IN THE
MATI’ER
OF:
PROPOSED
AMENDMENTS
TO
TIERED
APPROACH TO CORRECTIVE ACTION
OBJECTIVES (TACO)(MTBE):
35
ILL. ADM. CODE 742
)
)
)
)
RO0-19(C)
)
(Rulemaking-Land)
)
RECEiVED
CLERK’S
OF~V’)~
OCT
1
1
ZULU
•
STATE
OF ILLINOIS
PoJl~tto~
Control
Board
Dorothy M.
Gunn, Clerk
Illinois Pollution Control Board
James
R. Thompson Center
100
West Randolph
Street,
Suite 11-500
Chicago,
Illinois
60601
Robert
Lawley,
Chief Legal Counsel
Dept. ofNatural Resouróes
524 South Second Street
Springfield, Illinois
62701-1787
See Attached Service List
Matthew
J.
Dunn,
Chief
Environmental Bureau
Office of
the Attorney
General
188
W. Randolph,
20t1~
Floor
Chicago,
Illinois
60601
Amy
Jackson, Hearing Officer
Illinois Pollution Control Board
500 South Second
Street
Springfield,
Illinois
62706
NOTICE OF
FILiNG
PLEASE TAKE NOTICE that I have
filed
today with the Illinois Pollution Control Board the Supi~lernental
Comments
and Exhibits
ofthe
ENVIRONMENTAL
PROTECTION AGENCY,acopyofwliichis herewith
servethiport
you.
ENVIRONMENTAL
PROTECTION
AGENCY
OF
TE
STATE
OF
ILLiNOIS
By
Dated:
October
8, 2001
Illinois Environmental Protection
Agency
1021
N. Grand
Ave.
E.
P.O. Box
19276
Springfield, Illinois
62794-9276
(2
17/782-5544)
BEFORE
THE
POLLUTION CONTROL
BOARD
OF THE
STATE OF ILLINOIS
NOTICE OF
FILiNG
KimberijA.Geving
Assistant Counsel
Division
of
Legal Counsel
•
RECEIVED
CLERK’S OFF~’~
•
OC11~~Qüi
BEFORE THE ILLiNOIS POLLUTION
CONTROL BOAI~TEOF
ILLINOIS
•
•
•
•
•
Pollution control Board
iN THE MATTER OF:
•
)
)
PROPOSED AMENDMENTS TO TIERED
)
ROO-19(C)
APPROACH TO CORRECTIVE ACTION
)
(Rulemaking-Land)
OBJECTIVES (TACO)(MTBE):
)
35
ILL. ADM. CODE 742
SUPPLEMENTAL COMMENTS
The Illinois Environmental Protection Agency (“Illinois EPA”), by its attorney, Kimberly
Geving, and at the request ofthe Illinois Pollution Control Board (“Board”) in its September 6, 2001
First Notice Opinion and Order in the above-captioned matter, respectfully submits these Supplemental
Comments to the Board.
In its discussion ofthe Proposed Amendments regarding MTBE that were sent to First Notice via
the September
6th
Opinion and Order, the Board stated that “While the Agency has provided the Board
with information supporting the proposed standards, the record is lacking a detailed explanation ofthe
calculations employed by the Agency in reaching the proposed numbers.”
(Proposed Rule. First Notice
Opinion and Order dated September 6, 2001
at pages
4-5).
Page
5 ofthe Board’s Opinion and Order
specifically requested the illinois EPA to provide supplementation for its MTBE proposal during the first
notice period.
The Illinois EPA maintains that its proposal
was
technically substantiated on the record.
However, in the interest ofestablishing a more complete, technically sound record, the Illinois EPA
offers two attachments that we believe further explain how the objectives were established.
The first
attachment (Exhibit 1)’ provides a very detailed description ofthe Health Advisory that was proposed by
‘Exhibit
1
was
also submitted to the Boardthis
year
during
the
Part 620 regulatoryproceedings.
In
that
proceeding, it was labeledas Exhibit V to the Illinois EPA’s Statement ofReasons in R0l-14.
1
the illinois EPA in 1994 forMTBE and the scientificjustification forthe advisory.
The Health Advisory
served
as a base for determining remediation objectives for groundwater in this proceeding.
Exhibit
1
explains in detail how the numbers for MTBE were derived.
Additionally, Exhibit
1
includes Illinois
EPA responses to significant comments that were receivedregarding the health advisory proposal for
MTBE, further substantiating its scientific basis.
Exhibit 2 provides supplementation for how the illinois
EPA calculated the soil rernediation objectives for MTBE in
Part 742.
The Illinois EPA maintains that its proposed remediation objectives for MTBE in both soil and
groundwater have been scientificallyjustified.
The illinois EPAhopes that these Supplemental
Comments and attachments further clarify for the Board how the calculations were performed.
WhEREFORE, the Illinois EPA submits these Supplemental Comments to the Board for its
consideration
and
respectfully requests the Board to
adoptthe objectives proposed by the Illinois EPA in
theirentirety.
•
ILLiNOIS ENVIRONMENTAL
PROTECTION AGENCY
•
•
By~~th(L/4d4~Z
•
A5S~5~fltCOUlLl
if
•Dated:
October
5,
2001
1021 North Grand Ave. East
P.O. Box 19276
Springfield, illinois
62794-9276
•
(217)782-5544
•
THIS
FILING
SUBMITTED ON RECYCLED PAPER
2
Page
18/July,
1994
Eitviinnmen.za!
NOTICE OF
HEALTH ADVISORY FOR
•
METHYL
TERTIARY-B
UTYL ETHER
(MTBE)
_________
•
•
EXHIBIT
Prepared
by
•
•
Office
of
Chemical
Safety
•
-
Illinois EPA
June9,
1994
REASONFOR
ACTION
As
a
result
of
routine
monitoring
of
public
water
supply systems,
the
gasoline
additive
Methyl
Tertiaxy-Butyl
Ether
~MTBE) has been
detected
at
least in two
public
water supplies.
Therefore,
the
Illinois Environmental
Protection
Agency
(Agency) is announcing its intention to issue a health advisory, pursuant to 35
illinois Administrative Code
Part 620
Subpart
F:
Health Advisories,
for Methyl
Tertiary-Butyl
Ether.
According to Section
620.605 of Subpart F,
the
Agency shall
issue
a health advisory
for
a chemical
substance if all of
the following conditions are
met:
1)
A communny
water
supply well
is sampled
and a substance is
detected and confirmed
by
resampling;
2)
There
is no
standard under Section
620.410
for
such
chemical
substance;
and
3)
The
chemical
substance is
toxic or harmful to human health according
to the
procedures of Appendix
A,
B,
or
C.
-
The Agency
has determined
that
all
three
conditions have been
met,
prompting the
issuance of
this draft proposal
for
a
health advisory.
By
this issuance,
the
Agency is
opening
a
30-day
public
comment period, until Au~iist22.
1994,
regard-
ing
this health advisory draft.
Upon closing the
public
comment
period,
the
Agency
will
consider all
comments
received
and amend
the
health advisory if warranted.
The final health advisory will then
be published in the Environmental Register
(the
flhi.nois Pollution Control Board News)
with responses to
comments
received.
An abbreviated version of the final
health
advisory will also
be published in local newspapers which serve
communities in whose public
water
supply systems MTBE
has been detected.
PROPOSED
GUIDANCE
LEVELS
Section
620.605 of Subpart
F prescribes the
methods for developing health advisories for carcinogens and no~ncarcino-
gens.
Since
the
Agency
has
determined
that
there. is
insufficient
evidence of
the
carcinogenicity
of MTBE
at
this
time
(discussed
in
the
attachment
to
this
notice),
the
method
for
developing
a
health advisory
for
noncarcinogens
was
used.
Briefly, this method
specifies that
the USEPA’s maximum contaminant level
goal (MCL.G)
is the guidance level, ifavailable,
or
the
human
threshold
toxica.nt advisory
concentration (W~TAC)must
be determined
using the procedures
contained
in
Appendix
A of Section 620.
USEPA has not published an
MCLG for
MTBE, therefore
the
Agency
used
the
Appendix
A
procedures to calculate
the HTTAC.
Appendix A specifies in
prescribed order
the
toxicological
data
to be used in developing the 1-ITTAC,
ranging from a
verified
Reference
Dose developed
by
USEPA to a laboratory
animal
study of subchronic
duration in which only
a lowest
observable
adverse
effect level
(LOAEL)
has
been
determined.
This preferred
order reflects increasing
uncertainty in the
toxicological
database
regarding
a
chemical’s
potential
to
cause
adverse
health
effects
in
humans,
and
is
manifested
in
increasingly large safety factors which are applied to the data to calculate the HTTAC (maximum
10,000-fold safety factor).
In
the
case
of MTBE,
the
Agency
has selected
the only study available
in
which
the
test animals
were exposed by
the
oral
route of exposure
as the
basis
for the HTTAC.
Among other findings, this 90-day
subchrooic study reported increases
-..3
..._,-;~...._.
~
;,.
—*1
~
~
:.,..~,,4:....,
,L
1,,.,,,,..
1.,~..
...C
irv~
_.,t~....l,l
~
,
~.,,I.
r~
using this subchronic study in
which only
a LOAEL
was
determined,
the
language
of Subpart
F
specifies the
application of
safety
factors totalling to
10,000
to
the
animal
data,
resulting
in the HTI~ACguidance
level
of 0.07
mgI!,
or
70
parts per
billion
(ppb).
The details of
the derivation of the HTTAC
are
presented in
the attachment
to
this
notice.
At
this point
it
is
necessary
to discuss an aspect of the
evolving science of risk
assessment
which
has a bearing
on
this
notice.
The
Agency
has
been
informed
verbally,
by
USEPA personnel
that
in
most
cases
USEPA
no
longer
favors
the
Environme!Lta!
Register No.
484
July,
1994/Page 19
calculation of acceptable
exposure
values
for
humans
by
using laboratory a.aimal
data divided
by
uncertainty
factors
totalling
~
10,000.
This
preference
will
be
included
in
a
chapter
in
the
book
Essential
Elements
(in
press;
ILSI
Press,
1994).
Instead,
USEPA
now
prefers
to
utilize
uncertainty
factors
totalling
to
no
more
than
3,000.
The
Agency
agrees
with
this
approach
in
general,
except
in
cases where
the
overall toxicity database for
a
chemical
is very weak.
In
the
case of MTBE,
the database
contains enough
laboratory
animal
data
to determine
that
there
are not major
toxicity
gaps which
would
warrant
the
use
of
a
10,000-fold uncertainty
factor.
The Agency
is
therefore
also using
an overall
uncertainty
factor,
of
3,000
to
calcu.late
a guid.ance
level
for
MTBE.
Use of a
3,000-fold safety factor with the same
laboratory animal data described above
results
in
a
HTTAC
21.Iidance
level
of
0.23
mg/f,
or
230
ppb.
The
details
of
the
derivation
of
this
HTTAC
are
also
presented
in the attachment to
this notice.
Since
there is no
provision in the
language
of
Subpart F
for the
use
of
a
3,000-fold uncertainty
factor in
the
derivation
of
the
WrTAC,
the Agency
is proposing toutilize
HTTACS derived by
both
a
3,000-fold and
a
10,000-fold
uncertainty factor
in
the
health advisory
for
MTBE.
It
is
proposed
that the
HTTAC derived using
the
10,000-folduncertainty factor
(70
ppb)
be
a
precautionary
health advisory concentration and
the H1TAC
derived
using
the
3,000.-fold uncertainty
factor (230 ppb)
be the
final health
advisory
concentration.
The precautionary health advisory
would be
a level
in a public water
supply
below
which
no
action
would
be
necessary
and
above
which caution
should
be
exercised
by
the
public
water
supply
(such
as
increased sampling of the water
and identification of the potential
source(s)), while the
final health advisory would
be a
level
above which the
public water
supply should begin
actions to decrease
the concentration or utilize
an
alternate water supply.
The Agency
is
requesting comment. on the
use of this approach when
a total
uncertainty factor of
10,000-fold
is utilized
to
calculate a health advisory.
SUPPLEMENTARY INFORMATION
Section
620.605 also
specifies
that
the
health
advisory
must contain
a
general
description of
the
characteristics
of
the
chemical
substance and
its potential
adverse health
effects.
General D~criotion
of
MTBE
MTBE (Chemical Abstracts Service Number 1634-04-4), also known as
2-methoxy-2-methylpropane, is a colorless liquid
with a disagreeable taste and odor.
Its
taste in water
can be recognized
at
approximately 0.7
rug/f (700
ppb) (Connecticut
DEP), although
recent research
suggests
that some
pecple
may
be able
to detect its presence
in the
range of 0.25 mg/f and
possibly as
low
as 0.04
mgI!
(API,
1993).
It
has
a high solubility in water,
approximately 48,000 mg/! (von
Burg,
1992).
Because
of
this high
solubility,
it has
a high propensity
to move
through
soil ‘with
infiltrating rainwater
and snowmelt and
to
potentially reach
groundwater.
•
Its
main
use
is as an octane
booster in unleaded
gasoline;
it also has minor uses
as
an intermediate in the
production of
other chemicals,
especially
isobutene,
and’
as
a
treatment to dissolve gallstones.
Its
use has been increasing
recently
due
to
requirements under the Clean Air Act Amendments of
1990
for metropolitan areas which are not
in compliance with carbon
monoxide
standards to
increase the
percentage of oxygenated
fuel in
gasolines, especially
in the wintertime.
As
a result,
it has been estimated that approximately 20
of the gasoline sold in the United States contains MTBE, at
levels ranging from
2
to
15
in the gasolines.(Costantini,
1993).
Potential
Adverse Health
Effects of
MTBE
•
Relatively
few
reports of adverse
effects
of MTBE
on
humans
exist,
and
testing
for the
full
range
of possible health
effects
in
laboratory
animals
has not yet
been completed.
Summaries
of
the
acute,
reproductive and
developmental,
arid
chronic
toxicity data for MTBE
are presented.
Acute
Toxicity
-
Other than
a single
report
in
the
medical
literature of acute
kidney
failure
due to
leakage of MTBE
during
gallstone
treatment
(Ponchon.
1988),
there
is
no
information
regarding
the
effects
of short-term,
high
level
eApUSLLfe
U)
Mt
~n
Ui
numaa~.
toe
u.ai.a from
Laocr~Loryanirual
stuc1e~uiuicate
Lnat
uus
encmlcai
~
not very
tox1~.
during
briefexposures, with lethal doses
in
the
range
of 3,000-4,000 ppm by
oral
exposure
(about one pint for
an adult
human)
and 24,000-40,000 ppm (in air) by
inhalation exposure (this would
be within the
explosive range in air) (Reese
and
Kimbrough,
1993; von
Burg,
1992;
USEPA,
1993).
The
toxic
effect
in
both exposure
types was
central
nervous
system depression.
MTBE does ~ot
appear
to
cause
skin
irritation except
in cases of previously
damaged skin,
and eye
irritation
and opacity of
the cornea has been
reported
(von Burg,
1992).
Fag!
20/filly,
1994
Ein~ronmenWJ
Register
No.484
~r~duc~e
and
Developme!ital Toxicity
-
The
reproductive
effects
of
MTBE
have
been
~ported
in
three
studies,
and
reproductive
and
developmental toxicity
has
been
assessed
in
a
fourth,
using
rats,
mice,
and/or
rabbits.
No
significant
effects
were
reported
in
two of
the
reproductive studies (Biles
~
~.,
1987;
Conaway
~
i!.,
1985),
and
the
third reported effects
on
offspring (reduced body
weight and
reduced weight
gain
in rat
pUps,
and slightly
reduced
pU~
survival)
only at doses
which were also
toxic
to the
parents
(Neeper-Bradley,
1991).
Similarly,
the
reproductive and
developmental
study also reported offspring effects (reduced numbers of viable implantatio~sandior live births,
reduced
body
weight,
decreased
ossification, and
increased
incidence
of cleft
palate
in mouse
pups)
only
at
doses
toxic
to
the
adults (Tyl
and
Neeper-Bradley,
1989).
This makes
it di~cultto say whether
the
effects
on
reproductive performance
were tnily
an effect of MTBE on
the offspring,
or whether
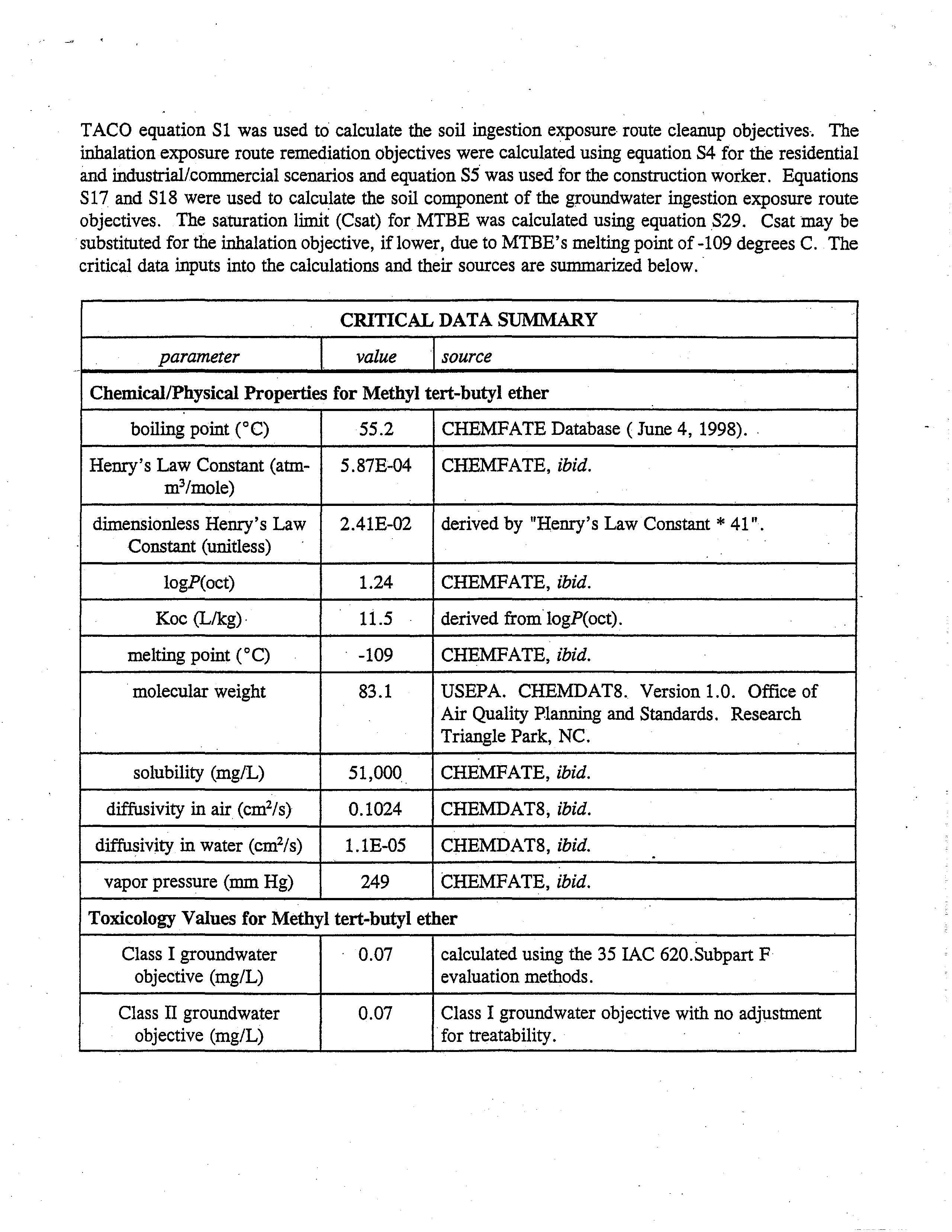
these effects resulted from
the
toxicity to the
parents.
Since
the
doses
which showed these toxic
effects were
high (3,000-4,000 ppm), the potentialfor
human
reproductive
effects
at
the
much lower anticipated environmental
exposure
levels
is extremely small.
Chr~rnic
Tpxicity
-
There are no
studies of the effects
on hurnanc
exposed
to
MTBE for longperiods, although anecdotal
reports of
increased
complaints of headache,
nausea,
vomiting, eye
irritation,
and respiratory
problems
have
surfaced
recently
in certain
areas
in
conjunction
with wintertime
MTBE increases in gasoline.
These complaints are
the
subject
of on-going
research.
There is
only
one
90-day subchronic
study in
laboratory
animals exposed
by
the oral
route,
which
was
the study
Finally
selected
to
derive
the
WITAC
by
the Agency after following
the procedures
of
Appendix
A.
This
study
is evaluated
in
depth
in
the
attachment
to
this
notice.
There
are
several
animal
subchroàic
and
chronic
studies
using
the
inhalation
route
of
exposure,
primarily
evaluating
the
neurotoxic
effects
of
MTBE.
In
one
study
(Greenough
~
.~J.,
1980)
in
which
the
maximum dose tested
was
1,000 ppm for 6
hrs/day,
5
days/wk, for
13
weeks, no
significant
effects
(other
than
anesthesia
following dosing
at high concentrationS) were reported.
In another study (Dodd and Kintigh,
1989), in which
the
ma.ximum
dose
tested was
8,000 ppm (same
dosing regimen),
slight
changes
in blood chemistry,
increased
serum
cortisone
levels
in
both
sexes,
reduced weight
gain, increased kidney,
liver, and adrenal
gland weights, and sporadic
neurotoxic
effects
were
at
doses of
4,000
andlor
8,000 ppm.
There
is also
a recently
completed
lifetime
cancer
bioassay
in
mice
and
rats
(Burleigh-Flayer ~
~j., unpublished; Chun ~
~.,
unpublished), the
details
of which are
evaluated
in
the
attachment to
this
notice.
FOR
FrJRTRER
INFORMATION.
COMMENTS
Persons
who
wish
to
receive
further information
about this
notice
or who wish
to provide
comment
on
its content.~
are.
requested
to
contact:
illinois Environmental Protection
Agency
Office
of
Chemical
Safety
P. 0.
Box
19276
2200
Churchill Road
Springfield,
Illinois
62794-9276
2171785-0830
Environiflen~al
Register
No.
484
-___________________
july,
1994”Page2l
ATTACHMENT
TO
NOTICE
OF
HEALTH
ADVISORY FOR
METHYL
TERTLA..RY-BUTYL ETHER
(MTBE)
OVER
VIEW
OF THE
KEY STUDIES
In
the
only
oral
study (Robinson
et al.,
1990),
rats were
given 0,
100,
300,
900,
or
1,200
mg/kg
(ppm)
by
gavage.
Rats
given
1,200
ppm
exhibited profound anesthesia
after
dosing throughout
the study,
but
recovered
after
the
dbse
within
two
hours
and
suffered
no
aftereffects.
Body
weight
decreased
with
increasing
dose,
with
the difference
between
treated
and
control
rats being statistically
significant
at
1,200 ppm.
Other
measurements
showing statistical significance
included:
decreased
blood
urea
nitrogen
(BUN)
and
serum
creatinine
(measures
of
kidney
function) a~t
all
doses;
increased
serum
cholesterol
at
all
doses; increased
kidney weight
at
300
ppm and above;
increases in several other organ weights at
900
ppm
and above;
and changes
in
blood parameters
at
1,200 ppm.
Microscopic examinations
revealed
effects
only
at
1,200
ppm,
where degenerative
changes
in the
kidneys of the
male rats were noted.
Finally,
loose stools and diarrhea
were
seen
at
all
doses
throughout
the study.
Viewing
the
results of
this
study,
it would
appear that the
kidney
is the target
organ of
MTBE.
However,
these results
must be
interpreted
carefully.
The decreases
in BUN
and
serum
cre.atinine probably have
no
adverse effect on
the
animals
(decreased
kidney
function is
often signaled
by
increases in these parameters),
and
may
even indicate an
increase
in kidney
function.
The
increased
kidney
weights
seen
at
300
ppm
and
above
are
not
in
themselves
an
adverse
effect,
only
an
indication
of a
possible
adverse effect
at even higher doses or longer
exposure
times.
Finally,
the
microscopic
changes seen
at
1,200
ppm
in
males
are
often
seen
in
male
rats
(and
only
male
rats)
exposed
to
certain
organic
chemicals,
due
to
overproduction
of a
unique
protein
in
the
male
rat
kidney.
Thus,
it
is not
clear
at
this
time whether MTBE
is toxic
to
the
kidney.
It would appear
that
a
no
observed
adverse effect
level (NOAEL)
has
not been
determined by
this
study,
since increased
serum cholesterol
and
diarrhea
were
observed
at
all
doses.
Thus,
the
100
ppm
dose
would
be
considered
to
be
the
lowest
observable
adverse
effect
level (LOAEL)
for MTBE.
The
procedure
for calculating
a
health advisory
for drinking
water
in
the
groundwater
quality standards
(35
Ill. Adm.
Code
620,
Subpart
F)
gives
preference
to
oral
studies
which
determine
a
NOAEL
or
LOAEL,
and
this
study
may
be considered
to
develop the
health
advisory
for MTBE.
A
lifetime
inhalation
cancer
bioassay
has
recently
been
completed
with
mice
and
rats,
but
the
results
have
not
been
published
(Burleigh-Flayer ~
~j.; Chun ~
~j.).
The Agency
has been
given summaries
of
the
studies
submitted
to
USEPA
by
the
USEPA
contact
for MTBE.
These
results
are
briefly
summarized,
but since
the studies
are
still
undergoing
review
it
must
berealized
that
this information
is
preliminary.
Both
species
were
exposed toO,
4.00,
3,000,
or
8,000
ppm
in air.
As
in the
oral
study above,
the
male rats
experienced
an
increased
incidence of kidney degeneration.
This became
the
leading cause
of
deathjn
male
rats,
and resulted in early
termination
of the
3,000 and
8,000
ppm
male
groups.
The other main cause of
death
in
male rats
was
leukemia,
seen
in
both
the
control
and
400
ppm
group.
(In fact,
the
incidence
in
the
control group
was
higher, 33/50,
than
in
the
400
ppm
group, 22/50.)
Non-cancer effects of
MTBE
included symptoms
of
central
nervous system depression
in
both sexes of
rats
at
3,000 and
8,000
ppm, but
not at
400
ppm,
and an
increased
incidence of kidney
degeneration
in
male
rats
at
400
ppm.
The only
tumors which were related to
MTBE
exposure
were
rumors
in
the kidneys of male
rats
in
the
3,000 and
8,000 ppm
groups.
These
tumor types are
also
thought
to
be
related to
the
overproduction of the
male
rat
protein,
and
the
significance
of
these
results
for
humans
is
questionable.
In
the
mouse
study, symptoms of central
nervous
system depression
similar
to
those seen
in rats
were
observed-at 3,000
and 8,000
ppm.
Increases
in
liver
and
kidney weights
were also seen
at
these doses, and
an
increase in
the
number of liver
cells
(nonc.ancerou.s),
an indication of toxic effects
on
the liver,
was
reported at 8,000 ppm.
The only
tumors found
in excess
of controls were
liver
tumors
in females
in
the
8,000
ppm
group.
However,
the significance
of
this
finding
for
humans
is
also
questionable,
since
this
tumor type
is
common
in
the
strain
of
mouse
used
in
this
study,
and
is
known
to occur
in
~
I. ~&4U
~Ly
U.~L&
In reviewing
the
results
of these studies,
it
is difficult to
say
whether MTBE
presents a carcinogenic
hazard
to
humans.
However,
the
noncancer
effects
may
be
relevant for
determining a health
advisory level
for MTBE.
In
this
regard,
the
rat
study
has
produced
a
LOAEL of
400
ppm
based
on
kidney
effects
in
male
rats
(this
dose
may
be
a
NOAEL
given
the
questionable
significance
of
this effect
for
humans),
while the
mouse
study
has
produced
a
NOAEL of
400
ppm.
The
mouse
Page 22/ July,
1994
Environmental_Reg&er
No.
484
portion of
this study
may
be
considered
to
develop
the
health
advisorj
for
MTBE,
once
it
has
F.nis~~USEPA’s
review
procesS.
-
DERIVATIO~’(OF THE HEALTH
ADVISORY
FOR MTBE
The first step
in the derivation of a heslth advisory is to determine
whether the
chemidai
pre~entsacarcinogenic
hazard
to
humans.
To
date,
there
have
been
no
investigations
whether
there
is
an
increased
incidence
of
cancer
in
humans
associated
with exposure
to
MTBE.
As
discu~àedabove,
there
is some evidence
that
MTBE causes
tumors
in
laboratory
animals,
but
the
types
of
tumors
found
in
the
rat
and
mouse
cancer
bioassays
may
not
provide
good
evidence
of
a
carcinogenic
hazard
to
hunianc since these tumors may
be species-specific responses with little or
no
relevance
to humans.
Furthermore,
these studies
are
still undergoing
review by
USEPA
and a
final determination of
the
usability of
the
results
for determining the
carcinogenic
hazard
to humans
has not been made.
Therefore,
the
Agency
has determined at
this time
that
the
derivation
of
the
health
advisory
for
MTBE
will
be
based
on
the
non-cancer
effects
of
this
chemical.
This
derivation may be changed
in the future,
depending
on
the USEPA’s ~determinations,
once the cancer
bioassay data
have been published and
the
weight-of-evidence for human
carcinogenic potential
has
been
determined.
In deriving a health advisory
to
protect against a health effect for which there is a threshold dose below which
no
damage
occurs
(i.e., noncarcinogenic
effects),
Section
620.605 specifies that
USEPA’s maximum cont~rnin~nt
level
goal
(MCLG).
if
available,
is
the
health advisory concentration.
USEPA has not publisheda MCLO for MTBE, therefore,
the
Agency
must
calculate
the
human
threshold
toxicant advisory
concentration
(WITAC)
as
the
health advisory
concentration, using
the
procedures specified
in
Appendix
A
of Section 620.
Appendix A specifies in subsection
(a)
that
the
ifITAC
is calculated
a~sfollows:
RTL4C=RS~~~~
w
Where:
HTTAC
=
Human threshold toxicant
advisory
concentration in
milligrams
per liter
(mg/I);
RSC
=
Relative
source
contribution,
the
relative
contribution of
the
amount
of
the
exposure
to
a
chemical
via drinking
water when compared
to the
total
exposure
to
that
chemical
from
all
sources.
Valid chemical-specific data
shall
be
used if available.
If valid
chemical-specific
data
are not available,
a
value of 20
(=0.20)
must be
used;
ADE
=
Acceptable
daily exposure of
substance
in milligrams
per
day (mg/d)
as
determined
pursuant
to
subsection
(b);
and
W
=
Per
capita daily
water consumption equal
to
2
liters
per
day
(Lid).
Subsection
(b) of
Appendix
A
specifies
that
the
ADE
be calculated using,
in specified
order~
USEPA’s
Verified
Oral
Reference
Dose (an
estimate
of
a
daily
exposure to
a
chemical
which is
expected to
be without
adverse
effect
for
humans,
including
sensitive
subgroups,
for
a
lifetime
of
exposure);
a
NOAEL
which
has
been
identified
as
a
result
of
human
exposures;
a
LOAEL which
has
been identified as
a
result of human
exposures;
a
NOAEL. which
has
been determined
from
studies with laboratory
animals; and
a LOAEL
which has been
determined from studies
with laboratory
animals.
There is no
Verified
Reference Dose currently available from USEPA.
As mentioned
above, there
is a
paucityof studies
on
the
adverse
effects
in
humans exposed
to
MTBE.
Thus,
the Agency has
determined that
a
NOAEL
or
LOA.EL
based
uQ
QLW)AL1
CA~’.J~U~~ 1~OUL
4VU~C
d~. i.W~ UU~.
L
~1C~U1C,
U~G
i~#i.:. L~aU.~L
tiC
U~Cte
i~tJh.h~
I
d~JI..JtJ
~
~
the
studies reviewed
by the
Agency,
the
90-day
rat
subchronic
study
and
the
cancer
bioassay (noocarcinogenic
effects) are
the most
appropriate animal
studies
for calculation of the
ADE.
It
is then
necessary
to
determine
which
study
is
the
most
valid
for
purposes
of calculating the
ADE.
Subsection
(c)
of
Appendix
A
specifies
criteria
for
establishing
the
validity
of
data
from
animal
studies,
leading
to
determinations
of high,
medium,
or
low validity.
High validity
studies
are
those
using the
oral
route
of exposure and which
Environmertta!
Register
No.
484
July,
l994/Page
23
meet
specified
criteria
depending
on
the
type
of
study,
and
are
to
be
used
preferentially
if availabie.The
rat
90-da’~
subch.roaic study was conducted
using
the
oral
route,
while
the
cancer
bioassay was
an
inhalation
study.
Therefore,
only
the
subchronic study could
be
a
high validity study.
However,
the
requirements
for a high validity
subchronic
study
include,
among
other
things,
a
study
using
two
species
and determining
a well-de5ned NOAEL.
The
90-day
rat
subchronjc study
used only
one
species
and
only determined a LOAEL,
as discussed
above.
Having
no high validity
study, the
Agency
must
determine which
of
the
two
studies is most
appropriate
for calculating
the
AIDE.
Subsection (c)
goes
on
to
specify
that
in
order for
a
subchrocic
study in which
a
LOAEL
is
determined
to
be
deemed
a
medium validity study,
the
study
must satisfy
all
other standards
for a high validity
study.
This is not
the case
for
the
90-
day
rat subchronic study, since there was only
one
species tested.
Similarly,
in order
for a study
other
than
an
oral exposure
study
to
be deemed
a
medium
validity
study,
the
sttidy
must
satisfy
all other
standards
for
a
high validity
study
arid
use
appropriate
correction factors
for conversion to the
oral route.
However,
the
requirements
for a high validity
cancer bioassay
include,
among other things,
at
least
2.5
survival
at
18
months
in
mice
and
24
months
in rats.
This
was
not
the
case
in
the cancer
bioassay,
since
the male
rats
in
the
3,000 and 8,000
ppm groups were
terminated
early due to
excessive
mortality.
Thus,
both
candidate
studies
are
defined
as low
validity studies,
and
the
90-day
rat
subchronic
study
is
selected
because
exposure
was
by
the
oral
route.
The
determination
of
the
ADE
from
the
subchronic
study is made
using
the
language
of subsections
(b)(5)
and
(b)(6).
Subsection (b)(6)
specifies
that
for
substances
for
which
a NOAEL
is not
available,
onà-tenth of
the
LOA.EL
is
substituted
for the NOAEL
in subsection
(b)(5).
Subsection
(b)(5)
specifies that
if
studies
of low validity
must
be used,
the
ADE
must
be
calculated
using
1/1000
of the
NOAEL.
The
overall
result
of
the procedures
in
these two subsections
is
that
the
ADE
is
1/10,000
of the
LOAEL,
times the
average
weight
of an
adult
human,
70kg:
ADE= lOOmg/k1g/dxlQkg
=0 7m
Id
10,000k2Jd
S
At
this point,
the calculation of the
TTTAC
would
proceed according
to
the formula
listed above.
However, the
Agency
has been
informed by
USEPA personnel
that in
most
cases
USEPA now prefers
to
calculate acceptable exposure
values
for
humans
by
using laboratory
animal
data divided by
no
more than
a 3,000-fold uncertainty factor;
a
10,000-fold uncertainty
factor would
be
used
only
where
the
overall
toxicity
database
is
very weak
for a
chemical.
The Agency
agrees
with
this
emerging USEPA approach.
Since
the MTBE
database
contains
enough
laboratory
animal
research
to
indicate that
there
are
not
major
toxicity
data
gaps
which would
warrant
the
use
of
a
10,000-fold
uncertainty
factor,
the
Agency
is
also
calculating
the
ADE
using a 3,000-fold uncertainty
factor:
ADE= 10C~ng/kg/dx70kg 2.3n~gJd
3,000
-
Finally,
the
determination of the
HTTAC
is straight-forward, since
there are no
chemical-specific data
available
for the
RSC
term:
H7TAC= 0i0x0.7mgJd007~
2.0~/d
Or:
0’0x23m
Id
HITAC=
—
~‘
=023ing/~
‘~
(~3th3
The
final
step
in
detei-rninirig
the
health advisory
is
to
compare
the
HTTAC
value
calculated
from
the
Appendix
A
procedures
to
the
chemical’s
Practical
Qu.antit.ation
Limit
(PQL).
In
the
case
of
MTBE,
no
USEPA
SW-846
analytical
method
specifies
a
PQL
for this chemical.
However,
the
Agency’s Division of
Laboratories
has
determined that a detection
limit of
0.005
mg/f
is
appropriate
for water
samples.
Therefore,
the
HTTAC
value
is above
the
detection
Limit.
Page 24/July, 1994
Envfrvnme~aa1R.!gt~arr
No.
484
The Agency has decided to
issue
a
two-part
health
advisory.
The precautionary
health ad~isory~ôncentration
for
Methyl Tertiary-Butyl
Ether
(MTBE)
is
0.07 mg/I
or
70
parts per billion
in drinking
water.
People
can
be
exposed
to
this concefltration of MTBE
in drinking
water
over a
70
year
lifetime.
Above thi~concentration, appropriate caution
should be exercised
by
the
Public
Water Supply,
such
as increased
frequency of sampling and
identification of the
MTBE
source(s).
The final
health advisory
concentration
is 0.23 rag/I or 230 parts per billion
in drinking water.
Above this
concentratiofl,
the Public
Water Supply should begin actions
to
decrease
the
amount
of MTBE
in
the
system.
REFERENCES
API, American Petroleum Institute.
1993.
Odor Threshold
Studies Performed
with Gasoline
and
Gasoline
Combined with
MTBE,
ETBE,
and
TAME.
API
publication
Number
4592.
Biles,
R.
W.,
Schroeder,
R.
E.,
and
Hold.sworth,
C.
E.
1987.
Methyl
Tertiary
Butyl
Ether
Inhalation in
Rats:
A
Single
Generation
Reproductive Study.
Toxicol.
md.
Health
3:
519-534.
Burleigh-Flayer,
H.
D.,
Chun, I.
S.,
and
Kintigh,
W.
J.
(unpublished).
Methyl Tertiary
Butyl
Ether:
Vapor
Inhalation
Oncogenicity
Study
in CD-i Mice.
Submitted
to
USEPA,
Docket
No.:
OPTS-42098.
Chun,
J.
S.,
Burleigh-Flayer,
H.
D.,
and
Kintigh,
W.
I.
(unpublished).
Methyl
Tertiary
Buryl
Ether:
Vapor
Inhalation
Oncogenicity
Study
in
Fischer
344
Rats.
Submitted
to USEPA,
Docket
No:
OPTS-42098.
Con.away,
C.
C., Schroeder,
R. E.,
and
Snyder,
N.
K.
1985.
Teratology Evaluation of Methyl Tertiary-butyl Ether in
Rats
and
Mice.
3.
Toxicol.
Environ, Health
16:
797-809.
Connecticut
Dept.
of
Environmental
Protection.
(undated).
Action
Level
for
Methyl
Tertiary
Butyl
Ether
(MTBE)
in
Drinking
Water.
Prepared
by
H.
V.
Ran, C.
3.
Dupuy,
and
D.
R.
Brown,
Connecticut
Dept. of Health Services.
Costantini,
M. 0.
1993.
Health
Effects
of
Oxygenated
Fuels.
Environ.
Health
Peispectives
Suppl.
101
(Suppl. 6):
151:.
160.
Dodd,
D.,E.
and Kintigh, W.
J.
1989.
MethyL Tertiary ButyL
Ether (MTBE):
Repeated
(13-Week) Vapor Inhalation Study
in
Rats
with
Neurotoxicity
Evaluation
(unpublished
study).
Union
Carbide,
Bushy
Run
Research
Center
for
MTBE
Committee.
TSCATS
403189.
EPAJOTS
#
FY1-OTS-0889-0689.
Cited
in
Revised
and
Updated
Drinking
Water
Quantification
ofToxicological
Effects
for Methyl Tert-Butyl Ether
(MTBE), Final
Draft.
USEPA;
ECAO-CIN-D023, July,
1993.
Essential
Elements
(in press).
ILSI
Press,
Washington,
D.C.
1994.
-
Greenough,
R.
3.,
McDonald,
P.,
Robinson,
P.,
et
al.
1980.
Methyl
Tertiary-Butyl
Ether
(Driveron)
Three
Month
Inhalation Toxicity in
Rats.
Project No.
413038.
Unpublished
report submitted
to
Cbemische
Werke Hôls AG,
Marl,
West
Germany.
230
p.
Cited
in
Revised
and Updated
Drinking Water
Quantification of Toxicological
Effects
for Methyl
Tert-
Butyl
Ether (MTBE),
Final
Draft.
USEPA;
ECAO-CIN-D023,
July,
1993.
Neeper-Bradley,
T.
L.
1991.
Two-Generation Reproduction Study
of
Lnhaled
Methyl
tert-Butyt
Ether
in
CD
Sprague-
Dawley
Rats
(unpublished
study).
Union
Carbide,
Bushy
Run Research
Center.
Cited
in
Revised
and Updated Drinking
Water
Quantification of
Toxicological Effects
for
Methyl Tert-Butyl
Ether
(MTBE),
Final
Draft.
USEPA;
ECAO-CIN-
D023,
July,
1993.
Ponchon, T.,
Baroud, 3.,
Pujol, B.,
Valette,
P. 3.,
and
Perrot,
D.
1988.
Renal
Failure
during Dissolution of Gallstone by
......Z..~.
...
.....j.
~
............Z
—.
—.
Reese,
E.,
and
Kimbrough,
R.
D.
1993.
Acute Toxicity of Gasoline and
Some
Additives.
Environ.
Health
Perspectives
Suppl.
101
(Suppl.
6):
115-131.
Robinson,
M., Bruner,
R.H.,
and
Olson,
G.R.
1990.
Fourteen
and
Ninety-Day Oral
Toxicity
Studies of Methyl Tertiary-
Butyl
Ether
in Sprague-Dawley Rats.
Journal
of
the
American College of Toxicology
9(5):
525-540.
Enviroflinefl1~12IRegister
No.
484
July,
1994/Page
25
Tyl,
R.
W..
and
Neeper-Bradley,
1.
L.
1989.
Developmental
Toxicity
Study of
Inhaled
Methyl.
Tertiar)T
Butyl
Ether
in
~D-l
Mice.
Project
Report
52-526.
Prepared
by
Bushy
Run
Research
Center,
Union Carbide
Corporation
for the Methyl
fertiary
Butyl
Ether
Committee,
Washington.
D.C.
Microfiche
No.
0TS0000689..1.
Cited
in
Revised
and
Updated
Drinking
Water
Quantification
of
Toxicological Effects
for Methyl Tert-Butyl
Ether (MTBE),
Final Draft.
USEPA;
ECAQ-
-
CIN-D023,
July’,
1993.
USEPA.
1993.
Revised
and Updated
Drinking Water
Quantification of Toxicological
Effects
for Methyl TertButyl
Ether
(MTBE),
Final
Draft.
USEPA;
ECAO-CIN-D023, July,
1993.
von Burg,
R.
1992,
Toxicology
Update.
Methyl Tert-Butyl
Ether.
I.
AppI.
Toxicol.
12:
73-74.
•
State ofillinois
--__ENVIRONMENTAL
PROTECTION AGENCY
~vfaryA.
Gade, Director
2200
Churchill
Road, Springfield, IL 62794-9276
217/785-0830
-
November 4,
1994
G.A. Van Gelder,
DVM,
Ph.D., ABVT
Manager, Toxicology
Health, Safety and Environment
Shell
Oil Company
One Shell
Plaza
P.O.
Box 4320
3ouston,
TX
77210
•Dear Dr. Van Gelder:
•
This letter confirms
the meeting to evaluate comments received regarding the
Illinois Environmental
Protection Agency’s proposed Health Advisory for MTBE
which we discussed over the telephone.
The meeting is scheduled for November
14,
1994,
beginning
at
12:30.
The room
is available until
5:00 PM,
if
necessary.
The meeting will
be held
in Room 031
on Floor
8, James
R. Thompson
Center,
100 W. Randolph,
Chicago,
Illinois,
60601.
I
have enclosed an agenda for the meeting,
a copy of the Health Advisory
Section of the Illinois Groundwater Quality Standards,
and a summary of the
Agency’s opinions on two key issues which have emerged from the comments.
I’m looking forward to
a productive meeting.
Please call
(217/785-0830)
if
you have any further comments or questions.
Sincerely,
T—
Thomas
C. Hornshaw,
Ph.
D.
Manager, Toxicity Assessment Unit
Office of Chemical
Safety
f:\psf\epa8566lmtbe.mtg
Attachment
Printid
on
R!qvlad
Paper
MTBE Meeting Agenda
12:30
-
12:45
12:45
-
1:45
1:45
-
2:00
2:00
-
3:15
3:15
-
3:30
Introductions and Background
Key Issues (LOAEL vs.
NOAEL,
RSC)
Break
Other
Issues (Tase/Odor Threshold,
Uncertainty
Factors, 2-Tier Vs. Single Advisory, Edits
Wrap-up
•
RESPONSES
TOSIGNTF1CA.NT
COM1VIENTS
REGARDING
PROPOSAL FOR
HEALTH
ADVISORY
-
FOR
METHYL
TERTIARY-BUTYL
ETHER
The Illinois
Environmental Protection Agency
(Agency) has received three
cowments in response
to the
Notice of Health
Advisory
for
Methyl
Tertiary-Butyl
Ether
(MTBE),
published
in
the
Illinois
Environmental
Register No.
484,
July,
1994.
The
comments
were received
from
the
American
Petroleum Institute
(API),
the Methyl Tertiary Butyl Ether Task Force
(Task Force),
and
Shell
Oil
Company
(Shell).
The
comments
cover
several
technical
and
typographical
subjects,
the
most
significant
of
which
address
the
Agency’s
determination
of
a
Lowest
Observable Adverse
Effect
Level
(LOAEL)
versus
a
No
Observable
Adverse
Effect
Level
(NOAEL)
and
the uncertainty
factors
which
result from
this
determination,
and
the Agency’s
use of the default value of 20
as
the Relative Source
Contribution
(RSC)
term
versus the use
of an
RSC
derived
from chemical-specific
data in the calculation of the Health Advisory.
The
Agency’s
responses
to these key
issues
are
presented in this paper.
LOAEL
vs. NOAEL
API
and
Shell disagree
with the Agency’s
characterization of the
diarrhea
and
elevated
serum
cholesterol
reported at
the
100
mg/kg
dose
in
the Robinson
et al.
(1990)
study
as
a
LOAEL.
In
reviewing
the
results
of
this
study,
the
Agency determined
that the
authors’
reports
that
“treated
rats
in
all
dose
groups
also
displayed
diarrhea
throughout
the exposure
period”
and
their
findings
that
“females
exposed to
all
dose levels exhibited
significant increases
in
serum
cholesterol”
indicated
that
the
study
had
not
identified
a
No
Observed
Adverse
Effect
Level.
This
determination
is
an
outcome
of
the
evaluation
of the
validity
of
the
candidate
studies
required
by
the
Groundwater
Quality
Standards
regulation
when
animal
studies
must be
used
to
develop
a
Health
Advisory.
This
evaluation
was
discussed
briefly
in
the
July,
1994
Notice,
and will
be
expanded for
explanation of the Agency’s
rationale.
Section
620.
Appendix
A(c)(1)(A)(iii),
which
identifies
the
elements
necessary
for
High
Validity
Studies,
requires:
Data
from
animal
subchronic
studies
with
a
minimum
of
3
dose
levels
and
control,
2
species,
both
sexes,
4
animals
per dose per
sex for non-rodent
species
or
10
animals
per dose per sex for rodent species,
a
duration of at least
5
ofthe
test species’
lifespan,
and
a well-defined NOAEL
(emphasis
added).
The Agency determined that the reports of diarrhea in all animals
and
elevated serum cholesterol
in
females
in
all dose
groups
could
not
be
called
a
“well-defined
NOAEL”
for
purposes
of
establishing
High
Validity
for
this
study.
Thus,
the
lowest
dose
tested,
100
mg/kg,
was
API
and
Shell
have
commented
that the
results
of the
study
should
not
be
interpreted
in
this
manner.
Both claim
that the occurrence of diarrhea
in treated
animals
is
not well-documented
or
described
in
the
Robinson
study,
that
diarrhea is
a
common
observation
in
rats
dosed
with
-
corn
oil,
and
it
is
a questionable
endpoint for extrapolation
to
low-dose lifetime
health
effects.
Both
also
claim
that
the
modest
increases
in
serum
cholesterol
in
the
female
rats
are
not
indicative of a meaningful health effect,
arguing
that the authors’ statistical evaluation incorrectly
attributes
a significant
difference
for the
300
mg/kg
dose,
that
there
is no
compelling evidence
for
a
dose
response,
that
only
the
900
mg/kg
dose
in
males
achieved
values
significantly
different from
controls,
and
that the increases are near
the range of
normal
variability.
Finally,
API argues
that the diarrhea and
elevated serum cholesterol
are
not significant results,
citing
the
authors’ conclusions
that the
study indicated that dose levels below those which induce anesthesia
(1200 mg/kg)
do
not
result in
significant pathophysiological
changes.
The
Agency remains
unconvinced
that
the Robinson
et al.
study
has
identified
a
well-defined
NOAEL.
Regarding
the occurrence of diarrhea,
we have
interpreted
the
authors’
reports of
diarrhea
in
“treated
rats
in
all dose groups”
to
mean
all
groups
receiving doses of MTBE,
but
not
those
receiving
the vehicle
control
(corn
oil).
Thus,
we believe that the diarrhea
is
likely
to be treatment -related,
at least
in females;
this belief is supported
by the findings of the 14-day
study
also
reported
in
this
paper,
in
which
“by
the
third
day
of
dosing,
all
treated
animals
displayed loose stools
which continued throughout
the remainder of the exposure period.
‘
We
have
reviewed
the
National
Toxicology
Program’s
report
on
the
lifetime cancer bioassays
of
gavage
vehicles in
male
Fisher
rats,
which
included corn
oil,
and
find
no
mention of diarrhea
as
an effect of corn oil (NTP,
1994).
Finally,
we have
relied on
the experience of one
of the
Agency’s
Office
of
Chemical
Safety
toxicologists,
who
reports
that,
in
over
8
1/2
years
of
experience
in
an
industrial
toxicology
laboratory,
the
occurrence
of
diarrhea
in
rats
in
conjunction
with corn oil vehicles was very infrequent (Morrow,
1994).
While we cannot rule
out the possibility that
the diarrhea reported by Robinson et al.
was vehicle-related,
we continue
to
believe
that
this
effect was a
result of the MTBE exposure.
Regarding the elevated serum cholesterol findings,
the Agency acknowledges that the statistical
significance
of
the
300
mg/kg
dose
in
female
rats
is
questionable
and
possibly
incorrectly
reported,
and
that
there
is
no
obvious
dose-response
relationship
among
the female treatment
groups even though all but the 300 mg/kg group is significantly greater than controls.
However,
we
maintain
that these results
are
potentially indicative of a
real
effect in
the rats;
it
is possible
(although
unlikely)
that
the effect may
plateau
relatively quickly,
such
that
the
dose-response
relationship
is defined at doses
below those tested in
this
study.
Further,
we again note that the
results
of
the
14-day
study
reported
in
this
paper
also
include
elevated
serum cholesterol
in
females
of most treatment groups.
Regarding the biological significance of the diarrhea and elevated serum cholesterol and whether
these endpoints
are
relevant for
extrapolating to human health risks, the Agency
maintains
that
•
such
effects
are
relevant for
use in
developing the Health Advisory.
While
neither endpoint is
relatively
serious,
diarrhea
can
be• deleterious
to
the
organism
over
time
by
contributing
to
dehydration, electrolyte imbalance, and/orpoor nutritional status,
and
elevated cholesterol, while
not
in useil
a
olologicany serious
ei1~eL,
is
a
CauLioti
IOr
inure
serious
CilectS
over
Li-file.
Willie
the
authors’
concluded
that
dose levels
below
those
which
induce
anesthesia
do
not
result
in
significant pathophysiological changes,
the Agency would be very
uncomfortable
using
a dose
which does
not induce
anesthesia as
the basis
for developing
a Health
Advisory.
We
continue
or
described
in
the
Robinson study,
that
diarrhea
is
a
common
observation
in
rats
dosed
with
corn
oil,
and
it is
a
questionable endpoint
for extrapolation to
low-dose
lifetime health effects.
Both
also
claim
that
the
modest
increases
in
serum
cholesterol
in
the
female
rats
are
not
indicative of a meaningful
health effect,
arguing that the authors’
statistical evaluation
incor~.ectly
attributes
a
significant difference for the
300
mg/kg dose,
that there is no
compelling
evidence
for
a
dose
response,
that
only
the
900
mg/kg
dose
in
males
achieved
values
significantly
different from
controls,
and that the increases are
near the
range
of
normal variability.
Finally,
API argues
that the
diarrhea and
elevated
serum cholesterol
are
not
significant
results,
citing
the
-
authors’
conclusions that the study indicated
that dose levels below
those which induce anesthesia
(1200 mg/kg)
do
not result in
significant pathophysiological
changes.
The
Agency remains
unconvinced
that the
Robinson
et
al.
study
has
identified
a
well-defined
NOAEL.
Regarding
the occurrence of diarrhea,
we have
interpreted
the authors’
reports
of
diarrhea
in
“treated rats
in
all
dose groups”
to
mean
all
groups
receiving doses of MTBE,
but
not
those
receiving
the vehicle
control
(corn
oil).
Thus,
we
believe that the
diarrhea
is
likely
to be treatment
-related, at least in females; this beliefis
supported
by the findings of the 14-day
study
also
reported
in
this
paper,
in
which
“by
the
third
day
of
dosing,
all
treated animals
displayed loose stools
which continued throughout
the remainder of the exposure period.”
We
have
reviewed the National
Toxicology
Program’s
report
on
the
lifetime
cancer bioassays of
gavage vehicles
in
male
Fisher
rats,
which
included corn
oil,
and
find
no
mention of diarrhea
as
an effect of corn
oil (NTP,
1994).
Finally,
we have relied
on
the experience of one of the
Agency’s
Office
of Chemical
Safety
toxicologists,
who
reports
that,
in
over
8
1/2
years
of
experience
in
an
industrial
toxicology
laboratory,
the
occurrence
of
diarrhea
in
rats
in
conjunction with corn oil vehicles was very
infrequent
(Morrow, 1994).
While
we cannot rule
out the possibility
that the diarrhea reported by Robinson et al.
was vehicle-related, we continue
to believe that
this
effect
was
a result of the MTBE
exposure.
Regarding the elevated serum cholesterol findings,
the Agency acknowledges that the statistical
significance
of the
300
mg/kg
dose
in
female
rats
is
questionable
and
possibly
incorrectly
reported,
and
that
there
is
no
obvious
dose-response
relationship
among
the female
treatment
groups even though all but the 300 mg/kg group is significantly
greater than controls.
However,
we
maintain
that these results
are
potentially
indicative of a
real
effect in the
rats; it is possible
(although
unlikely)
that the
effect may
plateau
relatively
quickly,
such
that
the dose-response
relationship
is defined at doses below
those tested in this study.
Further, we again
note that the
results of the
14-thy
study
reported
in
this
paper
also
include
elevated
serum
cholesterol
in
females of most treatment
groups.
Regarding the biological significance of the
diarrhea
and
elevated serum cholesterol
and
whether
these endpoints
are
relevant for extrapolating to human health risks,
the Agency
maintains
that
such
effects are
relevant for use
in developing the Health Advisory.
While neither endpoint is
relatively
serious,
diarrhea
can
be
deleterious
overtime
to
the
organism
by
contributing
to
dehydration, electrolyte imbalance,
and/or poor nutritional status,
and elevated cholesterol, while
not
in
itsett a
oloioglcany serious ertect,
is
a cauuon ror more senous
ertects over
time.
While
the
authors’
concluded
that
dose levels
below
those
which
induce
anesthesia
do
not
result
in
significant
pathophysiological changes,
the Agency
would
be
very uncomfortable
using
a
dose
which does
not
induce
anesthesia
as the basis for
developing
a Health Advisory.
We
continue
to
believe that
the
100
mg/kg
dose,
as
a
LOA.EL,
is
the
most
relevant
value
to
use
in
the
-
development of the Health Advisory.
This
reasoning
plus the relative paucity of data regarding
the ingestion of MTBE,
argues for
the continued
use of the 3000-fold
uncertainty
factor as the
most
appropriate
value
for
the final Health
Advisory.
•
•
•
—
MTBE
RELATIVE
SOURCE
CONTRIBUTION
TERM
The
comments
of
both
API
and
the
Task
Force
addressed
the
Agency’s
use of
the default
value
of 20
as
the Relative
Source
Contribution
(RSC)
term,
which
is
specified
in
Section
620.
Appendix
A(a).
(This
is
also
a
standard
TJSEPA
default
assumption,
used
in
risk
assessments
to
account
for
all
other
exposures
to
a chemical
other
than
direct
ingestion
in
drinking
water,
such
as through the
diet,
ambient air,
the workplace,
and
volatilization
from
the household water supply).
Both
comments
cite
a
tJSEPA
study
(tJSEPA,
1993)
which estimates
the
amount
of
MTBE
exposure
experienced by
the general
public during
activities
other
than
drinking
water,
such
as
working,
outdoor
exercise,
refueling,
driving,
etc.
This
study
is
proposed
to
be
used
as
chemical-specific
data
instead
of the
default
value
to
account
for
exposures to
MTBE other
than
via
direct ingestion of drinking water.
If this
study
is
used
to
define
the
RSC term,
the
range
of
weighted
annual
MTBE
ambient
air
concentrations
of
0.04
-
0.07
mg/rn3
would
result
in
a
RSC
term of approximately
45
-
70
for
drinking
water
exposures.
Depending
on
the final determination of the
RSC
term,
the Health Advisory
(HA)
for
MTBE
would
then
be
in
the
range
of
0.52
-
0.80
rng/l,
instead of the proposed
0.23
mg/l
using
a
20
RSC
term.
While
the
Agency
agrees
with
the
data
presented
in
the
USEPA
study,
it
cannot
agree
that
these
data
fully
account
for
all
other
sources
of MTBE
contributing
to
a
person’s
daily
exposure.
Use
of
only
this
study
to
account
for
inhalation
exposures
does
not
consider
inhalation
exposures which
will
Occur in the home as a result of volatilization of MT.BE
from
the household
water supply during uses
of the supply for purposes
other than
drinking.
Since
the
Agency
is
not aware
of studies
evaluating
such
exposures,
an
evaluation of the
indoor
inhalation pathway
was
undertaken using
data reported for trichloroethylene
(TCE).
• The
transfer
of
volatile
organic
chemicals
(VOCs),
including
TCE,
from
water
to
air
has
been
studied
by
several
investigators
(Andelman,
1985;
McKone,
1987;
McKone
and
Knezovich,
1991).
Of particular
interest
for
this
analysis
are
studies
which
measure
the
transfer of VOCs
during
showering
since this
activity is
likely
to
be
the
greatest
contributor
to
indoor
VOC
exposure due
to
the
temperature,
amount
of water
used,
turbulent
flow,
and
the relatively
small
volume of
air
in
the
bathroom.
Therefore,
the McKone
and
Knezovich
study,
which
measures
the
evolution
of
TCE
into
a
bathroom’s
air
during operation
of
the
~
~
~
~4~l~r.,’t
r’f
ti,’
~
,.-,‘.~
-..e
~c’rt,i~
~
‘.1.,.-.
showering.
This
study
evaluated
the
effects
of
shower
temperature
and
duration
on
the
transfer
efficiency
of TCE from
water
to
air,
concluding
that
the
transfer
efficiency
is
61 ±
9
and
that
inhalation
exposures
in
the shower
could
be
equivalent
to
an
ingestion
exposure
of from 1~4liters per day.
Assuming
that
the
transfer, efficiency of any
VOC
for
which
transfer
efficiency
has
not
been
measured is directly proportional
to that of another VOC having a measured transfer effici~ncy,
the
transfer
efficiency
of MTBE
from
water
to
air
can
be
estimated
from
the
TCE
data
by
comparing
the overall
mass
transfer coefficients
from
water
to
air (K~)for
both
chemicals.
McKone
(1987)
has
shown that K~
can
be
approximated by:
K~
r
2.5
+
RT
~1-’,
where
LD~L
2/3
~3A
2/3
J
DBL
=
diffusion
coefficient
in
water (m2/s),
=
diffusion coefficient
in air
(m2/s),
R
=
universal gas
constant,
0.0624 torr-m3/mol-K,
T
=
temperature,
303K (air temperature in
hot shower),
and
H
=
Henry’s law constant
(torr-m3/mol).
The
diffusion
coefficients
of
TCE
and
MTBE
were
calculated
according
to
methods
recommended
in Lyman
(1982), assuming
a water temperature of 37°C
and an air
temperature
of 30°Cto
be
representative
of hot
shower
conditions.
The
calculated values
for
TCE
and
MTBE for DBL are
1 .094E-09 m2/s
and
9.870E-lO m2/s,
respectively,
and for
DBA are 9.40E-06
m2fs
and
9.28E-06
m2/s,
respectively.
Substituting the calculated
DBL
and
DBA
values
and
Henry’s
law
constants of 6.9 16 torr-m3/mol
for
TCE
and
4.484
torr-m3/mol for MTBE
into the overall
mass transfer coefficient
equation,
•
values
for
K~were calculated ‘to be
4.236E-07
m2/s
and
3.950E-07 m2/s for
TCE
and MTBE,
respectively.
The ratio of the
two
K~values of
0.9325,
when
compared to the
measured TCE
transfer efficiency of
61
,
suggests an
MTBE
transfer efficiency of approximately
56.89.
Once the transfer
efficiency
has
been determined,
an estimate of a resident’s
cumulative
daily
intake
from
showering (CDI~)
can
be calculated for
any
VOC
water
concentration (Cv)
using
reasonable estimates of water use
during showering
andthe volume ofthe shower, plus
standard
USEPA
assumptions
for body
weight
(BW,
70
kg)
and
breathing
rate
(BR,
20
m3/d
=
0.014
m3/min).
For this exercise,
it
is assumed
that the resident’s shower duration (SD)
is
10
min/d,
the shower flow rate
(PR)
is
10
11mm, and
the volume
(V)
of the shower is 2.3
m3.
The CDI3
for
any
C~
is calculated from:
CDI5
=
r
(PR
x
SD
x
Transfer Efficiency)
x
BR
x SD
1
x C~,.
L
VxBW
•
j
After
substituting,
the
CDIS
for
any
C~
becomes:
LL)L~
=
(U.049
111g/d)
x
~
This
shower inhalation
intake
can
be
compared
directly
with
the
daily
ingestion
intake
(CDI1)
of
the
VOC
from
drinking
water
for
the
same C~by
again
employing
standard
USEPA
assumptions
for BW
(as
above)
and
daily
water
intake
(WI,
2.0
lId).
The
CDI1 is
calculated
from:
—
-
-
•
CDt1
=WIxC~,
•
•
-
BW
which becomes
after substitution:
•
CDt1
=
(0.029
lIkg/d) x
C~.
These
two
CDIs are now
directly
comparable
for
any
water concentration of MTBE.
The
ratio
of CDIS to CDI1 is 1.69,
suggesting that the resident’s
daily
showering contributes approximately
169
of the
daily
exposure to MTBE compared
to the exposure due to
ingestion
alone.
This
is
equivalent
to
an
additional
ingestion
intake of (169
x 2.0
lId),
or 3.38
lId.
An evaluation of other non-ingestion household
water uses
(cooking,
toilet use,
washing
dishes
and
clothes,
humidifier, etc.) is not as straightforward as the evaluation of showerexposures due
to greater
variability
in the frequencies ofthe activities/uses.
McKone (1987) estimates that the
ratio of the indoor inhalation
dose to the
drinking
water ingestion
dose for
VOCs
ranges from
1.5
-
6.0
(includes
showering
and all
other
inhalation
exposures).
As
estimated above,
the ratio
for showering
alone
is
1.69 for
MTBE,
which
suggests
that the ratio for all indoor
inhalation
exposures
must be
greater than
1.69.
Assuming
that
the
other indoor
inhalation
exposures
are
at least one-sixth to one-fifth the magnitudeof the shower exposure, it can be assumed that these
exposures’
ratio
to
the
drinking
water
ingestion
exposure
is
at
least
0.31,
or
31
of
the
ingestion exposure.
Thus, these exposures contribute at least the equiva.lentof 0.62
l/d
of direct
ingestion,
and the
total adjusted
intake
due to
in-home
water use for purposes
of a chemical-
specific RSC
should be
at least
(3.38
lId
+
0.62
lid
+
2.0 lId),
or 6.0
lid.
The data from USEPA (1993)
can now be used to calculate the remainder of the resident’s
daily
exposure
to
MTBE.
This
exposure
is
the
result
of
ambient air
exposures
plus
indoor
air
exposures which
are~
due to
an MTBE-contaminated water supply
(i.e.,
exposure to MTBE
which originated from the ambient air and is then inhaled in the residence,
workplace,
and
other
buildings).
These
calculations
have
been completed
using
the
USEPA
data for
a
6-month
oxyfuel season, which predicts 0.04 mg/rn3
and
0.07 mg/rn3 as the
Low
and High
annual
average
MTBE air concentration,
and
the
standard
USEPA assumption for breathing rate as above.
The
CDI
(in mg/d)
resulting from ambient air exposures (CDI~3
can
be -calculated
from:
CDIA
=
BR x
Annual
Average Concentration,
which results in
estimates of 0.8
mg/d
and
1.4
mg/d
for the
Low
and High
annual
averages,
respectively.
itie
rinal
step in
the development
or a cheimcal-specitic
RISC is
to
apportion
the contributions
of the Acceptable Daily Exposure
(ADE)
of 2.3
mg/d of MTBE between
ambient air
and
the
home water supply.
As
calculated from
theUSEPA data,
the ambient air exposures
contribute
between
0.8
mg/d and
1.4
mg/d
of the 2.3
mg/d
ADE.
This
leaves
between
(2.3
mg/d
-
1.4
mg/d or 2.3 -0.8
rng/d),
or 0.9
mg/d to
1.5
mg/d to be
contributed by
the home water supply.
As calculated above,
the equivalent
exposure
intake
value for the water supply is at-least 6.0
l/d.
Distributing
the
0.9
mg/d
to
1.5
mg/d portion of the
ADE
for
home and
water use
into
the
adjusted exposure
value of at
least 6.0
lId, the Health Advisory concentration for MTBE
using
chemical-specific
RSC
data
can be
no
more than 0.15 mg/l
to
0.25
mg/i.
Since the value
for
the Health
Advisory
originally proposed by
the Agency,
0.23
mg/i,
falls
within
this
range,
the
Agency proposes
to
adopt the Health Advisory
as originally
proposed.
REFERENCES
Andelman,
J.B.
1985.
Inhalation
exposure
in
the home
to
volatile
organic contaminants of
drinking
water.
Sci.
Total
Environ.
47:443.
Lyman, W.J.
1982.
Handbook of Chemical Property Estimation Methods.
W.J.
Lyman, W.F.
Reehl,
and D.H.
Rosenblatt,
Eds.
(McGraw-Hill Book Co.,
NY).
McKone, T.E.
1987.
Human exposure to volatile organic compounds in household
tap
water:
the indoor inhalation pathway.
Environ.
Sci.
Technology.
21:1194.
McKone,
T.E.
and
Knezovich,
J.P.
1991.
The
transfer
of trichioroethylene
(TCE)
from
a
shower to
indoor
air:
experimental measurements
and
their implications.
J.
Air Waste
Manage.
Assoc.
40:282.
Morrow,
Leslie D.
1994.
Personal communication.
NTP.
1994.
Comparative
Toxicology
Studies
of Corn
Oil,
Safflower
Oil,
and
Tricaprylin
in Male
F344/N
Rats
as
Vehicles for
Gavage.
National Toxicology
Program,
Technical
Report
Series,
No.
426.
U.S.
Dept.
of
Health
and
Human
Services,
Public
Health
Service,
National
Institutes of Health.
April,
1994.
-
Robinson, M.,
Bruner,
R. H.,
and
Olson,
G.
R.
1990.
Fourteen-
and ninety-day oral
toxicity
studies of Methyl
Tertiary-Butyl
Ether in Sprague-Dawley rats.
3.
Amer.
Coil.
Toxicol.
9:525.
USEPA.
1993.
Assessment of Potential Health
Risks
of Gasoline
Oxygenated
with
Methyl
•
Tertiary
Butyl
Ether
(MTBE).
EPA/600/R-93/206.
Office
of
Research
and
Development.
November,
1993.
P:\psf\epa5566~MTBEHA
ILLINOIS
ENVIRONMENTAL
PROTECTION
AGENCY
1021
NORTH
GRAND
AVENUE
EAST,
P.O.
Box
19276,
SPRINGFIELD,
ILLINOIS
62794-9276
RENEE
CIPRIANO,
DIRECTOR
DATE:
October 2,
2001
TO:
TAC-ON
File
FROM:
Tom Hornshaw/~f~
SUBJECT:
Soil Remediation Objective Recommendation
Methyl
tert-butyl ether
(CAS #1634-40-4)
•
CONFIDENTIAL
The
Toxicity Assessment Unit(TAU) has been asked to recommend cleanup objectives for
methyl tert-
butyl ether (MTBE).
Groundwater
objectives havepreviously been establishedaspresentedin theNotice
of
Health
Advisory for Methyl tertiary-butyl ether,
developed using methodology prescribed in
35
IAC
620.Subpart F,
and
published in the
Environmental
Register,
No. 484,
pages 18-24, July,
1994.
Because
soil remediation objectives for MTBE
are
not listed in 35
IAC Part
742 (TACO),
the determination of
the soil cleanup objectives recommendation was
also referred to the TAU.
Calculation
of the soil
remediation objectives
was
accomplished
through
use of the risk-based
soil
screening level (SSL) equations from 742.Appendix C,Table
A ofTACO. Defaultexposure durations
and
contact rates from 742.Appendix C, Table B ofTACO were used in these calculations.
The results
are
presented in the following table.
Residential Values for
Industrial/Commercial
Construction
Worker
Soil
Component of
the
Soil
Values for Soil
Values for Soil
•
Groundwater Ingestion
Route
Chemical
•
Ingestion
Inhalation
Ingestion
Inhalation
Ingestion
Inhalation
Class
I
Class U
AOL
Name
(mg/kg)
(mg/kg)
(mg/kg)
(mg/kg)
(mg/kg)
(mg/kg)
(mg/kg)
(mg/kg)
Methyl
tert.butyl
ether
780k
g,800b
20~000~
8,800k’
•
2,000k
140
0.32k
0.32
.
=
Calculated value
corresponds
to
a
target
hazard quotient
of 1.0.
b
=
Soil
saturation concentration (Csat).
*
Indicates
that
the
AOL
is less
than
or
equal
to
the
specified
remediacion
objective.
GEORGE
H.
RYAN, GOVERNOR
TACO
equation Si was used
to
calculate
the
soil ingestion
exposure-
route
cleanup
objectives-.
The
inhalation exposure route remediation objectives were calculated
using
equation S4 for
the residential
and industrial/commercial scenarios
and equation
S5
was used for the construction worker.
Equations
S17
and
SiS
were used
to
calculate the
soil component of
the groundwater ingestion exposure
route
objectives.
The saturation
limit
(Csat) for
MTBE was calculated
using
equation S29.
Csat may be
• substituted for the inhalation objective,
iflower,
due to MTBE’s melting point of-109 degrees C.
The
critical
data
inputs
into
the calculations and their
sources
are
summarized below.
CRITICAL
DATA
SUMMARY
parameter
value
source
Chemical/Physical Properties
for Methyl
tert-butyl
ether
boiling
point (°C)
55.2
CHEMFATE Database (June 4,
1998).
Henry’s
Law
Constant (atm-
m3/mole)
5.87E-04
CHEMFATE,
ibid.
•
dimensionless Henry’s
Law
Constant (unitless)
2.41E-02
derived by
“Henry’s
Law Constant
*
41”.
.
•
logP(oct)
1.24
CHEMFATE,
ibid.
Koc
(L/kg)-
•
11.5
•
derived from logP(oct).
melting
point (°C)
•
-109
CHEMFATE,
ibid.
molecular weight
.
83.1
•
~
USEPA.
CHEMDAT8.
Version 1.0.
Office of
Air Quality
Planning and Standards.
Research
Triangle Park,
NC.
solubility
(mg/L)
51,000
CHEMFATE,
ibid.
difflisivity in air
(cm2/s)
0.1024
CHEMDAT8,
ibid.
diffusivity
in
water
(cm2/s)
1.1E-05
CHEMDATS,
ibid.
vapor pressure (mm Hg)
249
CHEMFATE,
ibid.
Toxicology
Values for
Methyl tert-butyl
ether
Class I groundwater
objective (mg/L)
•
0.07
calculated
using
the
35
IAC 620.Subpart F
evaluation methods.
Class
II groundwater
objective
(mg/L)
0.07
Class I groundwater objective with no adjustment
for treatabiity.
CRITICAL
DATA
SUMMARY
parameter
value
source
RfDCI,JO~IIC
(mg/kg-day)
.
1 .OE-02
Developed by the Toxicity
Assessment Unit (TAU)
using procedures
specified in
35
IAC 620 Subpart
F.
RfDchronic
target
~
•
increased
cholesterol
and
diarrhea
TAU,
ibid.
.
R.tl)~ubchronic
(mg/kg-day)
1 .OE-02
RfDChrofljc
adopted as the
R±D~.JbChrOnLC.
RfDsubchronic
target
•
increased
•
cholesterol
and
diarrhea
same as
RfDchroalc.
•
~
(mg/m3)
•
•
3.0
•
Integrated Risk
Information
System
(IRIS), National
Center
for Environmental
Assessment, USEPA,
Accessed via Internet 6/22/98.
•
RfCCZ.JOP.IC
target
•
.
liver
and
kidney
effects,
prostration,
andeye
irritation.
IRIS,
ibid.
•
•
•
•
.
~
RfCrebc~oalc
(mg/m3)
3.0
RfCchropjc
adopted
as the
RfCSUbC~OulC.
fC~.j~c~j0njc
target
~
•
liver
and
kidney
effects,
prostration,
and eye
irritation
same as
RfC~~001~.
.
.
.
-
•
•
TH/mtbetac.wpd
STATE
OF ILLINOIS
)
COUNTY
OF SANGAMON
)
)
PROOF OF SERVICE
I, the undersigned, on oath statethat Ihave served the attached Suntlemental Comments
and Exhibits upon the
person to whom it is directed, by placing
a copy in an envelope
addressed to:
-
Dorothy M.
Gtmn,
Clerk
IL.
Pollution
Control Board
James R.
Thompson Center
100
W. Randolph, Ste
11-500
Chicago, Illinois 60601
Robert Lawley, ChiefLegal Counsel
-
Dept. of
Natural
Resources
524
South
Second
Street
Springfield, Illinois 62701-1787
See Attached Service List
and
mailing it
from
Springfield, Illinois
on
/O
...g~
/
SUBSCRIBED AND SWORN
TO BEFORE ME
Matthew J.
Dunn, Chief
Environmental Bureau
Office
of
the Attorney General
188
W. Randolph,
20th
Floor
Chicago, Illinois 60601
Amy Jackson, Hearing Officer
Illinois
Pollution
Control Board
500
South Second Street
Springfield,
Illinois 62706
with sufficient postage affixed.
)~d~4~th
this~!~day
of
~
Notary Public
•
fl”rrwrrrrr’.’?’..
.
-.-
OFFICIAL
SEAL
BRENDA
BOEHNER
~:
NOTARY
PUBLIC.
STATE
OF
ILuNOIl,L~
+MY COMMISSION
EXPIRES
11-14-2001
t
THIS FILING
IS SUBMITTED ON RECYCLED PAPER
RO0-19
SERVICE LIST
PROPOSED AMENDMENTS TO
TIERED APPROACH
TO CORRECTIVE
ACTION OBJECTIVES
May 21,
2001
C
9
0
0
‘-S
‘-S
c-n
fname
company
loan 0.
Joan C.
Anderson,
Ltd.
Sieve
Admiral Environmental
Services,
inc.
Chris
CIC1
Brin
Environmental Depi. Manager
•
Midwest
Engineeting
Service-s
William 0.
Sidlw, Austin, Brown&Wood
Matthew
1.
Chief, Environmental Bureau
Office
of
the Auorney General
Kimberly
A.
IBM,
Division
of
Legal Counsel
Steven
lOOT
•
Design& Environment
Dorothy
Clerk of the Board
-
lllinpis Pollution Control Board
Douglas F.
Urania, Anlialy. Schloemer &
Associates, Inc.
Sidley, Austin, Brown&
Wood
Address
PMB
#202.
4700 Gilbert Road, Suite47
2025
5. Arlington Heights Road,
Suite
103
9801
Higgins Road,
Suite
515
4243 West
166th Street
Bank One Plaza
10 Souih
DearbornStreet
188 W.
Randolph
Street,
20th
Floor
1021
N. Grand Avenue East
P.O. Box
19276
2300 South Dirksen Parkway
lnan~e
Anderson
Anderson
Bianco
Curley
Dickett
Dunn
Geving
Gobelnian
Gunn
lambley
Heyde,
Esq.
lodge, Esq.
Jackson
Jacobs
Janiison
Keefer
Larson
Lawley
-
Mankowaki
lvlarszalek
citystate
Western
Springs,
1L
Arlington
Heights,
IL
Roseinool, IL
Oak Forest, IL
• Chicago, 1L
Chicago, IL
Springfield, IL
Springfield,
IL
100W.
Randolph St., Suile
11-500
Chicago,
IL
0
a
7
0
C
0
‘a
a
-n
C
a
I-
r
0
En
-u
0
I—
I-
a
-l
0
0
0
-I
0
I-
03
0
0
+
(12
03
a
Co
a
a
03
-I
a
0)
0
0
PS)
S...
0
C
0)
zip
60558
60005-4141
60018
60452
60603
60601
62194-9276
62764
60601
60631-2801
60603
62705.5776
62704
63101
62563
61820
61110-0827
62701
60473
62707
John M.
Katherine
D,
Amy
Richard
George
Don
Jeffrey
Robei-t T.
Bob
Mark
1-lodge,
Dwyer
& Zernan
illinois
Pollution Control
Board
Thompson Coburn
-
Hanson Engineers
Illinois Stale Geological Survey
?vlissnian,
Slanley & Associates
Chief Legal Counsel
Department of Natural
Resources
EPI
Andrews Environmental Engineering
•
8501
W. Higgins Road,
Suite 280
Bank One Plaza
10 South Dearborn
Street
3150 Roland Avenue
P0. Box5776
600
South Second Street,
Suile 402
One Firsistar Plaza
3971
Bison
Trail
615 East Peabody Drive
333 Easi State Sired
P.O. Box4327
524 South Second Street
16650 South Canal Sireet
3535 Mayflower Blvd.
Chicago, IL
Chicago,
LL
Springfield. IL
Springfield,
IL
St. Louis, MO
Rochester,
1L
Champaign,
IL
Rockford,
IL
Springfield, IL
South Holland, IL
Springfield, IL
-U
00
R00-19
SERVICE LIST
PROPOSED
AMENDMENTS TO
TIERED APPROACH
TO CORRECTIVE ACT1ON
OBJECTIVES
May21,
2001
Nienkerk
Peterson
Reott
Richardson
Rinser
Sargis
—Schick——-
Soulter
Steinhnnr
Trivedi
Vanes
Vlahos
Vogel
Walton
Yonkauski
Zolyak
3140
Finley
Road
Downers Grove1
60515
IL
215 East Adams
Springfield,
IL
62701
One
1BM Plaza, 39th Floor
Chicago.
lL
60611
10 South
Dearborn
Chicago,
IL
60603
150
North Michigan,
Suite 2500
Chicago,
IL
19
South
LaSalle Street,
Suite
1203
Chicago,
IL
ruv~
Cnnlh
flirlccen
Pssrlcwav
•
Snrinafield. IL
60601
60603
62764
Monte
Clayton Group Services
Brooke
IERG
Raymond
T.
Jenner & Block
Diane H.
Commonwealth Edison
Environmental Services Department
David L.
Ross
&
1-lardies
Mñrk R.
Mauck
Bellande
& Cited’
—Randy—
•
•
Legal Department
Douglas 0.
Conestoga-Rovers &
Associates
Elizabeth
Weaver, Boos
& Gordon
Chelan
Trivedi
Associates,
lnc,
Rick
DAI EnvironmentaL, Inc.
Georgia
Office of Counsel
-
Niaval Training Center
-
Muselte I-I.
The Stolar
Parinership
Harry
Site-Remediation
Advisory
Comm..
Stan
Department of
Natural
Resources
Gary
Department of Defense
Regional
U.S. Army Env. Cenler
•8615
WeaL
Bryn Mawr
2021
Tirnberbrook Lane
2055
Steeplebrook Court
•
5 Revere Drive,
Suite 310
260
A Paul Jones Si.
Chicago,
IL
Springfield, IL
Naperville, IL
Northbrook, 1L
Great Lakes,
IL
60631
62702
60565
60062
60088
911
Washington
Avenue, 7th Floor
2520 Brooks
Drive
524
South
Second Street
5179
Hoadley
Road, Bldg.
E-4460
St.
Louis,
MO
• Decatur, IL
Springfield,
IL
Aberdeen, MD
63101
62521
62701
21010-5401
0
C-,
0
V
0
0
C
‘a
a
-U
C
Ej
,-
,-
0
in
0
I-
r
a
-I
0
0
-I
,-
03
a
+
03
P.)
03
a
03
a
a
03
-t
a
03
C
03
0
C
03
-U
a
C
—I
C
—I
D
r
-u
0
(A
I-.-
0
‘-S
c-n
-o
C
(4
C
14
2-