri~ier
water
was
24
to 37
and
26
to 58
p.g/L, respectively.
The
corresponding range for detecting the odor
ofMtBE
in
OFW was
43
to 71
p.g/L.
These
tests
December 1997
were conducted
by having
nine trained analysts undergo six “triangle
tests”
for several
concentrations,
in which
each
analyst determined
the odd case
when
blindly presented with
either
two
blanks
and
one
spiked
sample or one blank
and two
spiked samples.
It cannot be
determined
from
this
small,
noi~-representative
sample what
percentage
of the general population would be
able to detect MtBE
in
their drinking
water at these concentrations.
However, these taste
and
odor threshold
data are
consistent
with
those reported
by Young
et
al. (1996)
and API
(1993).
This
study by Dale et al.
(1997) found people more sensitive to taste than odor, which is
consistent
with
the
API
(1993)
findings for MtBE taste and
odor thresholds, but in the opposite
order to
that found by Young
et al. (1996).
Collectively, these
data support
a range of20 to 40
as an approximate
“threshold” for organoleptic
responses.
However, some
subjects
in
this
study were able
to detect MtBE at much lower
concentrations; thus,
in
a
general population,
some
unknown
percentage ofpeople
will
be likely to detect the taste
and
odor of
MtBE
in
drinking
water at
concentrations
below 20
p.g/L.
.
The
study
by Dale et al. (1997) went beyond simply mea~uring
taste
and
odor
thresholds.
The
investigators
also asked four panelists
to
describe
the
taste
and,odor ofMtBE in OFW at
concentrations ranging from
2
p.g’L
to
190
~gfL.
At
concentrations
of 2-5 ~.tgfL,
the consensus
judgment ofthe
panelists
was
that
the
taste
of
MtBE
in
OFW could
be
described
as “sweet,”
At
concentrations of2l-190
p.gJL, the
characterization, was
either”solvent”
or
“sweet
solvent.”
Similar characteristics applied at
concentrations of21-190
j.tg/L for the odor ofMtBE in
OFW.
The
panelists
were
also
asked to
rate
the intensity
ofthe
taste and
odor,
which
can become
“objectionable”
at a sufficiently
high
intensity.
The panelists considered the
taste
of
MtBE
in
OFW objectionable at a concentration ofapproximately 50
ji.gJL
and
the odor objectionable at
approximately 90-100 jigJL.
It is noted
that
these tests were conducted
with non-chlorinated
water at 25
degrees C.
Chlorinationwould
likely raise the thresholds for the
taste
and odor of
MtBE in water,
and higher
temperatures (e.g.; for showering) would likely reduce these
thresholds.
It is not possible to
identify
point
threshold
values for the
taste
and odor of
MtBE
in
drinking
water, as the concentration will
vary
for different individuals, for the same
individuals
at
different
times,
for
different
populations,
and for
different
water mnatrices,.temperatures,
and
many
other variables.
Nevertheless, it seems reasonable to offer a range of20-40. ~.ig/’L
as
advisory
guidance
for
helping
to
ensure consumer
acceptance of the
taste
and odor of
MtBE
Ui
drinking
water.
7.0
CHARACTERIZATION
OF HAZARD AND DOSE RESPONSE
7.1
Hazard Characterization
There are
very few data on human responses to
MtBE.
In controlled studies,
there
were no
observable responses
to short-term (1
hour)
exposures to low concentrations of
MtIBE
in air,
23
December
1997
although women felt the
air
quality was
substandard (Cain
et al.,
1994; Prali et al.; 1994):
Other
short-term human studies of MtBE are of limited value, because they evaluated effects under
conditions where MtBE
was
combined with
simultaneous
exposurds to other chemicals, such as
gasoline,
medicines
and/or anesthesia
(Allen
et al.,
1985; Hakkola et al.,
1996;
Suliani
et al.,
1985; Moolenaar et al.,
1994;
White
et al.,
1995;
Wyngaarden
1986).
Studies of
gasoline/MtBE
mixtures are
inconclusive, but suggest that
MtBE-containing gasoline
vapors may be
irritating
to
eyes, the respiratory system
and the
nervous system
(‘Hakkola et at, 1996; Moolenaar et al.,
1994; White et al.,
1995).
There have been no long-term
studies of
human
exposure to
MtBE.
Rodent
studies identify
the
kidneys, brain and
developing fetus as sensitive to MtBE.
The
neurotoxicity data from inhalation exposures in rats
(Daughtrey
et al.,
1997) showed
transient
CNS depression and
decreased motor
activity at high
1e~elsof
MtBE
(8,000
ppm).
However,
there
are
no
data to support the hypothesis
that MtBE dissolved in drinking
water has
adverse
effects
on the
nervous
system in
humans.
The collective
evaluation
ofthe reproductive
and
developmenl
studies
of
MtBE in~rrimals
indicate that
inhalation exposure can
result in
maternal toxicity and adverse effects
on the
developing
fetus (Bushy
Run Research
Center,
1991, 1989a,
l,989b;
Con.away
et
aL, 1985). The
fetal
toxicity in the mouse developmental
studies indicate
that it may be more sensitive to
inhalation of
MtBE vapors than
the
rat
or rabbit during gestation.
However, it is possible to
conclude that, at low
concentrations,
MtBE does not cause a developmental or reproductive
hazard
by inhalation in
three
different
animal.species.
This
also suggests that
humans
may not
be at
risk
when exposed to
very
low
concentrations
of
Mt.BE.
Effects on the
kidney
were observed in rats after
oral
and inhalation exposure to MtBE.
After
short-term oral
exposure, increases in
kidney
weights were noted (Robinson et al.,
1990), while
in
a longer term inhalation study, histopathological
abnormalities were apparent
(Chun et al.,
1992).
The oral
data
from the short-term study
are
confounded by the bolus gavage dosing
regime and
the less-than-lifetime duration ofthe
study, while
the
uncertainty
in
extrapolating
between
routes affects the
interpretation
of the
inhalation data.
-
The
use
of
inhalation data
to project effects
from
the
oral exposures is generally
not
desirable
but, in the case of
MtBE,
there is
qualitative similarity
in the effects observed
with
both r~utes.
However, when
using
the inhalation data to calculate
a
human
equivalent dose for
the risk
assessment
calculations,
additional uncertainty
is introduced by
the mathematical
conversion.
In
animals, there are two chronic inhalation
studies available, one
in
rats
causing increased
incidence ofrenal
and
testicular
tumors
(Chun et a!., 1992)
and
one
in mice inducing liver
tumors
(Burleigh-Flayer et al.,
1992).
By the oral route, there is
one gavage study
in rats producing a
dose-related increase in leukemia
and
lymphoma in
the
females
and an increase in testicular
tumors
in the
males (Belpoggi
et at,
1995). I~
addition,
formaldehyde,
a metabolite of
MtBE,
is
24
December 1997
an
animal carcinogen.
By inhalation
exposure, it induces
nasal tumors in rats (Kerns
et al.,
1983).
By
the
drinking water route of exposure,
one study
shows a dose-related
increase in
leukemia (Sof&itti et a!., 1989)
and another
study shows no
evidence
of
carcinogenicity (Til et
al.,
1989). I~
addition,
there is
some suggestive evidence of
carcinogenicity
of
TEA (another
MtBE metabolite)
—
an increased incidence of
renal tumors
in rats
and
an increase in thyroid
tumors
in.
the
female mice after drinking
water exposure.
Most ofthe
cancer
studies ofMtEE
and its
metabolites have
limitations,
such as high
mortality
among the treated
animals,
limited
reporting ofpathology and of
historical
tumor incidence, etc.
In spite ofthe
limitations, there are
some consistent tumor findings for
MtBE
and its
metabolites.
This
consistency contributes to the overall weight ofevidence.
A
statistically-significant
increase in
interstitial
Leydig cell adenomas of the
testes
was detected in the exposed
rats
after
both
inhalation
(Chun et a!.,
1992; Bird
et al.,
1997)
and
gavage exposures (Belpoggi et al.,
1995).
In addition, the elevation ofkidney
tumors
in
male
F344 rats
treated with TEA (a
metabolite ofMtBE), via drinking water (Cirvello
et at,
1995;
NIP, 1995)
supports
the increase
of
similar tumors
in
male rats after exposure to MtBE
byinhal4ion (Chun et al.,
1992; Bird et
a!.,
1997).
The similarity
in the
finding
of
a
dose-related increase
in
leukemia
of
rats (Sprague-
Dawley,
male
and
female
combined)
after exposure to formaldehyde
(also a ~etabolite of
MtBE)
via drinking water (Soffi-itti
et
a!., 1989)
and tht
increase of leukemi~J1ymphomas
in female rats
(same strain) afterexposure
to
MtBE
via gavage (Belpoggi et a!.,
1995),
suggests a
possible
involvement offormaldehyde in the leukemogenic effect of
MtBE.
However,
issues remain
unresolved
related to these ‘studies, which were conducted bythe same laboratory.
Both studies
provided limited reporting
and
no
information
on
historical incidence
of
leukemia5.
MtBE does not
appear
to be DNAreactive.
The
chemical has
been tested in an
array
ofboth
in
vitro
and
in
vivo
systems,
and
the
results have been
negative overall.
The possibility
that
the
genesis ofthe rat
kidney tumors
involves the
a-2u-globu.lin mechanism
is being investigated,
but, as yet, the evidence does not show that the
mechanism
accounts for the
tumors satisfying all
the EPA
criteria
(U.S.
EPA,
1991).
The observation of
nephropathy and toxicity
in association
with tumorigenicity
in the rat
kidney
suggests
that a
number
of factors, possibly including the
~-
2u-globulin mechanism, may be at
work.
The
observation
oftesticular
tumors from
MtBE
and,
thyroid tumors from
TEA suggest the
need
for examination of
disruption
of
pituitary
and thyroid
5Uulike NTP carcinogenicity studies, the histopathology diagnoses from
the
h1h2h~Qfl
studies
of MtBE
in
t’3.tS
and mice have
not
been subject to
a
fun
peer-review.
ALso, thereis a
major
difference
between the oral and
iiih2i~Ofl
carcinogenicity
studies
of
MtBE.
Lengthy
reports of the
inh~12tionstudies
of
MtBE in rats and mice
were submitted
to
EPA.
These reports (Burleigh-Flayer
et aL,
1990; Chun
et
aL,
1992) provide
significantly
more
information
than what is conmined in the
published
peer-reviewed
literature (Belpoggi
et aL,
1995;
Bird
et aL,
1997).
Based
on
these
reports,
we
can
conclude
that the
inhalation
studies were
conducted in conformance
with
Good
Laboratory
Practices, whiie
there
is
a lack of evidence
to back up
that the gavage study is
also
conducted in
conformance with
Good Laboratory Practices.
25
December
1997
hormone
fLinction1
as
such disruption is not
uncommon with
these
tumors
(Hill et al.,
1989;
USEPA,
1997).
It has
been suggested that MtBE-induced mouse
liver tumors
also may be
hormone-related (Moser et al.,
1996;
Bird
Ct
a!,
1997).
Although MtBE
is not mutagecic,
a
nonlinear mode ofaction
has not been established for
MtBE.
In the absence of sufficient mode of
action
information at the present
time,
it is prudent for EPA
to
assume a linear
dose-response for
MtBE.
Although
there
are
no studies
on the
carcinogenicity
of
MtBE
in
humans,
there
are
multiple animal studies
(by
inhalation
and
gavage routes
in
two
rodent species) showing carcinogenic
activity
and
there is supporting animal carcinogenicity data
for the metabolites.
The weight of
evidence
indicates that MTBE is an
animal carcinogen, and
the chemical poses a carcinogenic potential to humans (NSTC,
1997, page 4-26).
7.2
Characterization ofOrganolep
tic Effects
There
have been several
studies
of
taste and
odor
response
by humans.
There is typically
variation
among individuals
in these
responses
to a chemical,
and this
is the case
for MtBE.
The
studies
on MtBE have been ofa
few individuals each.
Larger
numbers
of
individuals
might
show the full
distribi±on
of
sensitivity
of
humans
which
re~ainsuncharacteiized.
Nevertheless,
the
existing studies were performed independently and
show
distributions that are consistent with
~
one
another.
This lends
confidence to the conclusion
that sensitive
individuals respond
to odor
and taste
at about 20 to 40 ,ug/L.
Other influences
on. consumer
perception and
acceptance
of the
taste and
odor ofMtlBE
contamination
ofwater
are
as yet uncharacterized.
These
include development of
tolerance,
exposure through food
and
beverage preparation or showering,
and
reaction to published
reports
of
contamination.
Moreover,
the presence or absence ofother natural
or water treatthent
chemicals
can
mask or reveal the taste
or odor
effects. Thus,
variable preexisting water
conditions
around
the
country
will increase variability
in the acceptability ofMtBE’s presence in
drinking
water.
7.3
Dose Response Characterization
There
are
no studies of
long-term
human
exposure
to
MtBE;
the
pertinent
data on
potential
adverse effects
are from rodent studies. The available
data do not provide sufficient information
on the potential toxic effects from
drinking
water exposure
and
support only an
uncertain view of
the quantitative dose
and
response relationship.
For quantitative assessment of
adverse health
effects
from
drinking
water exposure, the preferred data would be
frqm studies ofeffects
of
episodic
oral exposure throu~h
water or food.
For MtIBE, the data are either from inhalation
studies or from daily,
high dose (bolus), gavage
studies, using vegetable oil as a
vehicle.
Estimating drinking
water dose equivalents
based
on
inhalation
studies or
on bolus dosing’
studies in~oduces
significant uncertainties.
26
December
1997
The results of the Robinson et a!. (1990) study,
supported
by the inhalation exposure data of
Chun et al.
(1992) provide adequate
support for the conclusion
that MtBE may
exert adverse
effects on the kidney.
However, EPA does not have high
confidence in the use
ofthe Robinson
et al. (1990) study,
nor
any other
study presently available
for quantitation ofthe potential
noncancer or
cancer
effects of
MtBE.
Because
of the
lack
of
confidence in quantitative
estimation of
drinking
water
risk, this
Advisory does not recommend either a low-dose oral
cancer risk
number
or a low
end RfD.
Instead,
the Advisory
provides perspective by
showing the
margins
of exposure between observations ofthe range of
animal
effects and
water
coiicentrations.
Table
I summarizes this margin
of
exposure information.
A final health
advisory
will
be written
when
the database is improved
sufficiently
to allow greater confidence
in the integration of data. Since the production of
potable water is
a prerequisite for its use,
it is
evident that the
organoleptic (taste and
odor)
effects
ofMtBE should be
considered.
The
available
data (Prah et
al.
1994; Young et a!., 1996; Dale et al.,
1997; NSTC,
1997) suggest that
the
lower
range
for
the organoleptic effects ofMtBE is
20 to 40
p.g/L.
The values in Table
I show the lower
end
of
ranges ofobservation ofeffects
in animais tested for
cancer and noncancer responses.
Table
1
also shows the MoEs (i.e., the
ratios
ofthe
observed
numbers
to the sensitive range of
human
response
to odor and
taste
(20 to 40
j.ig/L).
The cancer
LED10
are
based on analyses of
the Belp.oggi et a!.
(1995),
Chun et
a!. (1992),
and Burleigh-
Flayer et
aL (1992)
studies
as described in section
5.2.2.1
above.
The noncancer NOAEL
values
are
based on analyses recounted in section
5.2.1.:
kidney
effects in a subchronic
gavage study on
rats,
reproductiveldevelopmental effects from inhalation
studies
in rodents, neurotoxicity for
frank, reversible effects in rats observed after short-term inhalation exposures.The ranges given
for
taste and
odor represent the low ends of the reported values for organoleptic responses to
MtBE
in water discussed
in section 6.0.
These
available data provide an estimate that the lower
range for the organoleptic
effects
of Mt~E
is about
15
to 39
~g/L
(taste
and odor)
from an
empirical observation.
Values are rounded to one significant number, 20’and 40 (odor and
taste),
to avoid the
appearance ofprecision that
use
of
two
significant
numbers
would give.
Since characterization
of the full disuibution of
sensitivity
is not provided by available data, the
nUmber?
should be
regarded as approximate, not precise.
For
the
same
reason1
a range is
presented.
The
data are
used
only
to estimate sensitive range and should not be
mistaken
as defining thresholds of
human
response.
In
practice,
the efforts ofwater
suppliers to
satisfy consumers
on
the acceptability
of
taste and odor of water,
also
will be influenced by considering
the effects
of
other chemical
in
local waters.
27
December 1997
7.4
Comparison ofMargins of Exposure
~ith
Potential Environmental Concentrations
and Guidance on Taste and Odor
Table ipermits comparison ofan observed
environmental
concentration with the observed
effects levels for test
animals to
calculate
a
margin of
exposure by
dividing
the
environmental
concentration into the value at
the
low end of
the range
for
an effect displayed.
lithe objective is to avoid unpleasant
taste and
odor,
this
Advisory
recommends that a
concentration in
the
range
of 20
to
40 ~ugJL
likely will protect sensitive members
of a population.
At 20
1L~g/L,the
margin
of
exposure
is
approximately forty. thousand
(40,000)
for
cancer effects
and
over one
hundred thousand
(100,000) for some noncancer effects.
At 40 ~g/L, the MoE
is
approximately twenty thousand (20,000) for cancer effects and sixty
thousand
(60,000) for some
noricancer effects.
In
the
case of noncancer
critical effects, the
lower
end
of the developmental
NOAEL-range was used as the
mirrirnum effect
level in the MoE calculatidn;
thecancer
value
was
calculated
using
the LED10 (95
lower
bound
ofthe, dose for
a
10
extra
risk)6.
Comparison indicates that there are
over fourto five orders of
i’nagnitude
between the 20 to
40 ~ug/Lrange and
concentrations
associated with observed ranges ofeffects in
animals.
There
is
little likelihood that an
MtBE
concentration of20 to 40 ~igJLin
drinking
water would cause
adverse effects
in
humans, reco~nizing
that some people
may
detect the chemical below
this
range.
It can be
noted
that
at
this
range of
concentrations,
the
margins of exposure are about
10
to 100
times greater than would be provided by
an EPA reference
dose (RfD)for noncancer
effects.
Additionally, they are in the range of
margins
of exposure typically provided by
National
Primary Drinking
Water
Standards
under the
Federal
Safe Drinking Water Act to
protect people from potential carcinogenic effects.
6~Based
on the
USEPA’s recently
proposed guidelines for carcinogen
±k
assessment
(U.S.
EPA,
1996), the
rationale supporting
the
use
ofthe
LED10
is that a
10
response
is
at or just below the limit of
sensitivity
for
discerning
a
siguiflcant difference in most
long-term
rodent studies.
The NOAEL
in. most
study
protocols is about
the
same
as
an LED5 or
LED1Q
—
the lower 95
confidence
limit
on a dose
associated with
a
5
or
10
increased
effect.
The
MoE
value for cancer
was
obtained by
dividing the concen~ation.
equivalent
to
the
LED~O
(23
mg/kg/day
equivalent
to 805,000 ~g/L)
by
20 ~g/T. to
obtain a MoE of 40,200.
The MoE
for
nonca±icer
effccta
was
obtained by dividing
the concentration equivalent to the
lower
end of the NOAEL for the
developmental
toxicity
range
(656
mg/kglday equivalent
to 2,292,500 ~g/L)
by
the
environmental
water concentration of 20
~g/L
to
obtain
an MoE of
114,625.
The
calculations assume
~ 70
kg body
weight and
2
Liday
water
consumption.
28
December
1997
Table
1.
Estimation of Margin of Exposure
for MtBE
on
Water
Concentration, 20-40
~tg/l’
Endpoint
.
~
Parameter
Concentration4
j.i.g/L
MoE
compared
to4O
jag/L
MoE
compared
to20~g1L
Noncancer
NOAEL
Kidney
3,500,000
90,000
180,000’
Neurological
7,400,000
185,000
370,000
Reproductive/Developmental
2,300,000
-
9,200,000
?.60,000
?..120,000
Canc&
LED1Q3
Rat Lymphoma and
Leukemia
(gavage)
in
females
805,000
.
20,000
40,000
RatKidney
Tumor
(inhalation) in males
6,230,000
160,000
320,000
Mouse Liver Tumor
(inhalation) in
females
11,025,000
280,000
550,000
tThe
margins
of
exposure
is
calculated
by
dividing
the
NOAELs
for
noncancer
endpoints
or
LEPO
for cancer
effects
by 40
~gfL
or
20
~g!L
which is the
low
end of the
taste
and odor threshold, respectively.
2The
data
from
Belpoggi
gavage
study
and
the
Chun
and Eurleigh-Flayer inhalation
studies were used in
the
calculation.
Air concentration
øf MtBE
in
ppm
was converted
tà mg/kg-day’by
the
NSTC
method:
I ppm
=
1.05
mg/kg-day
(NSTC,
1996,
See
also
4.2).
~The
LED1Q
is
defined as
the
95
lower
bound
on
dose for a
10
extra risk
which was
calculated
by
applying
the
tumor
incidence data to
the
multistage modeL
As
indicated
by
the NSTC
(1996),
a
lifetime adjustment
factor
of
2.37
i.e.,
(24/l8)~1was
applied to
the
mouse
liver
tumor data
to
account
for
the
short
duration
of the
study (18
months instead of
24
months).
In
addition,
as done
by
NTIS,
the rat
~dney
tumor
incidence in
the highest
exposure group was
excluded from’the risk
analysis because this exposure
group
was terminated at
82
weeks
(uot
102
weeks)
due
t~
extremely high
mortality.
4The
NOAEL
and
LED10 were
initially calculated
in
mg/kg-day and then converted to ~gfL,
assuming a
body
weight
of
70
kg
and
a
water
consuimption
rate
of
2
liters
per
day.
29
December
1997
8.0
REFERENCES
Allen, MJ., Borody,
T.J., Bugliosi, T.F., May,
GR., LaRusso, N.F.,
and J.L. Thistle.
1985.
Cholelitholysis~using
methyl
te-rtiary-butyl ether.
Gastroenterology
88: 122-125.
API.
1993.
American Petroleum Institute.
Odor threshold studies performed with
gasoline
and
gasoline
combined
with MtBE, EtBE and TAME.
Washington,
DC:
API ~
4592
ARCO.
1980.
ARCO
Chemical Company.
Methyl tertiary-butyl ether:
acute toxicological
studies.
Unpublished study for ARCO Research and
Development,
Glenolden, PA.
ARCO.
1995.
ARCO Chemical Company.
Methyl t-Butyl Ether (MtBE):
A status report of its
presence
and significance in
US
drinldng water.
Presented to the Office ofWater, U.S.
Environmental Protection Agency.
Presented
by ARCO
Chemical Company.
June
8,
1995.
ATSDR.
1996.
U.S.
Department
ofHealth and
Human Services’.
Toxicological profile for
methyl
tert-butyl ether;
Belpoggi, F.,
Soffritt
M.,
‘and C.
Maltoni.
1995’.
Methyl-tertiary-butyl ether
(‘MtBE)
—
a
gasoline additive
—
causes testicular and
lymphohaematopoietic
cancera
in rats.
Toxicol.
Ind.
Health
11:1-31.
Bevan,
C., Tyl, R.W.,
Neeper-Bradley,
T.L.,
Fischer,
L.C.,
Panson,
RD., Knéiss,
5.3., and L.S.
Andrews.
1997a.
Developmental toxicity
evaluation
ofmethyl
tertiary-butyl ether (MTBE)
by
inhalation
in mice
and rabbits.
I. Appi. Toxicol.
17(Sl):S21-S30.
Bevan, C.,
Neeper-Bradley, T.L., Tyl,
R.W., Fischer, L.C.,
Panson,
RD., Kneiss,
1.3.,
and
L.S.
Aridrews.
199Th.
Two-generation reproductive study ofmethyl
tertiary-butyl ether (MTBE) in
rats.
S.
Appi. Toxicol.
l7(S1):Sl3-S20.
Biles, R.W., Schroeder, RE.,
and
C.E.
Holdsworth.
1987.
Methyl tert-butyl ether inhalation in
rats: a single generation reproduction study.
Toxicol. Lid. Health.
34:519-534.
Bio/dynamics,
Inc.
1984.
The metabolic fate ofmethyl tertiary-butyl ether
(MtBE) following
acute
intraperitoneal
injection.
Project No. 80089.
Unpublished
report submitted
to
American
Petroleum Institute, Washington,
DC.
lSOpp.
Bio-Res. Lab.
1990a.
Bio-Research
Laboratories.
Pharmacokinetics of methyl
tertiary-butyl
ether
(MtBE) and
tert-butyl alcohol (TEA)
in
male and female Fischer-344 rats after
administration of
~4C-MtBE
by
iv,
oral,
and
dermal routes.
Report
~38842.
Sermeville, Quebec,
Canada: B ic-Research Laboratories.
December 1997
Bio-Res.
Lab.
1990b.
Bio-Researcb Laboratories.
Mass
balance of
radioactivity and
metabolism of methyl tert-butyl
ether (MtBE) in male
and female Fischer-344 rats after
administration
of ‘4C MtBE by iv,
oral, and
dermal
routes.
Report #38843.
Senneville, Quebec,
Canada:
B jo-Research Laboratories.
Bio-Res. Lab.
1990c.
Bio-Research Laboratories.
Pharmacolcinetics ofmethyl tert-butyl
ether
(MtBE) and tert-butyl
alcohol (TEA) in
male and female Fischer-344 rats after single and
repeat
inhalation nose-only
exposure
to
!4C*MtBE.
Report #38844.
$enneville, Quebec,
Canada:
Bio-
Research Laboratories.
Bio-Res. Lab.
1990d.
Bio-Research Laboratories.
Disposition of
radioactivity
ofmethyl
tertiary- butyl ether
(MtBE)
in
male
and
female Fiicher-344
rats
afternose-only inhalation
exposure to
‘4C-MtBE.
Report #38845.
Senneville, Quebec,
Canada: Bio-Research
Laboratories.
Bird,
M.G., Burleigh-Flayer, H.D.,
Chun, J,S., Douglas, S.F., Kii~iss,
3.3.
and L.S.
Andrews.
1.997.
Oncogenicity studie~
of
inhaled
methyl tertiary-butyl
ether (MTBE) in CD-i
mice
and F-
344
rats.
S. Appi.
Toxicol.
l7:(S
1): S45-356.
Borgho~S.f., Murphy, J.E.,
and M.A. Medinsky.
1996.
Development ofa physiologically
based phamiacokinetic model for methyl tertiary-butyl ether
and
terdary-butanol in male
Fischer-344 rats.
Fundam.
Appi. Toxicol.
30:264-275.
Brady,
J.F.,
Xiao, F., Ning, W.J.,
and
C.S. Yang.
1990.
Metabolism
of
methyl
tertiary-butyl
ether by
rat
hepatic
microsomes.
Arch.
Toxicol.,
64:157-160.
Burleigh-Flayer, H.D., Chun, J.S.,
and
W.J.
Kintigh.
1992.
Methyl
tertiary butyl ether
vapor
inhalation
oncogenicity study
in CD-i mice.
Report
9INOO13A..
Export,
PA: Bushy
Run
Research Center.
.
-
Bushy Run
Research Center.
1994.
Methyl
tertiaiy-butyl
ether:
in
vivo-
in
vitro
hepatocyte
unscheduled DNA synthesis
assay in mice.
Project
ID 93N13 16.
Export,
PA.
Bushy
Run
Research Center.
1993.
Methyl
tertiary-butyl
ether: bone
marrow
micronucleus
test
in mice.
Project
ID 93N
1244.
Export, PA.
Bushy Run
Research Center..
1991.
Two-generation reproduction study of inhaled methyl
tert-
butyl
ether
in
CD
Sprague-Dawley rats.
Final Report, August
13.
Project ID 53-594.
Export,
PA.
31
December 1997
Bushy Run
Research Center.
1989a.
Developmental toxicity study
of
ir~ha1edmethyl tertiary
butyl ether in
CD-I
mice.
Final Report,
July 20.
TSCATS/403 186.
EPS/OTS No. FYI-OTS-
0889-0689.
Export,
PA.
gushy
Run
Research Center.
1989b.
Developthental toxicity
study of
inhaled
methyl
tertiary
butyl ether in New
Zealand white rabbits.
Final
Report,
May
12. EPAJOTS No.
FYI-OTS-0889-
0689.
Export, PA.
Bushy Run Research Center.
1989c.
Methyl tertiarybutyl ether
repeated
exposure
vapor
inhalation study in
rats:
in vivo cytogenetic evaluation.
Project
Report
51-635.
Export, PA.
Cain,
W.S.,
Leaderer, B.P., Ginsberg,
G.L.,
Andrews,
L.S.,
Cometto-Muniz, I.E., Gent, S.F.,
Buck,
M., Bergiund, L.G., Mohsenin,V., Monhan,
E.,
and S.
Kjaergaard.
i994~
Human
reactions to brief
exposures
to methyl tertiary-butyl
ether.
(Unpublished data from
John lB.
Pierce
Laboratory,
New Haven, Connecticut).
Cederbaum, A.L
and
G.
Cohen.
1980.
Oxidative dernethylation oft-butyl alcohol by rat liver
microsomes.
Biochem. Biophys. Res. Comm.
97:730-736.
Chun J.S.,
Burleigh
Flayer, H.D.,
and
WJ.
Kintigh.’
1992.
Methyl
tertiary
ether: vapor
inhalation oncogenicity study. in Fisher 344 rats.
Export,
PA;
Bushy Run Research Center~
report 91NOO13B.
Cirvello, S.D., Radovsky, I.E. Heath,
D.R.
Famell and C. Lindamood, III.
1995.
Toxicity
and
carcinogenicity
oft-butyl alcohol in
rats’and
mice
following
chronic
exposure in drinking
water.
Toxicol. and Lid. Health.
11:15 1-165.
Conaway, C.C., Schroeder, RE., andN.K.
Snyder.
1985.
Teratology evaluation ofmethyl
tertiary-butyl ether in rats and mice.
I. Toxicol. Environ. Health.
166:797-809.
Dale, M.S.,
Moylan,
M.S., Koch,
B.,
and Davis,
M.K..
1997.
MTBE: Taste and
odor
threshold
determinations using the flavor profile rhethod.
Presented
at the Water
Quality Technology
Conference,
November 9-13,
1997.
Denver, CO.
Daughtrey, W.C., Gill, M.W.,
Pritts, I.M., Fielding Douglas,
I.,
Kneiss,
1.3.,
and
L.S.
Andrews.
1997.
Neurotoxicological
evaluation ofmethyl
tertiary-butyl ether in rats.
1. ofAppl. Toxicol.
17
(S1):S57-S64.
H~kkola,
M.,
Honkasalo,
M.L.,
and P. Pulkkinen.
1996.
N’europsychological symptoms among’
tanker drivers
exposed to
gasoline.
Occup.
Med.
46:125-130.
32
/
.
December 1997
Hazelton.
1989.
Hazelton Laboratories
America,
Inc.
Mutagenicity test on methyl
tertiary-butyl
ether.
Drosophila melanogaster sex-linked
recessive
lethal test.
Study
No.
1484-0-461.
Kensington, ~D.
Health Effect
Institute report (HEI),
1996.
The
potential health effects
of
oxygenates added
to
gasoline. A
review of the
current literature.
A special report
ofthe
Institute’s
oxygenates
evaluation
committee.
Health Effects Institute, Cambridge, MA.
In Interagency Oxygenated
Fuel Assessment.
1996.
Office ofScience and’ Technology
(OSTP) through
the
committee
on
Environment and Natural Science and Technology Council (NSTC).
Hill, RN., Erdreich,
L.S., Payn.ter, O.E.,
Roberts, PA.,
Rosenthal,
S.L.,
and Wilkinson,
C.F.
1989.
Thyroid follicular
cell
carcinogenesis.
Fundam.
Appi. Toxicol.
12:629-697.
Industrial Bio-Test Laboratories Inc.
1972a.
Absorption,
distribution,
and excretion study with
2,2-MMOP in
albino rats: (Unpublished data
from
Industrial Bio-Test LaboratOries Inc.,
Northbrook, flhinois,
to
Sun
Oil
Company).
IBT
No. E20~(A).
Industrial
Bio-Test
Laboratories.
1972b.
Absorption,
distribution, and excretion study with 2,2-
MMOP
in monkeys.
(Unpublished data from
Industrial Bio-Test Laboratories Inc., Northbrook,
Illinois,
to
Sun
Oil Company).
tBT
No. E200 (B).
IARC.
1995.
International Agency
for Research on
Cancer.
Foimaldehyde.
TIn:
IA.RC
mono~aphson the evaluation ofcarcinogenic risks
to
humans:
wood
dust and’formaldehyde.
Lyon, France: IARC.
62:217-362.
Johanson, G., Nihien, A.,
and A. Lof.
1995.
Toxicokinetics and acute effects
of
MtBE
and EtBE
in
male volunteers.
Toxicol. Lett.
82/83:713-718.
Suliani,
G.,
Gandini,G., Gabasio,
S.,
Bonardi, L., Fascetti, E.,
and L. Gremo.
1985.
Colelitolisi
chimica transcutanea con rhetil-ter-butjl
etere (MtBE).
La
Radidl. Med.
71:569-574.
Kerns
KiD., Pavkov K.L., Donofrio DJ., Gralla E.J.,
and J.A.Swenberg.
1983.
Carcinogenicity
of
formaldehyde in rats and mice after long-term inhalation exposure.
Cancer Res.
43 ~43
82-
4392.
Life Science Research, RornaToxicology
Centre S.P.A.
1989a.
Reverse
mutation in
Salmonella
typhimurium,
test substance: MtBE.
Report
No. 21600 1-M-03489.
Rome,
Italy.
Life Science Research, Roma Toxicology
Centre
S.P.A.
1989b.
Gene mutation in Chinese
hamster V79 cells, test substance:
MtBE.
Report No. 216002-M-03589.
Rome,
Italy.
33
December
1997
Life Science Research, Ron~’Toxicology Centre
S.P.A.
1989c.
Unscheduled DNA synthesis
(UDS) in primary
rat hepatoc~
tes
(autoradiographic method), test substance: MtBE.
Report No.
216003-M-03689.
Rome, Italy.
McKee, RH., Vergnes, I.S.,
Galvin,
I.E.,
Douglas, S.F.,
Kneiss,
1.1.,
and
ES.
Aridrews.
1997.
Assessment of the
in
vivo
rnutagenic
potential ofmethyl
tertiary-butyl ether.
I. Appl. Toxicol.
17 (S1):S31-S36.
Miller, MJ., Ferdinandi, IE.S., Klan,
M.,
Andrews,
L.S., Douglas, S.F.,
and 1.3. Kneiss,
1997.
Pharmacokinetics and disposition ofmethyl t-butyl ether in Fischer-344 rats.
I. Appl. Toxicol.
17(S1):S3-S13.
‘
Moo
lenaar,
R.L., Hefflin, BJ., Ashley, D.L.,
Middaugh,
LP.,
and
R.A. Etzel.
1994.
Methyl
tertiary
butyl ether in
human
blood
after exposure to oxygenated fuel in
Fairbanks, Alaska. Arch.
Environ.
Health 49(5):402-409.
(also
CDC
1993 a.
Centers
for Disease Control
and Prevention.
An
investigation
ofexposure to methyl
tertiary-butyl
ether
in
Fairbanks, Alaska.
Atlanta,
GA:.
• U.S.
Department
of
Health and Human Services,
National
Center
for Environmental
Health.
October 22,
1993.)
Moser, G.I:, Wang, B.A., Wolf, D.C., Moss, O.R.,
and
T.L.
Goldsworthy.
1996.
Comparative’
~:
short-term
effects
ofmethyl
tertiary-butyl
ether and unleaded gasolinevapor in female
B6C3F1.
mice.
Fundam.
Appi. Toxicol.
3 1:173-183.
NR.C.
1996.
National Research Council.
Toxicologlcal
and performance aspects ofoxygenated
motor vehicle fuels.
Washington,
DC: National
Academy
Press.
NSTC.
1996.
National Science
and Technology Council.
Interagency assessment
ofpotential
health
risks associated with
oxygenated
gasoline.
National
Science and Technology
Council
Committee
on
Environment and Natural Resources and Interagency Oxygenated
Fuels
Assessment Steering Committee.
NSTC.
1997.
National Science
and Technology Council
Committee
on
Environment and
Natural Resources.
Interagency Assessment
of
Oxygenated
Fuels.
NTP.
1995.
National
Toxicology
Program.
Toxicology
and carciriogenesis studies
of
t-butyl
alcohol
(CAS
Na. 76-65-0)
in F344iN rats
and B6C3F1
mice
(drinking
water studies).
Research
Triangle Park,
NC: National
Institute
ofHealth;
Technical Report Series
No. 436, NIH
Publication No. 94-3 167.
Prah,
J.D.,
Goldstein, G.M., Devlin,
R,
Otto, D.,
Ashley,
D., Hou~e,S.,
Cohen,
K.L., and
T.
Gerrity.
1994.
Sensory, symptomatic,
inflammatory,
and ocular
responses
to
and
the
34
r
December 1997
metabolism of methyl
tertiary-butyl ether
ma
controlled human
exposure
experiment.
Inhal.
Toxicol.
6:521-538.
Prescott-Mathews,
S.S., Wolf,
D.C., Wang, BA,
and S.J. Borghoff.
1997.
Methyl
tert-Butyl
Ether
Causes ~2u-globulin
Nephropathy and Enhanced Renal Cell Proliferation in Male F344
Rats.
Toxicol. Appi.
Pharm.
143:301-3 14.
Rao,
H.V.
and
G.L. Ginsberg.
A
Physiologically-Based Pharmacokinetic
Model Assessment of
Methyl
t-Butyl Ether in Groundwater for a Bathing and Showering Determination.
Risk Anal.
(In press)
Robinson, M., Bruner,
R.H.,
and
G.R
Olson.
1990.
Fourteen- and ninety-day oral toxicity
studies
of methyl
tertiary-butyl
ether in Sprague-Dawley rats.
1. Ar. Coil. Toxicol.
9:525-540.
Savolainen, H., Pfaffli, P., and E. Elovaara.
1985.
Biochemical
effects
of methyl
tertiary-butyl
ether in extended vapor exposure
in rats.
Arch. Toxicol.
57:285-288.
SellakumarA.R., Snyder C.A.,
Solomon
1.3.,
and
RE. Albert.
1985.
Carcinogenicity
of
formaldehyde and hydrogen chloride in rats.
Toxicol. Appi. PharmacoL
81:401-406.
Soffritti M., Maltoni C., Maffei, F., and B.. Biagi.
1989.
Formaldehyde: an experimental
multipotential carcinogen.
ToxicoL md. Health.
5:699-730.
Stoneybrook Laboratories Inc.
1993.
Activatedmouse lymphoma (L5178Y/TK/+/)
mutagenicity assay supplemented
with formaldehyde dehyrogenase
for methyl
tertiary butyl
ether.
Status Report
65579.
Princeton, NJ.
Til,
H.P., Woutersen R.A.,. Feron,
V.3.,
Hollanders, V.M.H., and
H.E.
Falke.
1989.
Two-year
drinking-water study
for formaldehyde in rats.
Food
Chem.
Toxicol.
27:77-87.
U.S. EPA.
1987.
Reference
Dose (RLfD):
Description and use in health risk assessments.
Integrated risk information system (IRIS): Appendix A. UnIted States Environmental Protection
Agency. Wpshington,
D.C.
Integrated risk information system documentation,
vol.
1.
EPAJ600/8-66/032a.
U.S. EPA.
1991.
Alpha
2~-g1abuiin
association
with chemically induced renal toxicity and
neoplasia in the male rat.
Risk. Assessment Forum.
United States Environmental Protectron
Agency.
Washington,
D.C.
EPAI62S/3
-91/01 9F.
35
December 1997
U.S. EPA.
1993.
Assessment ofpotential health risks
of gasoline oxygenated
with
methyl
tertiary-butyl ether (MtBE).
Office ofResearch. and
Development.
United
States Environmental
Protection Agency.
EPAJ600/R.93/206.
U.S. EPA.
1996.
Proposed guidelines
for carcinogen risk
assessment.
United States
Environmental
Protection Agency.
Federal Register
61(79):17960-180l I.
U.S. EPA.
1997.
Assessment of
thyroid follicular cell tumors.
Risk
Assessment
Forum.
United
States
Environmental
Protection Agency.
Washington,
D.C.
EPAJ63O/R-97/002.
USGS.
1996.
Occurrence ofthe
gasoline additive ~iftEE
in shallow
ground water
in urban
and
agricultural areas.
United States Geological Survey Fact
Sheet
114.95.
October.
Ward,
Jr., LB.,
Daiker,
D.H.,
Hastings,
DA,
Ammenheuser,
M.M.,
and
M.S. Legator.
1995.
Assessment of the
mutagenicity
of
methyl-tertiary butyl ether
at the
HPRT
gene in
CD-i
mice.
Toxicologist 15:79 (abstract).
Weil, C.S.
1970.
Significance of
organ-weight changes
in fo~d
safety evaluation.
In: Roe, F.J.,
ed., Metabolic
Aspects
ofFood Safety.
New York, NY,
Academic
Press, pp. 419-454.
White, M.C., Johnson, CA,
Ashley, D.L., Buchta, T.M.
and
D.J. Pelletier.
1995.
Exposure to
methyl tertiary-butyl ether from
oxygenated
gasoline in Stamford, Connecticut
Arch. Environ.
Health
50(3):l83-l89.
(also
CDC
1993b.
Centers
for
Disease
Control
and P±vention.An
investigation of
exposure.
to methyl
tertiary-butyl
ether among motorists
and
exposed workers in
Stamford, Connecticut.
Atlanta, GA: U.S. Department of
Health and Human
Services, National
Center for Environmental Health.
September 14,
1993.)
Williams, RT.
1959.
Detoxjcatjari
Mechanisms,
2nd Ed., p.
67, John
Wiley and
Sons, Inc.,
New York.
(As cited in Cederrbaum, A.I, G.
Cohen.
1980.
Oxidative d~methylationoft-butyl
alcohol by
rat
liver micrasornes.
Biachem. Biaphys.
Res. Comm.
97: 730-736.)
Woutersen R.A.,
van
Garderen-Hoetmer A.,
Bruijntjes
J.P.,
Swart A.,
and V.3. Feron.
1989.
Nasal tumors
in rats after severe injury to the
nasal
mucosa
and
prolonged exposure to
10 ppm
formaldehyde.
S. Appl. Toxicol.
9: 39-46.
Wyngaarden, J.B.
1986.
New
nonsurgical
treatment removes gallstones.
JAMA.
256:1692.
Young, W.F.,
Horth,
H.,
Crane, B.., Ogden, T.,
and
M.
Arnott.
199&
Taste
and
odor threshold
concentrations ofpotable water contaminants.
Water Res.
30:331-340.
36
Page
18/July,
1994
Eizvi;m’rrneith.i_R~j~tec
:•~34
NOTICE
OF
hEALTH
ADVISORY FOR
M~ETHYL
TERTIARY-BUTYL
EThER (MTBE)
Prepared
by
Office
of
Chemical
Safety
flhinois
EPA
June9,
~
REASON
FOR
ACTION
As
a
result
of
routine
monitoring
of
public
water
supply
systems,
the
gasoline
additive
Methyl Tertiary-Butyl
Ether
(MTBE)
has been
detected
at
least
in
two public water
supplies.
Therefore,
the
Illinois
Environmental
Protection Agency
(Agency) is
announcing its intention
to
issue a health advisory,
pursuant to
35
Illinois Administrative
Code Part
620
Subpart
F:
Health
Advisories,
for Methyl Tertiaxy-Butyl Ether.
According
to Section 620.605 of Subpart F,
the Agency shall
issue
a
health
advisory
for
a
chemical
substance
if all of the
following
conditions
are
met:
1)
A community
water
supply well
is
sampled and a substance
is
detected and
confirmed
by
resarnpling;
2)
There
is no
standard
under Section 620.410
for such chemical
substance;
and
3)
The chemical substance is toxic
or harmful
to
human
health
according
to
the
procedures of Appendix A,
B,
or
C.
The Agency
has determined
that all
three
conditions have
been
met,
prompting
the
issuance
of
this draft proposal
for
a
health advisory.
By
this
issuanc
,
the Agency is opening a 30-day public comment
period,
until Au~ust
22,
1994, regard-
ing this health
advisory
draft.
Upon
closing
the
public
comment period,
the Agency
will consider
all
comments
received
and amend
the health advisory if
warranted.
The final health advisory will then be
published
in
the Environmental Register
(the illinois Pollution Control
Board News)
with responses
to
comments received.
An
abbreviated version of
the
final health
advisory will also
be
published in
local
newspapers which serve
communities
in whose
public water
supply systems MTBE
has
been
detected.
PROPOSED
GUIDANCE
LEVELS
Section 620.605 of Subpart
F
prescribes
the
methods
for developing
health
advisories
for
carcinogens and
no,,ncarcino-
gens.
Since
the
Agency
has
determined
that
there
is
insufficient evidence
of
the
carcinogenicity
of
MTBE
at
this
time
(discussed
in
the
attachment
to
this
notice),
the
method
for developing
a
health
advisory
for noncarcinogens
was
used.
Briefly,
this
method
specifies
that the USEPA’s
maximum
contaminant level goal (MCLG) is the
guidance
level, ifavailable,
or
the
human
threshold toxicant
advisory concentration
(HTTAC)
must be
determined using
the
procedures
contained
in
Appendix
A of Section 620.
USEPA
has
not
published
an
MCLG
for MTBE,
therefore
the Agency used
the
Appendix
A
procedures
to calculate the
H~TTAC.
Appendix A
specifies
in
prescribed
order
the toxicological
data
to
be
used
in developing
the
HTTAC,
ranging
from
a
verified
Reference
Dose
developed by
USEPA to
a
laboratory
animal
study of
subchronic
duration
in which
only
a
lowest
observable
adverse
effect
level
(LOAEL)
has been
determined.
This
preferred
order
reflects
increasing
uncertainty
in
the
toxicological
database
regarding
a
chemical’s
potential
to
cause
adverse
health
effects
in
humans,
and
is
manifested
in
increasingly large safety factors
which are applied to
the
data to calculate
the
HTTAC
(maximum
10,000-fold safety factor).
In
the case
of MTBE,
the Agency
has selected
the only
study available
in which
the
test animals
were
exposed
by the
oral
route of
exposure
as
the
basis
for the
HTTAC.
A.mnong
other findings,
this
90-day
subchronic study
reported increases
.1~
~
~
:t~,.J:_~.
•L,.
~,..
~
...C t’f~
~
~
,.
,
—,,~•
~
using this subchronic
study
in
which only
a LOAEL
was
determined,
the
language
of Subpart
F
specifies
the application of
safety
factors
totalling
to
10,000
to
the
animal
data,
resulting in
the
HTTAC
guidance
level
of 0.07
mg/!, or
70
parts
per
—
billion (ppb).
The details
of the
derivation of the
1-fITAC
are presented
in
the
attachment
to
this
notice.
At this point
it
is
necessary
to discuss
an
aspect
of
the evolving
science of
risk
assessment
which has
a bearing
on
this
notice.
The
Agency
has
been
informed
verbally
by
USEPA
personnel
that
in
roost
cases
USEPA
no
longer
favors
the
EnvironmerLtal
Reg~erNo.
484
July,
l994/Page
19
calculation of acceptable
exposure
values
for humans
by
using
laboratory
animal
data
divided by
uri~crtainty
factors totalling
to
10,000.
This
preference
will
be
included
in
a
chapter
in
the
book
Essential
Elements
(in
press;
ILSI
Press,
1994).
Instead,
USEPA
now prefers
to
utilize
uncertainty
factors
totalling
to
no
more
than
3,000.
The Agency
agrees
with
this
approach
in
general,
except
in
cases where
the
overall toxicity database for
a
chemical
is very weak.
In
the
case of
MTBE,
the
database contains
enough laboratory
animal
data to
determine
that
there
are not major toxicity gaps
which
would
warrant
the
use of
a
10,000-fold uncertainty factor.
The Agency
is
therefore also
using
an
overall
uncertainty
factotof
3,000
to
calculate a
guidance level for
MTBE.
Use
of a 3,000-fold
safety factor with
the
same laboratory
animal
data described
above
results
in
a
HTTAC
~uidznce
level
of 023
mzif,
or
230
ppb.
The
details
of
the
derivation
of
this
1-fITAC
are
also
presented in
the
attachment
to
this
notice.
Since there
is no
provision
in
the
language
of
Subpart
F
for the use of a
3,000-fold
uncertainty
factor
in the
derivation
of
the H’TTAC,
the Agency is proposingto utilize
lfITAC5
derived by both a 3,000-fold and a
10,000-fold
uz~certainty
factor
in the health advisory for
MTBE.
It is proposed
that
the HTTAC derived
using
the
10,000-fold uncertainty factor
(70
ppb)
be
a
precautionary health
advisory
concentration and
the
HTTAC
derived
using
the 3,000-fold
uncertainty
factor
(230
ppb)
be
the
final health advisory concentration.
The
precautionary health
advisory would be
a
level
in
a
public water supply below
which
no
action
would
be
necessary
and
above
which caution
should
be
exercised
by
the
public
water
supply
(such
as
increased
sampling of the
water
and
identification of the
potential source(s)), while
the
final health advisory would be
a
level
above which the
public water
supply should
begin
actions
to
decrease
the concentration or utilize an
alternate water
supply.
The Agency is
requesting comment
on
the
use of
this
approach when
a
total
uncertainty
factor of,10,000-fold
is utilized to
calculate
a
health advisory.
SUPPLEMENTARY INFORMATION
Section
620.605
also specifies that
the
health
advisory
must
contain a
general
description
of,
the
characteristics
of the
chemical
substance
and
its
potential
adverse
health
effects.
General
Description
of MTBE
MTBE (Chemical Abstracts
Service Number
1634-04-4), also known as
2-methoxy-2-methylpropane, is a colorless liquid
with
a
disagreeable taste
and odor.
Its
taste in
water can
be
recognized
at approximately 0.7
tog/f
(700
ppb)
(Connecticut
DEP), although
recent research suggests
that
some
people
may
be
able to
detect
its presence
in
the
range of 0.25 mg/f
and
possibly as low
as
0.04
mg/f
(API,
1993).
It
has
a
high solubility in
water,
approximately
48,000
mg/f
(von Burg,
1992).
Because
of
this
high solubility,
it
has
a
high propensity
to move
through soil with
infiltrating
rainwater and
snowmnelt
and
to
potentially
reach
groundwater.
Its
main
use is as an
octanebooster
in unleaded gasoline;
it
also
has minor
uses as an intermediate
in
the production
of
other chemicals,
especially
isobutene,
and
as
a
treatment
to dissolve gallstones.
Its
use
has been
increasing
recently
due to
requirements
under
the
Clean
Air
Act Amendments of
1990
for metropolitan
areas whic~.
are not in
compliance with
carbon
monoxide
standards
to
increase
the
percentage
of oxygenated
fuel
in
gasolines,
especially
in
the
wintertime.
As
a
result,
it
has
been
estimated
that approximately 20
of the gasoline
sold
in the United States contains MTBE,
at
levels
ranging from
2
to
15
in
the
gasolines
(Costantini,
1993).
Potential
Adverse
Health
Effects
of
MTBE
Relatively
fe’.~’reports of
adverse
effects
of MTBE
on humans
exist,
and
testing
for the
full
range
of possible health
effects
in
laboratory
animals
has
not yet
been
completed.
Summaries
of
the
acute,
reproductive
and
developmental~
arid
chronic
toxicity
data
for
MTBE
are
presented.
Acute
Toxicity
-
Other than
a
single
report
in
the
medical
literature of
acute kidney
failure
due
to
leakage
of MTBE
during
gallstone
treatment
(Ponchon.
198S~. there
is
rio
information
regarding
the
effects
of
short-term,
high
level
to
Minc
in
numans.
tOe
aaLa
rrom
taooraiory
animal
stuci~s
inutcne
mat
10.15
Cncm.iCaL
~snot
very
tOX1~
during brief exposures,
with lethal doses in
the
range of
3,000-4,000
ppm
by oral
exposure
(about one
pint
for
an
adult
human)
and
24,000-40,000
ppm (in
air)
by inhalation exposure
(this would be
within
the explosive range
in
air) (Reese
and
Kimbrough,
1993;
von
Burg,
1992;
USEPA,
1993).
The
toxic
effect
in
both
exposure
types
was
central
nervous
system depression.
MTBE
does
cot appear
to
cause
skin
irritation except
in cases of previously
damaged
skin,
and
eye
irritation
and
opacity of
the
cornea
has been
reported
(von Burg,
1992).
Page 20/July,_1994
E,zyjronmentcl
Register
No.
484
~ri~ductive
and
D~v~1opn,~,taJ
Tpxicity
-
The
reproductive effects
of
MTBE
have
been
reported
in
three
studies,
and
reproductive
and
developmental
toxicity
has
been
assessed
in
fourth,
using
rats,
mice,
and/or
rabbits.
No
significant effects were reported
in two
of
the
reproductive
studies
(Biles ~
~j.,
1987;
Conaway
~
~j.,
1985),
and
the
third reported
effects on
offspring (reduced body
weight
and reduced
weight
gain
in rat pups,
and
slightly
reduc~d
pup
survival) only at
doses
which were also
toxic
to
the
parents
(Neeper-Bradley,
1991).
Similarly,
the
reproductive
and
developmental study also
reported offsprIng
effects (reduced numbers
of viable
implantations aodior
live
births,
reduced
body
weight,
decreased
ossification, and
increased incidence
of cleft
palate
in
mouse
pups)
only at
doses
toxic
to
the
adults (Tyl
and Neeper-Bradley,
1989).
This
makes
it difficult
to
say whether
the
effects
on
reproductive
performance
were truly an effect
of
MTBE
on the
offspring,
or whether these
effects
resulted from the toxicity to
the
parents.
Since
the
doses which
showed these toxic
effects
were high
(3,000-4,000
ppm),
the
potential
for
human reproductive effects
at
the
much lower anticipated environmental
exposure levels is extremely
small.
Chronic
Toxicity
-
There
areno
studies
of the
effects
on
hnrnar,c
exposed
to
MTBE for long periods,
although anecdotal
reports
of increased
complaints
of headache,
nausea,vomiting,
eye
irritation,
and
respiratory
problems
have
surfaced
recently
in certain areas in
conjunction
with
wintertime
MTBE
increases in
gasoline.
These complaints
are
the
subject
of on-going
research.
There is only one
90-day subchronic study
in
laboratory
animals
exposed
by the
oral
route,
which was
the
study
finally
selected
to
derive
the HITAC
by
the Agency
after
following the
procedures
of Appendix A.
This
study
is evaluated
in depth
in
the
attachment
to
this
notice.
There
are
several
animal
subchronic
and
chronic
studies using
the
inhalation
route
of
exposure,
primarily
evaluating
the
neurotoxic
effects
of MTBE.
In
one
study
(Greenough
~
~.,
1980)
in
which
the
maximum dose tested
was
1,000
ppm for
6
his/day,
S
days/wk, for
13
weeks,
no
significant
effects
(other
than
anesthesia
following dosing
at high concentrations)
were
reported.
In
another
study (Dodd and
Kintigh, 1989), in which the
maximum
dose tested
was
8,000
ppm (same dosing regimen),
slight
changes
in blood chemistry,
increased
serom
cortisone levels
in
both
sexes,
reduced
weight gain,
increased
kidney,
liver,
and
adrenal
gland
weights,
and sporadic ~eurotoxic
effects
were
seen
at doses
of
4,000
andlor 8,000 ppm.
There
is
aLso
a
recently
completed
lifetime
cancer
bioassay
in
mice
and
rats
(Burleigh-Flayer ~
~j.,
unpublished;
Chun ~
~.,
unpublished),
the details
of which are
evaluated
in
the
attachment
to
this
notice.
FOR
FtJRTffER
INFORMATION,
COMMENTS
Persons
who wish
to
receive
further information about
this
notice or who wish to provide
comment
on
its
contents
are
requested
to
contact:
illinois Environmental
Protection
Agency
Office of
Chemical
Safety
P.
0.
Box
19276
2200
Churchill Road
Springfield, Illinois
62794-9276
217/785-0830
Enviroamen~aJ
Regis-~er
No.
484
July,
l994i’Page
21
ATTACHMENT
TO
NOTICE
OF
IIALTH
ADVISORY
FOR
-
METHYL TERTIARY-BUTYL
ET~ER
(MTBE)
OVER VIEW
OF TF~E
KEY STIJt~
In
the
only
oral
study
(Robinson ~
~j.,
1990),
rats
were
given 0,
100,
300,
900,
or
1,200
mg/kg
(ppm)
by
gavage.
Rats
given
1,200 ppm exhibited
profound anesthesia
after dosing throughout the
study, but recovered
after
the dose
within
two
hours
and
suffered
no
aftereffects.
Body
weight
decreased
with
increasing
dose,
with
the
difference between
treated
and
control
rats
being
statistically significant
at
1,200 ppm.
Other
measurements
showing statistical significance
included:
decreased
blood
urea
nitrogen
(BUN)
and
serum
creatinine
(measures
of
kidney
function)
at
all
doses;
increased
serum
cholesterol
at
all
doses;
increased
kidney weight
at
300
ppm
and
above;
increases
in
several
other
organ weights
at
900
ppm
and
above;
and changes
in
blood
parameters
at 1,200 ppm.
Microscopic
examinations
revealed
effects
only
at 1,200 ppm,
where
degenerative
changes
in
the kidneys of the
male
rats
were
noted.
Finally,
loose
stools
and
diarrhea
were
seen
at
all
doses throughout
the study.
Viewing
the
results of
this
study,
it would
appear
that
the
kidney
is the
target
organ
of
MTBE.
However,
these
results
must
be interpreted carefully.
The
decreases
in BUN
and
serum
creatinine probably have
no
adverse
effect
on
the
animals
(decreased
kidney
function is
often
signaled
by
increases
in
these
parameters),
and
may
even indicate
an increase in
kidney
function.
The
increased
kidney
weights
seen
at
300
ppm
and
above
are
not
in
themselves
an
adverse
effect,
only
an
indication of a
possible adverse effect
at even higher doses or longer exposure
times.
Finally, the
microscopic
changes
seen
at
1,200
ppm
in
males
are
often
seen
in
male
rats
(and
only
male
rats)
exposed
to
certain
organic
chemicals,
due
to
overproduction
of a unique protein
in
the
male rat
kidney.
Thus,
it
is not clear
at
this
time whether MTBE is toxic
to
the
kidney.
It would appear that
a no observed adverse
effect
level
(NOAEL)
has
not
been
determined by
this
study, since
increased
serum cholesterol
and
diarrhea were
observed
at
all doses.
Thus,
the
100
ppm dose would be
considered
to
be
the lowest
observable
adverse
effect level
(LOAEL) for MTBE.
The
procedure
for calculating
a
health
advisory
for drinking
water
in
the
groundwater
quality
standards
(35
111.
Adm.
Code
620,
Subpart
F) gives
preference
to
oral
studies which determine
a
NOAEL or LOAEL,
and
this
study
may
be
considered
to
develop the
health advisory
for MTBE.
A
lifetime
inhalation
cancer
bioassay
has
recently
been
completed with
mice
and
rats,
but
the
results
have
not
been
published (Burleigh-Flayer ~
~J..;
Chun ~
flj.).
The Agency
has been given
summaries
of the
studies submitted
to
USEPA
by
the
USEPA
contact
for MTBE.
These
results
are
briefly summarized,
but
since
the
studies
are
still
undergoing
review
it
must
be
realIzed
that
this
information
is
preliminary.
Both
species
were
exposed
toO, 400,
3,000,
or
8,000
ppm
in air.
As
in
the oral
study above,
the
male rats experienced
an
increased
incidence of kidney
degeneration.
This became
the leading
cause of deathin
male
rats,
and
resulted
in
early
termination
of
the
3,000
and
8,000
ppm male
groups.
The other main
cause
of death
in
male
rats
was
leukemia,
seen
in
both
the
control
and
400
ppm
group.
(In fact,
the
incidence
in
the
control
group was
higher,
33/50,
than
in
the
400
ppm
group,
22/50.)
Non-cancer effects
of MTBE included symptoms
of central
nervous
system depression
in
both sexes of rats
at
3,000
and
8,000
ppm,
but not at
400
ppm,
and
an
increased
incidence
of kidney degeneration
in
male
rats
at
400
ppm.
The only
tumors which were related to
MTBE
exposure were
tumors
in
the kidneys of male
rats
in
the
3,000 and 8,000 ppm
groups.
These tumor
types
are also thought
to
be
related to the
overproduction of the
male
rat protein;
and
the
significance
of
these results
for humans
is questionable.
In
the
mouse
study,
symptoms
of central nervous system depression similar
to
those seen
in
rats
were observed
at
3,000
and
8,000
ppm.
Increases
in liver
and kidney
weights
were
also
seen at these doses,
and
an
increase in
the
number of liver
cells
(rloncancerous),
an indication of toxic effects
on
the liver,
was reported at 8,000
ppm.
The only
tumors
found
in excesS
of controls
were liver
tumors
in
females
in
the 8,000
ppm
group.
However,
the
significance of
this finding for humans is
also
questionable,
since
this
tumor type
is
common
in
the
strain
of
mouse
used
in
this
study,
and
is
known
to
occur
in
~L
~.
tCi.~Lt~CL)
th~Li
L~.Lc.
In
reviewing
the
results of
these
studies,
it is difficult to
say
whether
MTBE
presents
a carcinogenic
hazard
to
humans.
However,
the
noucaucer
effects may
be
relevant for
determining
a
health advisory level
for
MTBE.
In
this
regard,
the
rat
study
has
produced
a
LOAEL
of
400
ppm
based
on
kidney
effects
in
male
rats
(this
dose
may
be
a
NOAEL given
the
questionable significance of this effect
for
hurrtans),
while
the
mouse
study
has produced
a NOAEL of 400
ppm.
The
mouse
Pag.! 22/ July,
1994
Environmen&zl
Regfser No.
484
—,
portion
of
this study
may
be considered
to
develop
the
health advisorj
for
MTBE,
once
it
has
fui~s~edUSEFA’s
review
•
process.
DERIVATION
OF
THE
HEALTH ADVISORY
FOR
MTBE
The first step in
the derivation
of
a
health
advisory
is
to
determine whether the
chemical
presents
a
carcinogenic
hazard
to
humans.
To
date~there
have
been
no
investigations whether
there
is
an
increased
incidence
of
cancr
in
humans
associated
with
exposure
to MTBE.
As
discussed
above,
there is some
evidence
that MTBE causes
tumors
in
laboratory
animals,
but
the
types
of
tumors
found
in
the
rat
and
mouse
cancer
bioa.ssays
may
not
provide
good
evidence
of
a
carcinogenic hazard
to
hurn2n~since
these tumors may be species-specific responses with
little
or
no
relevance
to humans.
Furthermore,
these studies
are
still
undergoing
review by
USEPA
and a
final
determination of
the
usability of
the
results
for determining the carcinogenic
hazard
to
humans
has
not
been made.
Therefore,
the
Agency
has determined
at
this time
that
the
derivation of
the
health
advisory
for
MTBE
will
be
based
on
the
non-cancer
effects
of
this
chemical.
This
derivation
may
be changed
in
the
future,
depending
on
the IJSEPA’s
determinatiorLs,
once
the cancer
bioassay data
have been
published and
the
welght-1-evidence
for
human
carcinogenic potential
has been
determined.
In deriving a health
advisory
to
protect against a health effect for which there
is
a threshold dose below which no
damage
occurs
(i.e.,
noncarcinogenic
effects),
Section
620.605 specifies that
USEPA’s
maximum contaminant
level goal
(MCLG),
if
available,
is the
health
advisory
concentration.
USEPA has
not
published a MCLG
for
MTBE,
therefore, the
Agency must
calculate
the human
threshold
toxicant
advisory
concentration
(HiTAC)
as
the
health
advisory
concentration,
using
the
procedures
specified
in
Appendix
A
of
Section
620.
Appendix A specifies in subsection
(a)
that
the
HTTAC
is
calculated as
follows:
w
W~ere:
H’ITAC
=
Human threshold toxicant
advisory
concentration
in
miuigra~sper
liter
(mg/fl;
RSC
=
Relative
source
contribution,
the
relative
contribution of
the
amount
of
the
exposure
to
a
chemical
via
drinking water
when compared
to
the
total
exposure
to
that
chemical
from
all
sources.
Valid
chemical-specific
data shall be used
if available.
If valid
chemical-specific
data are
not available, a
value of
20
(=0.20)
must
be
used;
ADE
=
Acceptable daily exposure
of
substance in
milligrams per day
(mg/d)
as
determined pursuant
to
subsection
(b);
and
Per capita daily water
consumption
equal
to 2
liters per
day (lid).
Subsection
(b)
of
Appendix
A specifies that
the
ADE
be calculated using,
in specified
order:
USEPA’s Verified
Oral
Reference Dose (an
estimate of a daily
exposure
to
a chemical
which
is
expected
to be
without
adverse
effect for humans,
including
sensitive
subgroups,
for a
lifetime
of
exposure);
a
NOAEL which
has
been
identified
as
a
result
of
human
exposures;
a
LOAEL which has
been
identified
as
a result of human exposures;
a NOAEL which has been determined from
studies with laboratory
animals;
and a LOAEL which has been determined
from studies with
laboratory
animals.
There
is
no
Verified Reference Dose currently
available from
USEPA.
As
mentioned
above, there
is a
paucity of studies
on
the
adverse effects in
humans
exposed to
MTBE.
Thus,
the
Agency
has determined
that
a
NOAEL or LOAEL
based
Un
Ou.W.ML1
cap~uru~
i~
L1o~~v~u~u~c
~3LUL~sUu~c.
~UC~CLuLc,
L~ic
r’ui~
~
u’C
~
L~i~
~
~
‘.—~“
the studies
reviewed
by the
Agency,
the
90-day rat subcbronic study and
the cancer
bioassay (noocarcinogenic
effects)
are
•
-
the
most
appropriate
animal
studies
for calculation
of
the
ADE.
It
is then
necessary
to
determine
which study is
the
most
valid
for
purposes
of
calculating
the
ADE.
Subsection
(c)
of
Appendix
A
specifies
criteria
for
establishing
the
validity
of
data
from
animal
studies,
leading
to
determinations
of high,
medium,
or
low validity.
High validity
studies
are
those
using
the oral
route
of exposure
and which
Environmerl!aI
RegL~ter
No.
484
July,
l994/Page
23
meet
specified
criteria
depending
oo
the
type of
study,
and
are
to
be
used
preferentially
if
availabiè.~Therat
90-day
subchrocic study was conducted
using
the
oral
route,
while
the
cancer
bioassay was
an
inhalation
study.
Therefore,
only
the
subchronic study could
be a high
validity study
However,
the
requirements
for
a
high
validity subchronic
study include,
among
other
things,
a
study using
two
sc
es
and determining
a well-defined
NOAEL.
The
90-day
rat subchronic
study
used
only
one
species and only determined
a
LOA.EL,
as discussed
above.
Having
no high validity
study,
the
Agency
niust
determine which
of
the
two
studies
is
most appropriate
for
calculating
the
ADE.
Subsection
(c)
goes
on
to
specify
that
in
order for
a
subchronic stud~
in
which
a
LOAEL
is
determined
to
be
deemed
a
medium
validity study, the
study must satisfy
all
other
standards
for
a
high
validity
study.
This
is not
the
case
for
the
90-
day
rat
subchronic
study, since
there
was only
one
species tested.
Similarly, in order
for a study
other
than
an oral exposure
study
to
be
deemed
a medium validity
study,
the
study must
satisfy
all
other
standards
for
a
high
validity
study
and
use
appropriate
correction factors for conversion
to the
oral
route.
However, the
requirements
for a high validity cancer
bioassay
include,
among
other
things,
at
least 25
survival
at
18
months
in mice and
24
months
in rats.
This was
not the
case
in
the cancer bioassay, since
the male
rats
in the
3,000 and
8,000 ppm groups were
terminated
early due to
excessive
mortality.
Thus,
both
candidate
studies are
defined
as
low
validity
studies,
and
the 90-day
rat
subchronic
study
is
selected
because
exposure
was
by
the
oral
route.
The
determination of the
ADE
from
the
subchronic
study
is
made
using
the
language of subsections (b)(5)
and
(b)(6).
Subsection
(b)(6)
specifies
that
for
substances
for
which
a NOAEL is not
available, one-tenth ofthe
LOAEL
is substituted
for the NOAEL
in subsection
(b)(5).
Subsection
(b)(5)
specifies
that if studies of low
validity
must
be used,
the
ADE
must
be
calculated using l!l000 of
the NOAEL.
The overall
result of the
procedures in
these
two
subsections is
that
the
ADE
is
1/10,000 of
the LOAEL,
times the
average
weight
of an
adult
human,
70
kg:
ADE—
100~ng/~g/dx70kg
a
7mg/d
10,000kg/d
At
this
point, the calculation of the H’ITAC
would proceed according
to
the
formula listed above.
However,
the Agency
has been informed
by USEPA
personnel
that in
most cases USEPA now prefers
to calculate acceptable exposure
values
for
humans by using laboratory
animal
data
divided by
no
more
than
a 3,000-fold
uncertainty
factor;
a
10,000-fold
uncertainty
factor would
be
used
only
where
the
overall
toxicity
database
is very
weak
for
a
chemical.
The Agency agrees
with
this
emerging
USEPA
approach.
Since
the MTBE
database contains
enough
laboratory
animal
research
to
indicate
that
there
are
not
major
toxicity
data
gaps
which
would
warrant
the
use
of
a
10,000-fold
uncertainty
factor,
the
Agency
is
also
calculating the
ADE
using
a
3,000-fold uncertainty
factor:
4•4fJ~
10
/k~/dx7Qk~2
3m,gJd
3,000
Finally,
the determination of
the HITAC
is
straight-forward, since there are
no
chemical-specific data
available
for
the
RSC
term:
HrrAc=
01Ox0.7mJd0~~
2.OcJd
Or:
010x23m
Id
H7TAC=
~
oj3g/~
The
final
step
in
determining
the
health
advisory
is
to
compare
the
HTTAC
value
calculated
from
the
Appendix
A
procedures
to
the
chemical’s
Practical
Quantitation
Limit
(PQL).
In
the
ca~seof
MTBE,
no
USEPA
SW-846
analytical
method
specifies
a
PQL for this chemical.
However,
the
Agency’s Division of Laboratories
has determined
that
a
detection
limit of
0.005
mg/i
is appropriate
for water
samples.
Therefore,
the
HITAC
value
is above
the
detection limit.
Page
24/July,
1994
En~ironmeiUcJR~gi.~Thr
No.
484
The Agency has
decided
to
issue
a
two-part
health advisory.
The
precautionary
health
advisory~fãncentration
for
•
Methyl
Terti.ary-Butyl Ether
(MTBE)
is
0.07
mgi!
or
70
parts
per billion
in
drinking
water.
People
can
be expo~i
to
this
concentration
of
MTBE
in drinking water
over
a
70
year
lifetime.
Above
this
concentration,
appropriate
caution
•
should
be
exercised
by
the
Public
Water
Supply,
such
as
increased
frequency
of
sampling
and
identification of
the
MTBE
source(s).
The final
health advisory concentration
is 0.23
mg/i or
230
parts
per billion in
drinking water.
Above
this
•
concentration,
the
Public Water Supply
should
begin
actions
to decrease
the
amount
of
MTBE
in
the
system.
REFERENCES
API,
American Petroleum Institute.
1993.
Odor
Threshold Studies
Performed
with Gasoline and Gasoline
Combined with
MTBE,
ETBE,
and
TAME.
API
Publication
Number
4592.
Biles,
R. W.,
Schroeder,
R.
E.,
and Hold.sworth,
C.
B.
1987.
Methyl
Tertiary
Butyl
Ether
Inhalation in Rats:
A
Single
Generation
Reproductive Study.
Toxicol.
md.
Health
3:
519-534.
-
Burleigh-Flayer,
H.
D.,
Chun, J.
S.,
and
Kintigh,
W.
I.
(unpublished).
Methyl Tertiary
Butyl
Ether.
Vapor
Inhalation
Oncogenicity
Study
in CD-i
Mice.
Submitted to
USEPA,
Docket
No.:
OPTS-42098.
Chun,
I.
S., Burleigh-Flayer,
H.
D.,
and Kintigh, W.
J.
(unpublished).
Methyl
Tertiary
Butyl
Ether:
Vapor
Inhalation
Oncogenicity
Study
in Fischer
344
Rats.
Submitted
to USEPA,
Docket No:
OPTS-42098.
Conaway,
C. C.,
Schroeder,
R. E.,
and
Snyder,
N.
K.
1985.
Teratology Evaluation
of Methyl Tertiary-butyl Ether in
Rats
and
Mice.
J.
Toxicol.
Environ. Health
16:
797-809.
Connecticut
Dept.
of
Environmental
Protection.
(undated).
Action Level
for
Methyl
Tertiary
Butyl Ether
(MTBE)
in
Drinking
Water.
Prepared
by
H.
V.
Ran,
C.
J.
Dupuy,
and
D.
R.
Brown,
Connecticut Dept.
of Health
Services.
Costantini, M.
G.
1993.
Health
Effects of Oxygenated Fuels.
Environ.
Health Perspectives Suppl.
101
(Suppl. 6):
151-
160.
Dodd, D.
E.
and
Kintigh, W.
J.
1989.
Methyl
Tertiary Butyl
Ether
(MTBE):
Repeated
(13-Week) Vapor
Inhalation
Study
in
Rats
with
Neurotoxicity
Evaluation
(unpublished
study).
Union
Carbide,
Bushy
Run
Research
Center
for
MTBE
Committee.
TSCATS
403189.
EPA/OTS
#
FY1-OTS-0889-0689.
Cited
in
Revised
and
Updated
Drinking
Water
Quantification of
Toxicological
Effects
for Methyl Tert-Butyl Ether (MTBE),
Final Draft.
USEPA;
ECAO-CIN.D023,July,
1993.
Essential Elements
(in
press).
ILSI
Press, Washington,
D.C.
1994.
Greenough,
R.
J.,
McDonald,
P.,
Robinson,
P.,
et
a!.
1980.
Methyl
Tertiary-Butyl
Ether
(Driveron)
Three
Month
Inhalation Toxicity
in
Rats.
Project
No. 413038.
Unpublished
report
submitted to Cbemische Werke
Höls AG,
Marl,
West
Germany.
230 p.
Cited in Revised
and
Updated Drinking
Water
Quantification of Toxicological
Effects
for Methyl
Tert
Butyl
Ether (MTBE).
Final
Draft.
USEPA;
ECAO-CIN-D023, July,
1993.
Neeper-Bradley,
T.
L.
1991.
Two-Generation Reproduction
Study of
Inhaled
Methyl
tert-Butyl
Ether
in
CD
Sprague-
Dawley
Rats (unpublished
study).
Union
Carbide,
Bushy
Run Research
Center.
Cited
in Revised
and
Updated
Drw.king
Water
Quantification of Toxicological Effects
for Methyl
Tert-Butyl Ether
(MTBE),
Final
Draft.
USEPA;
ECAO-CIN
D023, July,
1993.
Ponchon,
T., Baroud,
J.,
Pujol,
B.,
Valette,
P.
1.,
and
Perrot,
D.
1988.
Renal
Failure
during Dissolution of Gallstone by
~
,...
~
~
__.___:
_
-•
Reese,
E.,
and
Kimbrough,
R.
D.
1993.
Acute Toxicity of Gasoline
and
Some
Additives.
Environ.
Health
Perspectives
Suppl.
101
(Suppl.
6):
115-131.
Robinson,
M.,
Bruner,
R.H.,
and
Olson,
G.R.
1990.
Fourteen and
Ninety-Day Oral
Toxicity
Studies of Methyl
TertiarY-
Butyl
Ether
in Sprague-Dawley Rats.
Journal of the
American
College of Toxicology 9(5):
525-540.
EnyirojzmerUi.zI
Regi.Sler No.
484
July,
l994/Page
25
Tyl,
R.
W.,
and
Neep~r-Bradley,T.
L.
1989.
Developmental
Toxicity
Study
of
Inhaled
Methyl
Tertiarf
Bury!
Ether
in
(Th
CD-I
Mice.
Project
Report
52-526.
Prepared
by
Busby
Run
Research
Center,
Union
Carbide
Corporation
for
the
Methyl
•
-
Tertiary
Butyl
Ether
Committee,
Washington,
D.C.
Microfiche
No.
0TS0000689-1.
Cited
i~Revi~ and
Updated
•
Drinking Water
Quantification
of Toxicological
Effects
for Methyl
Tert-Butyl
Ether (MTBE),
Final
Draft.
USEPA;
ECAO-
-
CIN-D023,
July,
1993.
-
•
-
-
•
-
USEPA.
1993.
Revised
and Updated Drinki
g Water
Quantification of Toxicoiogical
Effects
for Methyl Tert~BurylEther
•
(MTBE),
Final
Draft.
USEPA;
ECAO-CIN-D023, July,
1993.
von Burg,
R.
1992.
Toxicology
Update.
Methyl Tert-Butyl
Ether.
J.
Appl. Toxicol.
12:
73-74.
•
State of illinois
ENVIRONMENTAL PROTECTION AGENCY
Mazy
A.
Gade, Director
2200
Churchill
Road,
Springfield, IL 62794-9276
217/785-0830
-
November 4,
1994
G.A. Van Gelder, DVM,
Ph.D.,
ABVT
Manager, Toxicology
Health, Safety -and Environment
Shell
Oil Company
One
Shell
Plaza
P.O.
Box 4320
-
Houston, IX
77210
•
-
Dear Dr. Van Gelder:
This letter confirms the meeting to evaluate comments received regarding the
Illinois Environmental
Protection Agency’s proposed Health Advisory for MTBE
which we discussed over the telephone.
The meeting
is scheduled for November
14,
1994, beginning
at 12:30.
The room is available until
5:00 PM,
if
necessary.
The meeting will
be held in Room 031
on Floor
8,
James
R. Thompson
Center,
100 W. Randolph, Chicago,
Illinois, 60601.
I
have enclosed an
agenda for the meeting,
a copy of the Health Advisory
Section
of the Illinois Groundwater Quality Standards,
and a summary of the
Agency’s opinions on two key issues which have emerged from the comments.
I’m looking forward to a productive meeting.
Please call
(217/785-0830)
if
you have any further comments
or questions.
Sincerely,
T~-
Thomas
C. Hornshaw,
Ph.
0.
Manager, Toxicity Assessment Unit
Office of Chemical
Safety
f:\psf\epa8566\mtbe.mtg
Attachment
MTBE Meeting Agenda
12:30
-
12:45
12:45
-
1:45
1:45
-
2:00
2:00
-
.3:15
3:15
-
3:30
Introductions
and Background
Key Issues
(LOAEL
vs.
NOAEL,
RSC)
Break
Other Issues
(Tase/Odor Threshold, Uncertainty
Factors,
2-Tier Vs. Single Advisory,
Edits
Wrap-up
RESPONSES
TO
SIGNIFICANT
COMMENTS
REGARDING
PROPOSAL FOR
E~EALTH ADVISORY
-
FOR
METHYL
TERTIARY-BUTYL ETHER
The lUinois
Environmental Protection Agency (Agency) has received three
comments in response
to
the
Notice of Health
Advisory for
Methyl
Tertiary-Butyl
Ether
(MTBE),
published
in
the
Illinois
Environmental
Register No.
484,
July,
1994.
The
comments
were
received
from
the
American
Petroleum
Institute
(API),
the Methyl Tertiary Butyl Ether Task Force (Task Force),
and
Shell
Oil
Company
(Shell).
The
comments
cover
several
technical
and
typographical
subjects,
the
most
significant
of
which
address
the
Agency’s
determination
of
a
Lowest
Observable
Adverse
Effect
Level
(LOAEL)
versus
a
No
Observable
Adverse
Effect
Level
(NOAEL)
and the
uncertainty
factors
which result
from
this
determination,
and
the
Agency’s
use of the default value of 20
as
the Relative Source
Contribution (RSC) term-versus
the use
of an
RSC
derived from
chemical-specific data in the calculation of the Health Advisory.
The
Agency’s responses
to these key
issues
are
presented in
this paper.
-
-
LC)AEL
vs. NOAEL
API and
Shell
disagree with the
Agency’s
characterization of the diarrhea and
elevated serum
cholesterol reported
at the
100
mg/kg
dose
in the
Robinson
et
al.
(1990)
study
as
a
LOAEL.
In
reviewing
the results
of this
study,
the
Agency
determined
that the
authors’
reports
that
“treated
rats
in
all dose
groups
also
displayed
diarrhea
throughout
the
exposure period”
and
their
findings
that
“females
exposed to
all
dose levels exhibited
significant
increases
in
serum
cholesterol”
indicated
that the
study
had
not
identified
a No
Observed
Adverse
Effect
Level.
This
determination
is
an
outcome
of the
evaluation of
the
validity
of the
candidate
snidies
required
by
the
Groundwater
Quality
Standards
regulation
when
animal
studies
must be
used
to
develop
a
Health
Advisory.
This
evaluation
was
discussed
briefly
in
the
July,
1994
Notice,
and
will
be
expanded
for
explanation
of the Agency’s
rationale.
Section
620.
Appendix
A(c)(l)(A)(iii),
which
identifies
the
elements
necessary
for
High
Validity
Studies,
requires:
-
-
Data
from
animal
subchronic
studies
with
a
minimum
of
3
dose
levels
and
control,
2
species,
both sexes,
4
animals per dose-per sex for non-rodent
species
or
10
animals
per
dose per sex for rodent species,
a duration of at least
5
of the
test species’
lifespan,
and a
well-defined NOAEL
(emphasis
added-).
-
The Agency determined that the reports of diarrhea in all animals
and
elevated
serum cholesterol
in
females
in
all
dose
groups
could
not
be
called
a
“well-defined NOAEL”
for
purposes
of
establishing
High
Validity
for
this
study.
Thus,
the
lowest
dose
tested,
100
mg/kg,
was
API
and
Shell
have
commented
that
the
results
of the
study
should
not
be
interpreted
in
this
manner.
Both claim
that
the occurrence of diarrhea in
treated
animals
is
not well-documented
or
described
in
the
Robinson
study,
that
diarrhea
is
a
common observation
in
rats
dosed
with
-
corn
oil,
and
it
is
a questionable
endpoint
for extrapolation
to low-dose lifetime
health
effects.
Both
also
claim
that
the
modest
increases
in
serum
cholesterol
in
the
female
rats
are
not
indicative of a
meaningful health effect,
arguing
that the authors’
statistical evaluation incorrectly
-
attributes a
significant difference
for the
300
mg/kg
dose,
that there
is
no
compelling
evidence
for
a
dose
response,
that
only
the
900
mg/kg
dose
in
males
achieved
value-s
significantly
different from controls,
and that the increases are near
the
range
of normal
variability.
Finally,
API argues
that the
diarrhea
and elevated serum
cholesterol are not
significant
results,
citing
the
authors’ conclusions that the study indicated that dose levels below those which
induce anesthesia
(1200 mg/kg)
do
not
result
in
significant
pathophysiological
changes.
The
Agency
remains
unconvinced
that
the Robinson
et al.
study
has
identified
a well-defined
NOAEL.
Regarding
the occurrence of
diarrhea,
we have
interpreted the
authors’
reports of
diarrhea
in
“treated rats
in all dose
groups”
-
to
mean all groups receiving
doses of MTBE,
but
not
those receiving the vehicle control
(corn oil).
Thus,
we believe
that the
diarrhea
is
likely
to be treatment -related, at least in females;
this
belief is
supported by the fmdings of the 14-day
study
also
reported
in
this
paper,
in
which
“by
the
third
day
of
dosing,
all
treated
animals
displayed loose
stools
which
continued throughout the remainder
of the exposure period.”
We
have reviewed the
National
Toxicology
Program’s report
on
the
lifetime
cancer
bioassays
of
-
gavage vehicles in
male Fisher
rats,
which
included corn
oil,
and find
no
mention of diarrhea
as
an effect of corn oil (NTP,
1994).
Finally,
we have relied
on
the
experience of one of the
Agency’s
Office
of Chemical
Safety
toxicologists,
who
reports
that,
in
over
8
1/2
years
of
experience
in
an
industrial
toxicology
laboratory,
the
occurrence
of
diarrhea
in
rats
in
conjunction with
corn oil vehicles was very infrequent (Morrow,
1994).
While
we
cannot rule
out the possibility
that the diarrhea reported by
Robinson et
al.
was vehicle-related,
we continue
to
believe that this effect was
a result of the MTBE exposure.
Regarding
the elevated serum cholesterol findings,
the Agency acknowledges
that the statistical
significance
of
the
300
mg/kg
dose
in
female
rats
is
questionable
and
possibly
incorrectly
reported,
and
that
there
is
no
obvious
dose-response
relationship
among
the female treatment
groups even though
all but the 300 mg/kg
group is
significantly
greater than controls.
However,
we
maintain
that these results
are
potentially
indicative of a
real-effect in the rats;
it is possible
(although unlikely)
that
the
effect
may plateau relatively
quickly,
such
that
the
dose-response
relationship is defined
at doses
below
those
tested in this
study.
Further,
we again
note that the
results
of the
14-day
study
reported
in
this
paper
also
include
elevated
serum cholesterol
in
females of most
treatment groups.
-
Regarding
the biological
significance
of the
diarrhea
and elevated serum cholesterol
and whether
these endpoints
are relevant for
extrapolating
to
human
health risks,
the Agency
maintains
that
such
effects
are relevant for use
in developing
the Health Advisory.
While
neither
endpoint is
relatively
serious,
diarrhea
can
be~deleterious
to
the
organism
over
time
by
contributing
to
dehydration,
electrolyte imbalance, and/or
poor nutritional status, and elevated cholesterol, while
flOt.
in
itsett
a oioiuglcany
senous eiieei,
is
a.
CaULIOU
tor
1110cc
5ef10u5
eliecis
OVCf
LUhle.
WiLlie
the
authors’
concluded
that dose
levels
below
those
which
induce
anesthesia
do
not
result
in
significant pathophysiological
changes,
the Agency
would
be
very uncomfortable using
a dose
which
does not
induce anesthesia
as the
basis
for developing
a Health Advisory.
We continue
or
described
in
the
Robinson
study,
that
diarrhea
is
a
common observation
in
rats
dosed with
corn
oil,
and
it
is
a questionable endpoint for extrapolation
to
low-dose
lifetime
health effects.
Both
also
claim
that
the
modest
increases
in
serum
cholesterol
in
the
female
rats
are
not
*
indicative of a
meaningful
health effect,
arguing
that the authors’
statistical evaluation
incorr_ect.ly
attribu~es
a
significant difference for the
300
mg/kg dose,
that there
is
no
compelling evidence
for
a
dose
response,
that
only
the
900
mg/kg
dose
in
males
achieved
values
significantly
-
different from controls,
and
that the increases
are near the
range
of normal
variability.
Finally,
API
argues
that the
diarrhea
and
elevated serum cholesterol are not
significant
results,
citing
the
-—
authors’
conclusions thatthe study indicated that dose levels below those which induce anesthesia
(1200 mg/kg)
do
not result in
significant
pathophysiological
changes.
-
The
Agency
remains
unconvinced
that
the Robinson
et
al.
study
has
identified
a
well-defined
NOAEL.
Regarding
the occurrence of
diarrhea,
we have
interpreted the
authors’
reports
of-
diarrhea
in
“treated
rats
in
all dose
groups”
to
mean all
groups
receiving
doses of
MTBE,
but
not
those receiving the vehicle
control
(corn
oil).
Thus,
we believe
that
the
diarrhea
is
likely
to be treatment -related,
at least in females;
this beliefis supported by the findings of the 14-day
study
also
reported
in
this
paper,
in
which
“by
the
third
day
of dosing,
all
treated
animals
displayed
loose
stools
which continued throughout the remainder of the exposure period.”
We
have reviewed
the
National
Toxicology
Program’s
report
on the
lifetime
cancer bioassays
of
gavage vehicles
in
male Fisher
rats,
which included
corn
oil,
and find
no
mention of diarrhea
as
an effect of corn
oil (NTP,
1994).
Finally,
we have
relied
on the experience of one of the
Agency’s
Office
of
Chemical
Safety
toxicologists,
who
reports
that,
in
over
8
1/2
years
of
experience
in
an
industrial
toxicology
laboratory,
the
occurrence
of
diarrhea
in
rats
in
conjunction with corn oil vehicles was very infrequent (Morrow,
1994).
While
we cannot rule
out the possibility
that the diarrhea reported by Robinson et al.
was
vehicle-related, we continue
to believe
that
this effect was
a result of the MTBE exposure.
Regarding the elevated
serum cholesterol findings,
the Agency acknowledges that the statistical
significance
of
the
300
mg/kg
dose
in
female
rats
is
questionable
and
possibly
incorrectly
reported,
and
that
there
is
no
obvious
dose-response
relationship
among the female treatment
groups even though all but the 300 mg/kg
group is significantly greater than controls.
However,
-
we
maintain
that these results
are potentially indicative
of a
real effect in the rats; it
is possible
(although
unlikely)
that the effect
may
plateau
relatively quickly,
such
that
the
dose-response
-
relationship
is defined at doses below
those tested in this
study.
Further,
we
again
note that the
results of
the
14-day
study
reported in
this
paper
also
include
elevated
serum
cholesterol
in
females of most treatment
groups.
Regarding
the
biological significance of the diarrhea and elevated serum cholesterol
and
whether
these endpoints
are relevant for
extrapolating
to
human
health risks,
the Agency
maintains
that
such effects are
relevant for use in
developing
the
Health Advisory.
While neither
endpoint
is
relatively
serious,
diarrhea
can
be
deleterious
overtime
to
the
organism
by
contributing
to
dehydration, electrolyte imbalance, and/or poor nutritional status, and elevated cholesterol, while
not
in
itseit
a
biologically
serious errect,
is
a caution nor more serious ettects
over tune.
While
the authors’
concluded
that
dose levels below
those
which
induce
anesthesia
do
not result in
significant pathophysiological
changes,
the Agency
would
be very uncomfortable using
a
dose
which
does
not
induce
anesthesia as the basis
for developing
a
Health Advisory.
We
continue
to
believe
that
the
100
mg/kg
dose,
as
a
LOAEL,
is
the
most
relevant
value
to
use
in
the
—
development of the Health
Advisory.
This reasoning
plus
the relative paucity of
data regarding
-
the ingestion of
MTBE,
argues
for
the
continued
use of the 3000-fold
uncertainty
factor as
the
most appropriate
value
for
the final
Health
Advisory.
-
—
MTBE
RELATIVE
SOURCE
CONTRIBUTION
TERM
The
comments
of
both
API
and
the Task
Force
addressed
the
Agency’s
use
of the
default
value
of
20
as
the
Relative Source
Contribution
(RSC)
term,
which is
specified
in
Section
620.
Appendix
A(a).
(This
is
also
a
standard
USEPA
default
assumption,
used
in
risk
assessments
to
account
for
all
other
exposures
to
a
chemical
other
than
direct
ingestion
in
drinking
water,
such
as
through
the
diet,
ambient air,
the workplace;
and
volatilization
from
the
household
water supply).
—
-
Both
comments
cite
a
USEPA
study
(tJSEPA,
1993)
which
estimates
the amount
of
MTBE
exposure experienced
by the
general
public
during
activities
other
than
drinking
water,
such
as
working,
outdoor
exercise,
refueling,
driving,
etc.
This
study
is
proposed
to
be
used
as
chemical-specific
data
instead of the
default
value
to
account for
exposures
to
MTBE
other
than
via
direct
ingestion
of
drinking
water.
If this
study
is
used
to
define
the
RSC term,
the
range
of
weighted
annual MTBE
ambient
air
concentrations
of 0.04
-
0.07
mg/m3
would
-
-.
~-
-
result in
a
RSC
term
of
approximately
45
-
70
for
drinking
water
exposures.
Depending
on the
final determination
of
the
RSC
term,
the
Health
Advisory
(HA) for
MTBE
would then
be
in
the
range
of
0.52
-
0.80
mg/l,
instead of the
proposed
0.23
mg/l
using
a
20
RSC
term.
While
the
Agency
agrees
with
the
data
presented
in
the USEPA
study,
it
cannot agree
that
these
data
fully
account
for
all
other
sources
of
MTBE
contributing
to
a
person’s
daily
exposure.
Use
of
only
this
study
to
account
for
inhalation
exposures
does
not
consider
inhalation
exposures which
will
occur
in the home
as
a
result of volati~izationof
MTBE
from
the household water supply
during
uses of the supply
for purposes
other than
drinking.
Since
the
Agency
is
not
aware
of
studies
evaluating
such
exposures,
an
evaluation
of the
indoor
inhalation pathway
was
undertaken using
data
reported for trichloroethylene (TCE).
The
transfer
of
volatile
organic
chemicals
(VOCs),
including
TCE,
from
water
to
air
has
been
studied
by
several
investigators
(Andelman,
1985;
McKone,
-
1987;
McKone
and
Knezovich,
.1991).
Of
particular
interest
for
this
analysis
are
studies
which
measure
the
transfer
of VOCs
during
showering
since
this
activity is
likely
to
be
the greatest
contributor
to
indoor
VOC
exposure
due
to
the
temperature,
amount of water
used,
turbulent
flow,
and
the
relatively
small
volume
of air
in
the bathroom.
Therefore,
the
McKone and
Knezovich
study,
which
measures
the
evolution
of TCE
into
a bathroom’s
air
during
operation
of
the
~
~
~
‘~‘
~
~---~-~
~
~
showering.
This
study
evaluated
the
effects
of
shower
temperature
and
duration
on
the
-
transfer
efficiency of
TCE from
water
to
air,
concluding
that -the
transfer efficiency
is
61 ±
9
and
that
inhalation
exposures
in
the
shower could be
equivalent
to
an
ingestion
exposure
of from
1-4 liters per
day.
Assuming
that
the
transfer
efficiency
of any
VOC
for
which
transfer
efficiency has not
been
*
measured
is
directly proportional to
that of another VOC
having
a measured transfer effici~ncy,
the
t~ansfer
efficiency
of
MTBE
from
water
to
air
can
be
estimated
from
the
TCE
data
by
comparing
the
overall
mass
transfer
coefficients
from
water
to
air (K~)for
both
chemicals.
McKone (1987)
has
shown that K~
can
be
approximated by:
—
r
2.5
+
RT
~,
where
-
LDBLV3
RDBAV3J
DEL
=
diffusion
coefficient in
water (m2/s),
-
-
DEA
=
diffusion
coefficient in
air (m2/s),
R
=
universal
gas
constant,
0.0624
torr-m3/mol-K,
-
T
=
temperature,
303K (air temperature in
hot
shower),
and
H
=
Henry’s
law
constant (torr-m3/mol).
-
The
diffusion
coefficients
of
TCE
and
MTBE
were
calculated
according
to
methods
recommended
in
Lyman
(1982),
assuming a
water temperature of 37°Cand
an air temperature
of 30°Cto
be
representative
of hot shower
conditions.
The
calculated values
for
TCE
and
MTBE
for DEL are
1 .094E-09
m2/s
and 9.870E-lO m2/s,
respectively,
and
for DEA
are 9.40E-06
(::
m2/s
and
9.28E-06
m2/s,
respectively.
Substituting the
calculated DEL and
DBA values
and
Henry’s
law
constants of 6.916 torr-m3/mol
for
TCE and
4.484
torr-m3/mol for
MTBE
into the
overall
mass
transfer
coefficient equation,
values
for K~were calculated to be
4.236E-07
m2/s
and
3 .950E-07
m2/s for TCE and
MTBE,
respectively.
The ratio
of the
two
K~,values of 0.9325,
when compared to the measured TCE
transfer
efficiency of 61,
suggests
an
MTBE transfer
efficiency of approximately
56.89.
Once
the
transfer
efficiency
has
been determined,
an
estimate
of a
resident’s
cumulative
daily
intake
from
showering
(CDI~)can be
calculated for any
VOC
water
concentration
(Cv)
using
reasonable estimates of water use during
showering and the volume ofthe shower, plus standard
USEPA
assumptions for body
weight
(BW,
70
kg)
and
breathing
rate
(BR,
20
m3/d
=
0.014
m3/min).
For
this exercise,
it is
assumed
that the resident’s shower duration
(SD)
is
10
minld,
the shower flow rate
(FR)
is
10
lJmin,
and the volume
(V)
of the
shower is
2.3
m3.
The CDI~
for
any C~
is
calculated from:
CDIS
=
r
(FR x
SD
x
Transfer Efficiencv’
x
BR x
SD
1
x C~.
L
VxBW
After
substituting, the CDI~for
any C~becomes:
-
UJi~
=
(U.049
lilg/d)
x (J~.
-
This
shower
inhalation
intake
can
be
compared
directly
with the
daily
ingestion
intake
(CDI1)
of
the
VOC
from
drinking
water
for
the
same
C~by
again
employing
standard
USEPA
assumptions
for
BW
(as
above)
and
daily
water intake
(WI,
2.0
lId).
The
CDI1
is
calculated
from:
—
.
-
.
-.
CDI1=~xC~,
.-
-
-
BW
-
-.
which becomes
after substitution:
CDI1
=
(0.029
lJkg/d)
x
C~.
These
two
CDIs
are
now
directly
comparable for any
water concentration of
MTBE.
The ratio
of CDIS to CDI1 is
1.69,
suggesting that the resident’s
daily showering contributes approximately
169
of the
daily
exposure to
MTBE compared
to the exposure
due
to ingestion
alone.
This
is
equivalent
to an
additional ingestion intake of (169
x 2.0
lId),
or 3.38
lid.
An evaluation of other non-ingestion household water uses (cooking,
toilet use,
washing
dishes
and clothes,
humidifier,
etc.) is not as straightforward as the evaluation of shower exposures due
to greater
variability
in the frequencies of the activities/uses.
McKone
(1987) estimates that the
ratio
of the indoor inhalation dose to
the
drinking
water ingestion
dose for VOCs
ranges from
1.5
-
6.0
(includes
showering and all other
inhalation
exposures).
As
estimated above, the ratio
for
showering
alone
is
1.69
for
MTBE,
which suggests
that the
ratio
for
all indoor inhalation
exposures
must be
greater than
1.69.
Assuming that
the other
indoor inhalation
exposures
are
at least one-sixth to one-fifth the magnitude-ofthe shower exposure, it can be assumed that these
exposures’
ratio
to
the
drinking
water
ingestion
exposure
is
at
least
0.31,
or
31
of
-
the
ingestion exposure.
Thus,
these exposures contribute at least the equivalent of 0.62
l/d
of direct
ingestion,
and
the total
adjusted
intake
due
to
in-home
water
use for purposes
of a
chemical-
specific
RSC
should
be at least (3.38
l/d +
0.62
lId
+
2.0
lId),
or
6.0
lid.
The data from
USEPA
(1993)
can now be used to calculate the remainder of the resident’s
daily
exposure
to
MTBE.
This
exposure
is
the
result
of
ambient
air
exposures
plus
indoor
air
exposures which
are ~
due to an MTBE-contaminated water
supply (i.e.,
exposure to MTBE
which originated from the ambient air
and is
then
inhaled
in the residence, workplace,
and- other
buildings).
These
calculations
have -been
completed
using
the
USEPA
data
for
a
6-month
oxyfuel season, which predicts 0.04 mg/rn3 and 0.07 mg/rn3
as the
Low
and High
annual average
MTBE air concentration,
and the standard USEPA
assumption for
breathing
rate as above.
The
CDI
(in mg/d)
resulting
from
ambient
air
exposures (CDIx)
can be
calculated from:
CDIA
=
BR x
Annual
Average
Concentration,
which
results
in estimates
of 0.8
mg/d
and
1.4
mg/d
for the Low
and
High
annual averages,
respectively.
‘the
rinal step
in
the development or a ciienmcal-specitic R~C
is
to
apportion
the contributions
-
-
-
of the
Acceptable
Daily
Exposure
(ADE)
of 2.3
mg/d
of
MTBE
between ambient
air and
the
home water supply.
As calculated from the USEPA data,
the ambient
air exposures
contribute
between 0.8
mg/d
and
1.4
mg/d
of the 2.3
mg/d
ADE.
This
leaves between (2.3
mg/d
-
1.4
mg/d or 2.3
-
0.8
mg/d),
or 0.9
mg/d
to
1.5
mg/d
to be contributed
by
the home water supply.
-
-,
-
As
calculated
above,
the equivalent exposure
intake
value for the water supply isat-least-6.0
lid..-
Distributing
the
0.9
mg/d
to
1.5
mg/d
portion
of
the
ADE
for
home
and
water use
into
the
adjusted exposure value of at least 6.0
lfd,
the Health
Advisory concentration for
MTBE
using
chemical-specific
RSC data can
be
no
more
thar~0.15
mg/l toO.25 mg/l.
Since the value
for
the Health Advisory
originally
proposed by
the Agency,
0.23
mg/l,
falls
within this
range,
the
Agency proposes to adopt the Health
Advisory as
originally
proposed.
REFERENCES
Andelman,
J.B.
1985.
Inhalation
exposure in
the home to volatile
organic contaminants of
drinking
water.
Sci.
Total
Environ.
47:443.
Lyman, W.J.
1982.
Handbook of
Chemical Property
Estimation Methods.
W.J.
Lyman, W.F.-
-
Reehi,
and
D.H.
Rosenblatt,’ Eds.
(McGraw-Hill Book Co., NY).
-
-
McKone,
T.E.
1987.
Human exposure to volatile organic compounds
in household
tap
water:
the indoor inhalation pathway.
Environ.
Sci.
Technology..
21:1194.
‘~
McKone,
T.E.
and
Knezovich,
J.P.
1991.
The
transfer of trichloroethylene
(TCE)
from
a
shower to indoor air:
experimental measurements and
their implications.
J.
Air Waste
Manage.
Assoc.
40:282.
Morrow, Leslie
D.
1994.
Personal communication.
NTP.
1994.
Comparative
Toxicology
Studies
of
Corn
Oil,
Safflower
Oil,
and
Tricaprylin
in Male F344/N Rats
as Vehicles for Gavage.
National Toxicology Program, Technical
Report
Series,
No.
426.
U.S.
Dept.
of
Health
and
Human
Services,
Public
Health
Service,
National Institutes of Health.
April,
1994.
Robinson, M.,
Bruner,
R.
H.,
and Olson,
G.
R.
1990.
Fourteen-
and ninety-day
oral
toxicity
studies of Methyl Tertiary-Butyl Ether in Sprague-Dawley rats.
J.
Amer.
COil. Toxicol.
9:525.
USEPA.
1993.
Assessment of Potential Health Risks
of Gasoline
Oxygenated
with
Methyl
Tertiary
Butyl
Ether
(MTBE).
-
EPAI600/R-93/206.
Office
of
Research
and
Development.
November,
1993.
F:\psf\epaSS66’MTBEHA
CERTIFICIATE OF SERVICE
I,
the
undersigned,
CERTIFY
that
I
have
served
a
copy
of
the
TESTIMONY
OF
RICHARD
P. COBB,
P.G.
with Exhibits
upon:
Kay
Anderson
American
Bottoms RWTF
One American Bottoms Road
Sauget, Illinois
62201
Fredric P.
Andes
Barnes &
Thornburg
2600 Chase Plaza,
10
S.
LaSalle Street
Chicago,
Illinois
60603
Karen L. Bernoteit
IL Environmental Regulatory Group
215
E.
Adams
St.
Springfield,
Illinois
62701-1199
Chris
Bianco
Chemical Industry Council
9801
W. Higgins
Road, Suite
515
Rosemont, Illinois
60018
Christine
Bucko
AAG
188
W.
Randolph, 20th Floor
Chicago, Illinois
60601
Jack
Darin
Sierra Club, Illinois Chapter
200 N. Michigan,
Suite
505
Chicago, Illinois
60601
Stephen Davis
Illinois Department ofNatural Resources
524
South
Second Street
Springfield, Illinois
62704
William G.
Dickett
Sidley & Austin
Bank
One Plaza
10 South
Dearborn
Street
Chicago,
IL
60603
Albert Ettinger
Environmental Law & Policy Center
35 E.
Wacker Drive,
Suite
1300
Chicago, Illinois
60601-2110
Susan M. Franzetti
Sonnenschein Nath & Rosenthal
8000 Sears Tower, 233
South Wacker Drive
Chicago, Illinois
60606
-
-
-
James T. Harrington
Ross & Hardies
150 North Michigan,
Suite 2500
Chicago, Illinois
60601
John M.
Heyde
Sidley
& Austin
Bank
One Plaza,
10 So.
Dearborn St.
Chicago, Illinois
60603
Katherine Hodge
Hodge
& Dwyer
3150
Roland Ave., P0 Box 5776
Springfield,
Illinois
62705-5776
Richard
J. Kissel
Gardner,
Carton &
Douglas
321 N.
Clark Street, Suite 3400
Chicago, Illinois
60610
Sharon
Neal
ComEd
—
Unicom
Law Dept.
125
S.
Clark St.
Chicago, Illinois
60603
Jerry
Paulson
-
McHenry
County Defenders
804 Reginact
Woodstock, Illinois
60098
Irwin
Polls
Metropolitan Water
Reclamation District
600 lWest
Pershing Road
Cicero, Illinois
60804-4112
Cindy
Skrudkrud
4209
W. Solon
Road
Richmond,
Illinois
60071
Connie
Tonsor
IEPA
—
Div. Of Legal Counsel
P.O. Box 19296
Springfield, Illinois
62794-9276
Georgia Vlahos
Dept ofthe Navy, Naval Training Center
260lA
Paul
Jones Street
Great Lakes, Illinois
60088-2845
Musette H.
Vogel
The Stolar Group
The
Lammert Building
911
W.
Washington
St. Louis, MO
63 101-1290
Charles Wesseihoft
Ross
& Hardies
150
North
Michigan,
Suite
2500
Chicago, Illinois
60601
Stanley Yonkauski
IL. Department ofNatural Resources
524 South Second Street
Springfield,
Illinois
62701
by depositing said document in the United States Mail in
Springfield, Illinois on February 16, 2001.
C
~
Stephen C.
Ewart