BEFORE
THE ILLINOIS
POLLUTION
CONTROL BOARD
IN TIlE MATTER
OF
RCRA DELISTING
ADJUSTED
STANDARD
PETITION
OF PEORIA
DISPOSAL
COMPANY
)
AS 08-10
)
(Adjusted Standard
— Land)
)
(RCRA Delisting)
)
To:
See
attached
Certificate
of Service
PLEASE
TAKE NOTICE that
on this date we filed
with
the Clerk
of the Illinois
Pollution
Control Board
the
attached COMMENTS
OF BAKER & MCKENZIE
LLP,
copies
of
which
are
attached hereto and
herby served upon
you.
Dated: September
12, 2008
• Respectfully
submitted,
fo)
Wats6n
‘aker
& McKenzie
LLP
One Prudential
Plaza,
Suite 3500
130
East Randolph Drive
Chicago,
IL 60601
NOTICE
OF FILING
THIS DOCUMENT
IS
FILED ON RECYCLED
PAPER
CERTIFICATE
OF
SERVICE
The undersigned certifies
that copies
of
the
COMMENTS
OF BAKER & MCKENZIE
LLP
were served
on
this
12
th
day
of
September,
2008.
Upon
the
following
by hand
delivery
(original and
nine copies):
John Therriault
Acting
Clerk of the
Board
Illinois
Pollution
Control Board
100
West Randolph
Street, Suite 11-500
Chicago,
IL 60601
And upon the following
by U.S. First Class
Mail:
Carol
Webb
Hearing
Officer
Illinois
Pollution Control
Board
1021
North Grand
Avenue East
P.O.
Box 19276
Springfield,
IL 62794
William
D.
Ingersoll
Illinois
Environmental
Protection
Agency
1021
North Grand
Avenue East
P.O.
Box
19276
Springfield,
IL 62794
Brian
Meginnes
Janaki Nair
Elias,
Meginnes,
Riffle &
Seghetti, P.C.
416 Main
Street, Suite 1400
Peoria,TL
61602
Claire Manning
Brown,
Hay
& Stephens,
LLP
205 South Fifth
Street,
Suite
700
Springfield,
IL 62701
BEFORE
THE ILLINOIS
POLLUTION
CONTROL
BOARD
IN THE MATTER
OF
)
)
AS 08-10
RCRA
DELISTING
ADJUSTED
)
(Adjusted
Standard
— Land)
STANDARD
PETITION OF
PEORIA
)
(RCRA Delisting)
DISPOSAL
COMPANY
)
COMMENTS
OF
BAKER & MCKENZIE
LLP
Baker & McKenzie
respectfully
submits
the following
comments in
response
to the
above-captioned
Petition
of Peoria
Disposal Company
(“PDC”) for
a RCRA delisting
adjusted
standard (the
“Petition”):
1.
If
PDC’s
Petition
is granted, the
delisting
should
include a
requirement that
re
treated electric
arc furnace
dust stabilized
residue
(“EAFDSR”)
be analyzed
for all
metals
constituents.
The Technical Support
Document
for the RCRA Delisting
Adjusted
Standard
Petition
for
PDC
EAF Dust Stabilized
Residue (hereinafter
“Technical
Support
Document”)
states:
When
analysis
of
samples following additional
chemical
reaction
time does
not
indicate compliance
with the delisting
levels prior to the
storage time
limit, or the
trend
in re-sample
analytical results makes
evident
to PDC that reduced
concentrations
with
additional curing time
are unlikely,
PDC
would
re-process
the
“failed”
EAF dust treatment
residue
in
the same
manner
as is described
above for
untreated EAF dust.
The re-treatment
recipe would
be
determined based
on the
metals
concentrations
and
final
extraction
pH of
the
most recent
resample.
Sample collection of
re-treated EAF dust
treatment residue
would be
identical
to
that
proposed above
for
samples
collected
after
additional
curing time.
Consistent
with
the
existing
IEPA
sampling and analysis
requirements
for WSF
treatment
residues,
re-treated LAFDSR
would
be
analyzedfor
only those constituents
that
did
not
meet the delisting levels
prior to re-treatment.
(Technical Support
Document,
at 3-20
(emphasis
added).)
If
additional chemical
reaction time
or re-processing
is necessary,
all constituents
should
be
reanalyzed
to determine compliance
with
the
delisting
levels,
not just
those
constituents
that
did not meet
the delisting levels prior to re-treatment. Typically,
laboratories
do not analyze
for
individual metals but rather
a suite
of
metals.
As such, no additional burden would
be imposed
by
reanalyzing
for all constituents,
given
that the data is already available.
The proposed
practice raises the question
why would
one
not
report
all
of the data if it is readily available.
The
use
of
additional reaction times and/or the necessity
of
adding
supplementary
reagents subsequent to initial
treatment
efforts indicate that the primary treatment
of
the electric
arc furnace dust has
failed
to attain delisting standards.
This
initial failure indicates that the
expected chemical reaction
mechanisms
responsible for chemical stabilization have not occurred
or
have been significantly retarded.
PDC
may elect to use
cure time
(supplementary reaction time) or additional reagents to
augment the initial
treatment regime to attain delisting standards.
To
verify
compliance
with
the
delisting,
it is being proposed that re-treated residue need only be analyzed for those constituents
that have failed
to meet delisting standards prior to re-treatment. The assumption being made
is
that cure time or
additional reagent enhancements will only have a positive effect
on
the overall
treatment process.
This
is
an incorrect assumption. Supplemental treatment
may also
have
negative
consequences
on
constituents
of
concern that were previously in compliance delisting
standards.
PDC
explains that it commonly operates its treatment process using “reagent proportions
known through
experience to be at the lower end of the effective range for a specific waste type.”
Technical
Support
Document, at 3-19. When considering the complexities associated with
delisting
fourteen distinct metals and differing solubility behaviors, subsequent treatment (cure
and/or
augmentation
using additional additives)
can
create
non-optimal conditions that may
cause
previously stabilized analytes to become leachable; even when starting
from
the “lower
2
end of
the
effective
range.”
In other
words,
cure
and/or
re-treatment
activities
can
create
an
environment
that
is
optimal
for
one
particular
analyte
of interest
while
creating
an environment
that is
deleterious
to
another.
Chemical
reactions
continue
to occur
and
the
final product
is
not
the
same as
the product
that
was
originally
tested.
Therefore,
this necessitates
that
every
constituent
of
concern
must be
reevaluated
when using
cure
and/or
re-treatment
options.
Additional
treatment
activities
(cure
and/or
re-treatment)
represent
an entirely
new
treatment
regime
that is
unproven
and
requires
a complete
assessment
of all
fourteen
metals
to ensure
that
this
new treatment
is effective
for
all constituents
of concern.
2.
If
approved,
the
Petition
should
be required
to demonstrate
that the
EAFDSR
has
a
complete
reaction
mechanism
prior
to landfill.
RMT,
Inc.,
the
consultant
utilized
by PDC
for
assistance
in preparation
of
the
Petition,
has
been
granted
many
patents
for
the stabilization
of
materials
including
EAFD.
While
it
is not
clear
if any
of
the patent
claims
have
been
utilized
in the
demonstration
for this
petition,
of
particular
concern
is
the
absence
of
a
reaction
which
is
acknowledged
in patent
#5,037,479
(attached
hereto
at
Appendix
A) and
other
similar
patents
held
by
RMT,
Inc..
The patent
states
that:
Dry
mixing
of
the agents
with
the solid
waste
does
not
necessarily
cause
the
reactions
which
convert
lead,
cadmium
and
zinc
into substantially
non-leachable
foims.
This
reaction
may
not
occur
until the
mixture
is
analyzed
using
the
Extraction
Procedure
Toxicity
Test
and will
result
in the
material
not being
classqIed
as
EP
toxic.
The reactions
may
also
not
occur until
the
waste
mixture
is
analyzed
using a
water
leach
test,
which
is
designed
to simulate
the leaching
conditions
present
in
an industrial
landfill.
In
the field,
the
reaction
will
probably
occur
slowly
as
the waste
is
wetted.”
(App.
A,
at 12
(emphasis
added).)
Only
when
the TCLP
extract
is analyzed
does
the
waste
sample
undergo
stabilization
such
that
it
passes
the
test for
the regulated
chemicals.
In other
words,
the TCLP
test
itself
3
causes the reaction leading to
stabilization;
the lest results do
not indicate
that the thy
combination ofKO6] waste and additives proposedfor deregulation are stabilized.
While the
Petition
and
the Technical
Support
Document report that water
is used in
the
process,
the following language does not indicate that water is being used to facilitate reactions:
As noted
earlier
in
this Document, the
addition of
treatment chemicals and make
up water, which
facilitates
mixing and will eliminate
the potential
for airborne
waste emissions when the EAFDSR
is
deposited at the landfill face, will increase
the
average bulk density of the
EAF
dust
by
approximately
50%
(Technical Support Document,
at
2-3; see also id. at
3-14 (table summarizing
same).) PDC fails
to
explain if sufficient water is added to provide a medium in
which
the reactions can occur. It is
simply
used to facilitate mixing and eliminate the
potential
for
airborne
waste
emissions.
All
reactions need a
driving mechanism. Most stabilization processes utilized water
as
a medium
in
which the
reactions can occur. Other driving forces are possible, such as the use of heat or
pressure, but
without
further information, it is not possible to know how the EAFDSR is created.
If
the delisting is
granted, it should require that a demonstration be made that the liquid added,
or
whatever mechanism utilized, is
sufficient
to
drive the reactions and produce
a
stabilized
product.
Showing that
this process has occurred in a relatively dry state is critical to prove that
the
proprietary technology
actually stabilized the waste. Otherwise, the petitioner
is
just gaming
the TCLP in a
clever if not inappropriate way in order
to evade the clear
intent
of the regulations.
3.
Finally, if the delisting is approved, to be consistent with delistings granted by
USEPA,
reopener
language similar
to that found in the Heritage Environmental Services, LLC,
at
Nucor Steel
facility in Crawfordsville, TN delisting for K06l (40 CFR 261 Appendix IX Table
2)
should
be incorporated into this delisting.
4
In conclusion,
if
the
Board grants
the
Petition
and approves
PDC’s
requested
delisting,
the
requirements
described
above
should
be
included
in the
approved
delisting.
Respectfully
submitted,
Jo
atson
B
er
& McKenzie
LLP
One
Prudential
Plaza,
Suite 3500
130 East Randolph
Drive
Chicago,
IL
60601
5
Appendix A: Patent #5,037,479
United States Patent
5,037,479
Stanforth
*
August
6, 1991
Method for reduction of heavy metal leaching
from
hazardous
waste under acidic
and
nonacidic conditions
Abstract
A method of treating solid hazardous wastes containing unacceptable levels
of leachable metals
such as lead, cadmium and zinc includes
mixing the solid waste with a buffering agent selected
from
the
group
consisting
of
magnesium
oxide, magnesium hydroxide, reactive calcium
carbonates
and
reactive
magnesium
carbonates, and with an additional agent which
is an acid
or
salt
containing
an anion that forms substantially nonleachable fonns
of
the
metals,
which
additional
agent is selected from the group consisting
of
triple superphosphate,
ammonium
phosphate,
diammonium phosphate, phosphoric acid, boric acid and metallic,
iron so that under
both
acidic and nonacidic leaching conditions the metals in the mixture will be converted
to
substantially nonleachabl e
forms.
Inventors: Stanforth; Robert R.
(Madison,
WI)
Assignee: RMT, Inc. (Madison, WI)
1*1
Notice: The portion of the term of this patent subsequent to December
26, 2006 has been
disclaimed.
Appl.No.:
512641
Filed:
April 20, 1990
Current U.S. Class:
‘
588/318; 75/746; 405/1 29.25; 423/92; 423/1 02;
423/659;
588/257; 588/407;
588/412
Intern’l
Class:
,
BO9B
003/00
Field of
Search:
405/129
423/35,55,305,659,DIG.
20,792,92,102
75/722,586,746
106/735,691 588/257 210/751
References Cited
IReferenced Byl
U.S.
Patent Documents
4356030
Oct.,
1982
Halpin
75/586.
4652381
Mar.,
1987
Inglis
423/35.
4671882
Jun., 1987
Douglas
423/305.
4764284
Aug.,
1988
Jansen
423/35.
4878944
Nov.,
1989
Rolle et al.
75/25.
4889640
Dec.,
1989
Stanforth
210/751.
4950409
Aug.,
1990
Stanforth
405/129.
Other
References
1985,
AFS
Transactions,
“Methods
to Treat EP
Toxic Foundry
Wastes and
Waste
Waters,”
vol.
93,
PP.
737-740.
California
Cast
Metals Association,
Feb.
1989, “Detoxifying
Foundry
Sand,” Report
to
Members.
Nagle,
D.
L.,
R.
R.
Stanforth,
P. E. Duranceau
and
T. P.
Kunes, 1983,
AFS
Transactions,
“Treatment
of
Hazardous
Foundry
Melting
Furnace
Dust
and
Sludges,”
vol.
91,
pp.
71 5-720.
Turpin,
P.
D., T.
R.
Sta]zenburg,
W.
A.
Stephens,
and
T. P.
Kunes.
Primary
Examiner:
Straub; Gary
P.
Assistant
Examiner:
Chen; C.
Robert
Attorney,
Agent
or Firm: Lathrop
&
Clark
Claims
What
is
claimed
is:
1. A method
of
treating
solid hazardous
waste containing
unacceptable
levels
of
leachable
metals
selected from
the group
consisting
of lead
and
cadmium,
the
method comprising
the steps
of
mixing the
solid waste
with
at least
one
first gent
selected from
the
group
consisting
of
magnesium
oxide and
magnesium
hydroxide,
and an
additional
agent
which is triple
superphosphate,
so
that
under both
acidic
and
nonacidic
leaching
conditions,
the
metals in
the
mixture
will
be
converted
to substantially
non-leachable
forms.
2.
The method
of claim
I wherein
the solid
waste and
agents are
mixed
into a mixture
with
sufficient quantities
of
each
of the
agents
so that
a random
100
gram sample
of the
mixture
will
have
sufficient
agents to
limit cadmium
extraction
to
below 1.0
mg!L
and
lead
extraction
to
below 5.0 mg/L
when
analyzed
by
the Extraction
Procedure
Toxicity
Test,
and cadmium
leaching
to below
0.01 mg/L
and lead
leaching
to
below
0.05
mg!L
when
analyzed
with a
water
leach
test.
3. A
non-hazardous
solid waste
mixture
which
may be
safely disposed
of
in a landfill,
comprising
a
solid
hazardous
waste
containing
unacceptable
levels
of
leachable
metals
selected
from
the group
consisting
of lead and
cadmium,
mixed
with at least
one
fist agent selected
from
thegroup
consisting
of
magnesium
oxide and
magnesium
hydroxide,
and
an additional
agent
which
is
triple superphosphate,
in quantities
sufficient
so that
under
both
acidic
and
nonacidic
leaching
conditions,
the
metals
will be converted
to
substantially
nonleachable
forms.
4. The non-hazardous
solid
waste
mixture
of
claim
3 wherein
there
is a sufficient
quantity
of
each
of the agents
incorporated
into
the mixture
so that
when a
random
100 gram
sample
of the
mixture is analyzed
by
the Extraction Procedure
Toxicity Test, cadmium
is
extracted
at a
level
below
1.0
mg/L and lead
is
extracted
at a
level below
5.0 mg/L, and when
a random 100 gram
sample
of
the mixture
is
analyzed
using a
water
leach test, cadmium will leach
at a
level
below
0.01 mgIL and
lead will
leach at a
level
below 0.05 mg/L.
5. A method of treating solid hazardous waste containing
unacceptable levels
of
leachable
metals
selected from the group consisting
of lead and cadmium, the method comprising the
steps of
mixing the solid waste
with
at least one
first
agent selected from the
group
consisting
of
magnesium oxide, magnesium hydroxide, reactive calcium carbonates
and
reactive
magnesium
carbonates, and at least one additional agent selected
from the group consisting of triple
superphosphate, ammonium phosphate,
diammonium phosphate,
phosphoric acid,
boric acid and
metallic
iron so
that under
both acidic and nonacidic leaching conditions,
the metals in the
mixture will be
converted
to substantially non-leachable forms.
6.
The method of claim 5 wherein the
solid
waste and agents
are mixed intQ a mixture with
sufficient
quantities of each
of
the agents
so
that
a
random
100 gram sample of the mixture
will
have
sufficient agents to limit cadmium extraction to below
1.0 mg/L and lead extraction to
below 5.0 mg/L when analyzed
by
the Extraction Procedure Toxicity
Test, and cadmium
leaching to below 0.01 mg/L and lead leaching to below 0.05
mg/L
when
analyzed with a water
leach test.
7. A
non-hazardous solid waste mixture which may
be safely disposed of in a landfill,
comprising a solid hazardous waste containing unacceptable levels
of
leachable
metals
selected
form the group
consisting
of lead and cadmium, mixed with at least one first agent selected
from
the group consisting of magnesium oxide, magnesium
hydroxide,
reactive
calcium carbonates
and
reactive
magnesium
carbonates, and at least one additional agent selected
from
the
group
consisting of triple superphosphate, ammonium phosphate,
diammonium phosphate, phosphoric
acid, boric acid, and metallic iron in quantities sufficient so that under both acidic
and nonacidic
leaching
conditions, the metals will be converted to substantially
nonleachable forms.
8. The
non-hazardous solid waste mixture specified
in
claim
7 wherein there is a sufficient
quantity
of each of the agents incorporated into the mixture
so
that when
a random 100 gram
sample of
the mixture is analyzed
by
the Extraction
Procedure
Toxicity Test, cadmium
is
extracted at a level below 1.0 mg!L and lead is extracted
at
a levelbelow
5.0 mg/L, and when
a
random 100 gram sample of the mixture is analyzed using a water leach test,
cadmium will leach
at a level
below 0.01 mg/L and lead will leach at
a
level
below 0.05 mg/L.
9.
A method of
treating solid hazardous waste
containing unacceptable levels
of
leachable
metals
selected
from the group
consisting
of lead and cadmium, the method comprising
the steps of
mixing
the solid waste with at least one first agent selected
from the group consisting
of
magnesium oxide, magnesium hydroxide, reactive calcium carbonates
and reactive magnesium
carbonates, and at least one additional agent selected
from the group consisting of triple
superphosphate,
ammonium
phosphate,
dianrmonium phosphate, phosphoric acid
and boric acid
so
that under
both acidic and nonacidic leaching
conditions, the metals in the
mixture will be
converted to
substantially
non-leachable forms.
10.
The
method
of claim
9
wherein
the
solid waste
and agents
are
mixed
into
a
mixture with
sufficient
quantities
of
each
of
the
agents
so
that
a random
100 gram sample
of the mixture
will
have
sufficient
agents
to limit
cadmium
extraction
to below
1
.0
mg!L and
lead
extraction
to
below
5.0 mg/L
when analyzed
by
the Extraction
Procedure
Toxicity
Test,
and cadmium
leaching
to below
0.01
mg/L
and lead
leaching
to
below
0.05
mg/L
when
analyzed
with a water
leach
test.
11. A non-hazardous
solid waste
mixture
which
may be safely
disposed
of
in a landfill,
comprising
a
solid
hazardous
waste
containing
unacceptable
levels
of leachable
metals
selected
from
the
group consisting
of
lead
and cadmium,
mixed
with at
least one
first agent
selected
from
the
group
consisting
ofmagnesium
oxide,
magnesium
hydroxide,
reactive
calcium
carbonates
and
reactive
magnesium
carbonates,
and at least
one
additional
agent
selected
from
the group
consisting
of
triple superphosphate,
ammonium
phosphate,
diammonium
phosphate,
phosphoric
acid
and boric
acid
in
quantities sufficient
so
that under
both
acidic
and nonacidic
leaching
conditions,
the
metals
will
be converted
to substantially
nonleachable
forms.
12. The
non-hazardous
solid
waste
mixture
of
claim
11
wherein
there
is
a
sufficient
quantity
of
each
of the agents
incorporated
into
the mixture
so
that when
a
random 100
gram sample
of
the
mixture
is analyzed
utilizing
the
Extraction
Procedure
Toxicity
Test, cadmium
is extracted
at
a
level
below 1.0
mgJL
and
lead
is extracted
at a
level
below 5.0
mg/L,
and
when
a
random 100
gram
sample
of the mixture
is analyzed
using
a
water leach
test,
cadmium
will leach
at a
level
below
0.01
mg/L and
lead
will leach
at a level
below
0.05 mg!L.
13. A
method
of treating
solid
hazardous
waste containing
unacceptable
levels
of
leachable
metals
selected
from the
group consisting
of lead
and cadmium,
the method
comprising
the
steps
of mixing
the
solid
waste
with at
least one
buffering
agent, and
further mixing
it
with at least
one
additional
agent
selected
from the
group
consisting
of triple
superphosphate,
ammonium
phosphate,
diammonium
phosphate,
phosphoric
acid,
boric acid
and metallic
iron
so that under
both
acidic
and
nonacidic leaching
conditions
the metals
in the
mixture will
be converted
to
substantially
nonleachable
forms.
14.
A nonhazardous
solid
waste
mixture
which
may be safely
disposed
of in
a landfill,
comprising
a solid
hazardous
waste
containing
unacceptable
levels
of
leachable
metals
selected
from
the
group
consisting
of lead and
cadmium,
mixed with
at least
one buffering
agent
and
further
mixed
with
at
least
one additional
agent
selected
from the
group
consisting
of triple
superphosphate,
ammonium
phosphate,
diammonium
phosphate,
phosphoric
acid, boric
acid,
and
metallic
iron
in
quantities
sufficient
so hat under
both acidic
and nonacidic
leaching
conditions,
the
metals
will
be
converted
to
substantially
nonleachable
forms.
15.
A
method
of
treating
solid
hazardous
waste
containing
unacceptable
levels
of leachable
metals
selected
from
the
group
consisting
of lead,
cadmium
and
zinc,
the
method
comprising
the
steps of
mixing the
solid
waste
with
at least
one
first
agent selected
from the group
consisting
of
magnesium
oxide,
magnesium
hydroxide,
reactive calcium
carbonates
and reactive
magnesium
carbonates,
and at least
one
additional
agent
selected
from the
group
consisting
of
triple
superphosphate,
ammonium
phosphate,
diammonium
phosphate,
phosphoric
acid,
boric acid
and
metallic
iron
so
that under
both
acidic
and nonacidic
leaching
conditions,
the
metals
in the
mixture
will be
converted
to substantially non-leachable
forms.
16. The method
of claim 15 wherein
the solid
waste
and agents are
mixed, into a mixture
with.
sufficient
quantities of each of the
agents
so
that
a random
100 gram sample
of
the
mixture will
have
sufficient agents to limit
cadmium
extraction to below
1 .0 mg/L and lead
extraction
to
below 5.0 mg/L
when
analyzed by the
Extraction Procedure
Toxicity Test,
and
cadmium
leaching to
below
0.01 mg!L and
lead leaching to
below 0.05 mg/L
when
analyzed
with a water
leach
test.
17. A non-hazardous
solid waste
mixture which may
be
safely disposed
of
in
a
landfill,
comprising
a solid hazardous
waste containing unacceptable
levels
of leachable
metals selected
from
the group consisting
of
lead,
cadmium
and zinc, mixed
with
at least
one first agent selected
from
the
group
consisting
of magnesium
oxide, magnesium
hydroxide,
reactive calcium
carbonates
and
reactive
magnesium
carbonates, and
at least
one
additional agent selected
from
the
group
consisting of
triple superphosphate,
ammonium
phosphate, diammonium
phosphate,
phosphoric
acid,
boric
acid, and metallic
iron
in
quantities
sufficient so that
under
both
acidic
and nonacidic
leaching
conditions,
the
metals will be converted
to substantially
nonleachable
forms.
1
8.
The non-hazardous
solid waste
mixture
of
claim 17 wherein there
is a sufficient
quantity
of
each
of
the agents incorporated
into the mixture
so that
when
a random
100
gram sample of
the
mixture
is analyzed by
the
Extraction Procedure
Toxicity
Test, cadmium is extracted
at a level
below
1.0 mg/L
and lead
is
extracted
at
a level below 5.0
mg/L, and when
a random
100
gram
sample
of
the mixture is
analyzed using
a
water
leach test,
cadmium will leach
at a level below
0.01 mg!L and lead
will
leach at a level
below 0.05 mgIL.
Description
FIELD OF
THE INVENTION
This
invention pertains
generally to the
field
of
treating
solid hazardous waste,
and particularly
to the chemical
treatment
of solid waste
containing
unacceptable
levels
of leachable metals,
such
as lead,
cadmium
and zinc to control leaching
under
both acidic and
nonacidic reaction
conditions.
BACKGROUND
OF THE INVENTION
Safe disposal
of hazardous waste is
an increasing societal
problem. Once
a
hazardous
waste
is
generated, disposal
and treatment of the
waste
is
heavily
regulated
by
the
United
States
Environmental
Protection
Agency
because
of the potentially
serious
consequences
of improper
disposal or
treatment.
Foundries
typically generate
large
quantities
of solid waste which
is collected
as baghouse
dust.
These
wastes
often contain leachable
heavy
metals, such as lead,
cadmium
and
zinc. If the levels
of
leachable
heavy
metals
are
high,
the
wastes
are
classified
as hazardous
wastes
by
U.S.
EPA
or
the
state
regulatory
agencies.
Disposal
of hazardous
wastes
containing
high
levels
of
leachable
heavy
metals,
such
as
lead
or
cadmium,
is
expensive.
In
addition
to the
business
cost of
completing
paperwork
associated
with
the
regulation
of
hazardous
waste,
tipping
fees
are
usually
more
than
$150
per
ton.
Transportation
costs
are also
high
Since
hazardous
waste
must
often
be transported
some
distance
for disposal
in
approved
facilities.
These
costs
will
undoubtedly
increase
with
the
U.S.
EPA
prohibition
on
land
disposal
of
untreated
hazardous
waste
beginning
on
May
8,
1990.
Therefore,
a
method
of
treating
hazardous
waste
to
render
it
nonhazardous
could
result
in
tremendous
cost
savings
for
generators
of
hazardous
waste,
such
as foundries.
Solid
wastes
are
classified
as
hazardous
by
U.S.
EPA
for
a number
of reasons.
Certain
wastes
are
classified
as
hazardous
because
they
contain
chemicals
which
are
listed
by
U.S.
EPA
as
hazardous.
Other
wastes
are
classified
as hazardous
because
of
characteristics
of
the
waste.
These
characteristics
include
ignitability,
corrosivity,
reactivity,
and
Extraction
Procedure
(EP)
Toxicity.
Extraction
Procedure
Toxicity
is determined
using
the
Extraction
Procedure
Toxicity
Test (EP.
Toxicity
Test)
contained
in
40
C.F.R.
Part
261,
Appendix
11,
(1989),
the
disclosure
of which
is
hereby
incorporated
by
reference.
In the
near
future,
the
Toxicity
Characteristic
Leaching
Procedure
(TCLP)
will
replace
the
EP
Toxicity
Test.
The
EP
Toxicity
Test and
TCLP
determine
whether
a solid
waste
has
unacceptable
levels
of hazardous
substances,
such
as heavy
metals,
which
can
be
leached
from
the
waste
by
infiltrating
water.
Wastes
containing
leachable
lead
and
cadmium
are
cuffently
classified
as EP
Toxic
Waste
if
the level
of
cadmium
extracted
in an
EP
Toxicity
test
is
above
1.0
mgIL
or
if the
level
of lead
extracted
is
above
5.0
mg/L.
The
upcoming
ban
on
disposal
of untreated
hazardous
waste
will
require
that
wastes
that
are
hazardous
for
lead
and/or
cadmium
leaching
not
be land
disposed
without
treatment.
Some
states,
notably
Michigan,
also
classify
wastes
which
leach
high
levels
of
zinc
as hazardous.
The
EP
Toxicity
Test
and
TCLP
are
designed
to simulate
a
worst-case
leaching
situation.
These
leaching
conditions
would
typically
be
found
in the
interior
of
an actively
degrading
municipal
landfill.
In such
landfills,
the leaching
medium
is slightly
acidic,
with
a
pH of
about
5.
Additionally,
the
leaching
medium
is
heavily
buffered
by
volatile
organic
acids
(e.g.,
acetic
acid)
produced
by
the
facultative
anaerobic
degradation
of organic
matter.
In
fact, many
industrial
wastes
are
not
disposed
of under
acidic
conditions.
The
actual
leaching
conditions experienced
by
these
wastes
in
an
industrial
landfill
may
be quite
different
from
the
worst-case
situation
simulated
by the
EP
Toxicity
and
TCLP
tests.
In
general,
high
volume
hazardous waste,
such
as
foundry
baghouse
dust,
is
disposed
of
in a
situation
where
it
is
not
exposed
to a
heavily
buffered
acidic
leachate,
but
rather
to
unbuffered
waters
such
as
rain.
Tests
are
available
which
simulate
the
more
typical
disposal
situation
for
hazardous
wastes
such
as
foundry
waste.
These
tests
utilize
a
relatively
unbuffered
solution,
e.g.,
deionized
water,
to
provide
a better
simulation
of
leaching
as
it actually
occurs
in
the
environment.
Examples
of
nonacidic
or
not
heavily
buffered
acidic
leach
tests,
commonly
referred
to as
water
leach
tests,
include
the Indiana Water Leach Test, which
is also called the EP Water
Test (Indiana
Administrative Code
Title
329, Article 2,
Solid Waste Management
Rule
9);
the
U.S. EPA
Multiple
Extraction Procedure
(U.S. EPA, 1986, Test Methods for Evaluating
Solid Waste,
Volume I C,
Method
1320); the American Society
.of
Testing
Materials
Test
(ATSM Standards,
method D3987-85); the American Foundrymans
Society Leach Test (Ham,
R. K., W. C. Boyle
and
T. P.
Kunes, J. Env.
Eng.
Div. Amer.
Soc. Civil Eng., 107 EEL,
pp.
155-170, 1981); and the
University
of
Wisconsin
SLT
Test
(Ham, R. K., M. A. Anderson, R. Stegmann
and
R.
R.
Stanforth,
EPA
600/2-79/109, 1979).
For wastes that are not disposed
of in
acidic
environments, two separate leach
tests need to be
run to determine whether
the
waste
is hazardous according to regulatory
standards, and to
determine whether the waste could pose an actual environmental
risk when exposed
to nonacidic
leachate in a disposal facility. The EP Toxicity
Test
or TCLP test will define
the regulatory
status
of
the waste. A water leach test will
provide an indication of the actual
leaching potential
of the waste in the environment.
It is important to utilize both types
of
tests
because lead and zinc are amphoteric
metals.
Therefore,
at alkaline pH values, lead will
solubilize and may leach from waste
at concentrations
that exceed the drinking water standards (maximum
contaminant
levels) established
under the
Safe
Drinking Water Act. Lead levels may even exceed
the hazardous waste standards
at alkaline
pH values. Because zinc is also an amphoteric metal, it would
be
expected
that zinc could
also
leach
from
waste at alkaline
PH
values,
at levels of environmental concern.
It should be noted
that cadmium is not an amphoteric metal except at extremely
high pH and leaching
of cadmium
from treated
waste
under
nonacidic leaching conditions has
not
been
observed.
These considerations are important because one
method
that
has been used for treating
hazardous
wastes containing leachable metals
is the addition of alkaline materials
to
control
the pH of the
EP Toxicity test so that the metals will not be extracted at
levels above the hazardous waste
standards
when the
waste is
analyzed using the EP Toxicity Test
or TCLP test. A number of
alkaline additives have been used which chemically react with
metals in the waste
and
control
the leaching
test
pH.
(See,
for
example,
Hickock, E. A., and Associates.
1984, “Foundry Waste
Stabilization:
Laboratory Testing and Conceptual Equipment
Design”, Report
to Participating
Foundry
Groups, 47 pages; California Cast Metals Association,
1989, “Detoxifiing Foundry
Sand”,
Report to Members; Nagle,
D.L., R. R. Stanforth, P. E. Duranceau and
T. P. Kunes, 1983,
AFS
Transactions, Vol. 91,
pp.
715-720; Turpin,
P.
D.,
T. R. Stolzenburg, W.
A.
Stephens,
and
T. P.
Kunes,
1985, AFS
Transactions,
Vol. 93,
pp.
737-740; and
U.S. Pat. No. 4,889,640 issued
Dec.
26,
1989 to Stanforth).
Hazardous wastes containing leachable metals that are
treated with alkaline additives
currently
meet regulatory
standards for
land disposal. However, the tests used
for regulatory purposes, the
EP
Toxicity Test and TCLP, do not necessarily simulate
actual environmental conditions.
Consequently,
it is possible to treat a waste
with
chemical
additives to reduce
leaching of metals
such as
lead
and
cadmium in the
EP Toxicity Test and TCLP to render
it nonhazardous from
a
regulatory standpoint,
while
at
the
same time increasing the
pH to the point where lead
is
solubilized when a water leach test is run. For wastes
containing
zinc, it would
also be
expected
that
zinc could leach
at levels
of
environmental
concern. Since the water
leach test simulates
common
disposal conditions
in
the environment,
this
indicates
that it
is
possible
to create
an
environmental
problem
in the
process
of
solving a
regulatory
problem.
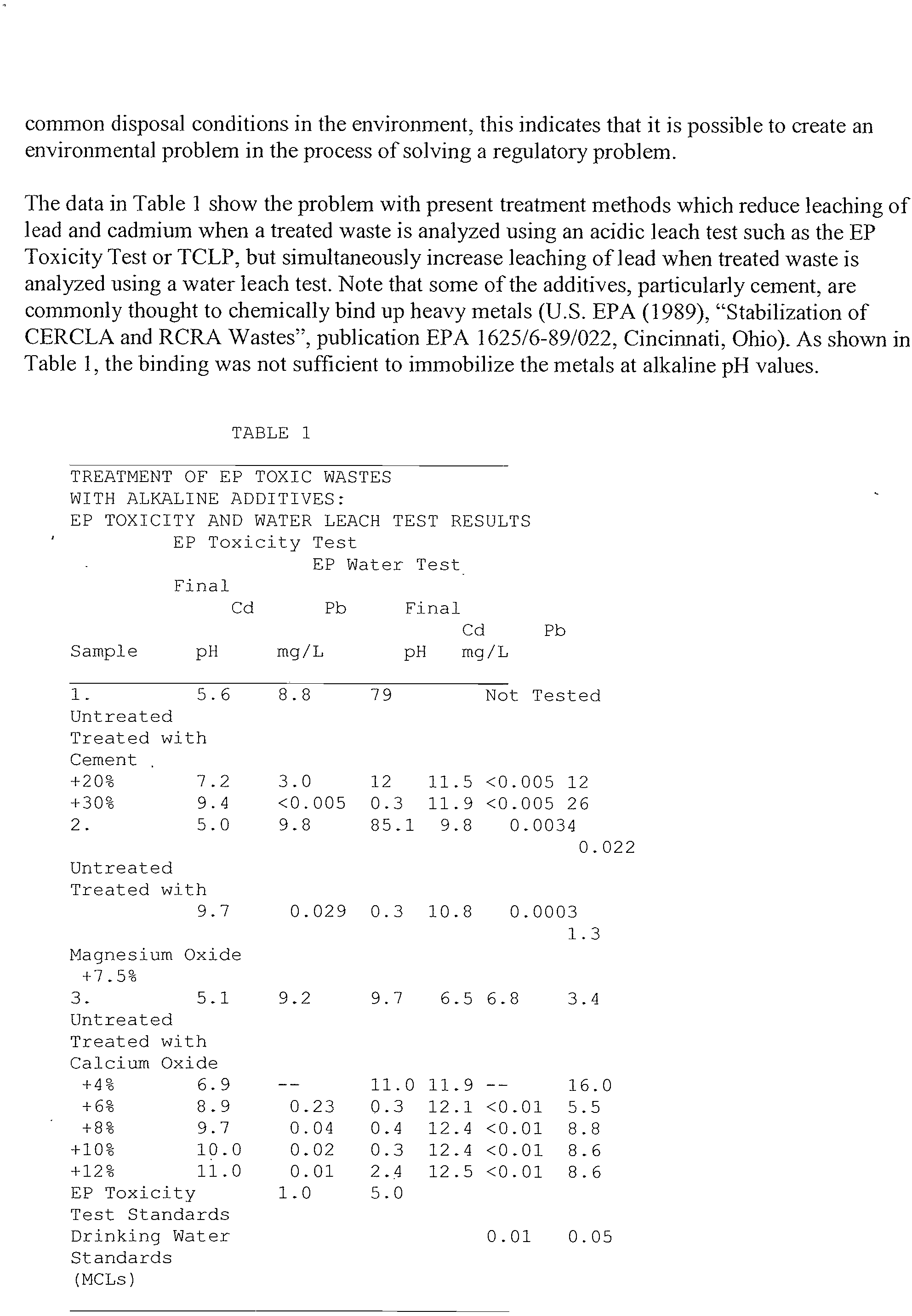
The
data
in Table I
show
the problem
with present
treatment
methods
which reduce
leaching
of
lead
and cadmium
when
a treated
waste
is analyzed
using an
acidic
leach test such
as the
EP
Toxicity
Test or
TCLP,
but simultaneously
increase
leaching
of lead
when treated
waste
is
analyzed
using
a water
leach
test.
Note
that some
of the additives,
particularly
cement,
are
commonly
thought
to
chemically
bind up
heavy
metals
(U.S.
EPA (1989),
“Stabilization
of
CERCLA
and RCRA
Wastes”,
publication
EPA 1625/6-89/022,
Cincinnati,
Ohio). As shown
in
Table 1,
the binding
was
not
sufficient to
immobilize
the
metals at alkaline
pH
values.
Untreated
Treated
with
Magnesium
Oxide
+7.5%
3.
5.1
9.2
Untreated
Treated
with
Calcium Oxide
+4%
6.9
16.0
+6%
8.9
5.5
+8%
9.7
8.8
+10%
10.0
8.6
+12%
11.0
8.6
EP Toxicity
Test Standards
Drinking
Water
Standards
(MCL5)
TABLE
1
TREATMENT
OF
EP TOXIC
WASTES
WITH
ALKALINE
ADDITIVES:
EP TOXICITY
AND
WATER
LEACH TEST
RESULTS
EP
Toxicity
Test
EP Water
Test
Final
Cd
Pb
Final
Cd
Pb
Sample
pH
mg/L
pH
mg/L
1.
5.6
8.8
Not Tested
Untreated
Treated
with
Cement
+20%
7.2
3.0
+30%
9.4
<0.005
2.
5.0
9.8
79
12
0.3
85.1
11.5
11.9
9.8
<0.005
12
<0.005
26
0.0034
9.7
0. 022
0.029
0.3 10.8
0.0003
1.3
9.7
6.5 6.8
3.4
-—
11.0
11.9
0.23
0.3 12.1
0.04
0.4
12.4
0.02
0.3
12.4
0.01
2.4
12.5
1.0
5.0
<0.01
<0.01
<0.01
<0.01
0.01
0.05
There are
single compound additives that can be used to control
leaching in both an EP Toxicity
Test and a
water leach test, that are not covered
by
this invention
disclosure,
for
example,
reactive calcium
carbonate
and
reactive
magnesium
carbonate as disclosed
in U.S.
Pat.
No.
4,889,640, which is incorporated herein by reference. However, a
problem with
reactive
calcium
carbonate is that it is less effective at preventing the leaching
of
lead
and cadmium, if the solid
waste
contains
high
levels
of
zinc, because zinc interferes with
the conversion of lead and
cadmium to
carbonate salts.
SUMMARY OF
THE INVENTION
In
accordance with the present
invention,
a method is disclosed for treating solid hazardous
waste containing unacceptable
levels
of leachable metals such as lead, cadmium
and
zinc,
which
includes the steps of mixing the solid waste with at least two additives, the
first a pH
buffering
agent and an additional agent which is an acid or salt containing an anion that
forms
insoluble
or
non-leachable forms of leachable metals such as lead, cadmium and zinc. The
pH
buffering
agent
is
selected
from
the group consisting of magnesium oxide, magnesium hydroxide, reactive
magnesium carbonate and
reactive calcium carbonate.
The anion contributing agent is selected
from the group consisting of
phosphoric
acid,
ammonium
phosphate,
diammonium phosphate,
boric acid, triple
superphosphate (TSP), other inexpensive
phosphate sources, and
metallic
iron.
The solid
waste and agents are mixed so that under both acidic and nonacidic leaching
conditions,
the metals will be converted to
substantially
non-leachable
forms.
Preferably,
the solid waste and agents are mixed into a mixture with a sufficient quantity of the
agents
so
that any 100
gram
sample of the
mixture has sufficient quantities
of
the
agents
to
limit
cadmium
extraction
to below 1.0 mg!L and lead extraction to below 5.0 mg/L when the sample
is
analyzed in accordance
with the
EP
Toxicity or TCLP Test, and at the
same
time, limit leaching
of lead to
below 0.05 mg!L and cadmium to
below
0.01 rngJL when analyzed with a water leach
test. This
method
would
also
be
expected to control zinc leaching under
both
acid and
nonacidic
conditions,
because like lead, zinc
is
an amphoteric metal.
Thus,
a primary
objective of the invention is to provide a method of treating solid hazardous
wastes containing
unacceptable levels of
leachable
metals
such as lead, cadmium and zinc to
reduce
the leaching of
lead,
cadmium,
and zinc and thereby render
the
waste
non-EP
toxic,
while
at
the same time
reducing lead and zinc leaching under the more typical nonacidic leaching
conditions
present
in the
environment.
A
second objective of the
invention
is
to make the treatment
method disclosed in U.S.
Pat.
No.
4,889,640 more
effective,
by overcoming the problem of zinc interfering with lead and cadmium
reaction
in wastes
which
also
contain zinc.
This
is accomplished
by
combining
the
reactive
calcium
carbonate
or reactive magnesium carbonate
with
an anion contributing agent.
A
third objective of
the invention is to provide a method
of
treating
solid
hazardous waste
containing
unacceptable
levels
of
leachable metals, such
as lead, cadmium and zinc, so
that
the
treatment
effectiveness does not decrease with the age of the treated waste.
A
fourth
objective
of
the
present
invention
is to provide
a method
for avoiding
the
generation
of
hazardous
waste
in
foundries
by
introducing
the
chemical
agents
into
the waste
stream
before
the
baghouse
collector,
or
other
covection
device.
Other objects,
features
and advantages
of the
invention
will
be apparent
from
the following
detailed
description
in which
a preferred
embodiment
of
the invention
has
been
selected
for
exemplification.
DESCRIPTION
OF
THE
PREFERRED
EMBODiMENT
The
present
invention
is
a method
of
treating
solid
hazardous
wastes
to
control
leaching
of lead,
cadmium
and zinc
under
both
acidic and
nonacidic
leaching
conditions.
The process
consists
of
mixing
the solid
waste
with
two
agents,
one
a
pH
buffer
and
the
other
an acid
or salt
containing
an anion
that
forms insoluble
or non-leachable
forms
of
the metals.
The
pH
buffering
agent
is
selected
from
the group
consisting
of magnesium
oxide,
magnesium
hydroxide,
reactive
magnesium
carbonate
and
reactive
calcium
carbonate.
The
term
“reactive”
as used
herein
means
a
form of
carbonate
compound
which
will
both
neutralize
the acetic
acid
in
the
EP
Toxicity
Test,
and
under
test
conditions
and
environmental
conditions
that
support
reaction
between
the
agents
and
metals
will
react
with the
lead,
cadmium
and zinc
in the
solid
waste.
This
reaction
reduces
the
leachability
of the
metals
by
converting
them into
substantially
non-leachable
forms,
most
likely carbonate
salts.
Conditions
which
support
reaction
between
the carbonate
compound
and
metals
include
the
presence
of
water
or
acetic acid.
Other
solvents
may
also
support
such reaction.
The term
“substantially
non-leachable
forms”
as used
herein
means
for a
form
of lead
which
will
not
leach
at above
5 mg/L
or a
form of
cadmium
which
will
not leach
at above
I mg/L
when
the
mixture
of waste
and agents
is
analyzed
using
the EP
Toxicity
Test.
The term
“EP
Toxicity
Test”
as
used
herein
includes
the
Extraction
Procedure
Toxicity
Test,
Toxicity
Characteristic
Leaching
Procedure
(TCLP)
and other
acidic
leach
tests.
The
term
“water
leach
test”
as
used herein
includes
the EP
Water Test,
U.S.
EPA
Multiple
Extraction
Procedure,
American
Society
of
Testing
Materials
Test,
American
Foundryrnen’s
Society
Leach
Test,
the University
of
Wisconsin
SLT
Test,
and other
nonacidic
water
leach
tests.
Sources
of reactive
calcium
and/or
magnesium
carbonate
include
(1)
water
softening
plant
sludge
(lime
sludge)
which
usually
includes
a
mixture
of calcium
carbonate,
magnesium
carbonate
and
calcium
magnesium
carbonate;
(2)
calcium
carbonate
which
is formed
in
a
solution
and
precipitated
out; and
(3) some
industrial
sludges
containing
calcium
carbonate
produced
by
water
treatment
processes,
e.g.
some
paper
mill
lime
sludges.
The
anion
contributing
agent
is
selected
from
the group
consisting
of
phosphoric
acid,
ammonium
phosphate,
diammonium
phosphate,
boric
acid,
triple
superphosphate
(TSP),
other
inexpensive
phosphate
sources,
and
metallic
iron.
In
the preferred embodiment,
solid waste
which
is
classified
as hazardous
due to
high
levels
of
leachable lead or
cadmium
is
treated
with a
combination
of magnesium
oxide
as a pH
buffer and
triple superphosphate (TSP) as an anion contributor. An appropriate mixture of these agents and
the solid waste will control leaching of the metals under both acidic and nonacidic conditions.
By
way
of
example, the treatment
of
wastes with
TSP
alone,
magnesium oxide
alone, and a
combination
of TSP and magnesium oxide is
shown
in Table 2. Treatment of
the waste with
TSP
alone was not successful in limiting cadmium leaching to below the hazardous waste standard of
1.0
mg/L
when analyzed using the EP Toxicity Test. Likewise, treatment
of
the waste with
magnesium
oxide alone limited leaching
of cadmium
and
lead from
the waste when analyzed
using the EP Toxicity Test;
however,
the treated waste still leached levels of lead above the
drinking water standard of 0.05 mg/L when analyzed using a water leach test. Treating the waste
with
a
combination of magnesium oxide and TSP resulted in a treated wastethat had low
leaching potential when analyzed with both the
EP
Toxicity Test and a water leach test. As
shown
in
Table 2, with the combined treatment, the concentration
of
lead and cadmium leached
from
the waste were below the
U.S.
EPA proposed treatment standards of 0.51 mg/L for lead and
0.14
mg/L
for cadmium when analyzed using the EP Toxicity Test, and concentration of both
metals were below the drinking water standards, or maximum contaminant levels of 0.05 mg/L
for lead and 0.01 mg!L for cadmium when analyzed
with
a water leach test. The other buffering
agents and anion contributing agents listed
above
could be utilized and would be
expected
to
react with
the lead, cadmium and zinc to
form
salts which would be substantially insoluble under
both acidic and nonacidic leaching conditions. Metallic iron could also be used as the anion
contributor.
However,
it
is
likely that metallic
iron
would
reduce
lead and cadmium
leaching
through
chemical reduction,
rather
than
by
the formation of insoluble salts.
It should be
noted that it would be expected that treatment
of
waste containing zinc with the
method of the present
invention
would control the leaching of
zinc
under both acidic and
nonacidic
reaction conditions.
TABLE 2
TREATMENT OF EP TOXIC
WASTES WITH
MAGNESIUM
OXIDE AND TRIPLE SUPERPHOSPHATE:
EP TOXICITY
AND WATER LEACH TEST RESULTS
EP
(or
ASTM)
EP Toxicity Test
Water
Test
Final
Cd
Pb
Final
Cd
Pb
pH
mg/L
pH
mg/L
Sample 1
Untreated
4.9
13
140
7.1 7.1
3.5
+7.5% MgO 9.6
0.12
0.6
10.4 <0.005
1.1
Sample 2
Untreated 5.0
7.0
100
8.7
0.031
0.10
25%
I’4g0
Sample
3
Untreated
5.3
0.59
260
8.6
<0.005
10.7
<0.005
0.250
+5%
MgO
&
9.2
<0.005
<0.1
10.4
<0.005
5% TSP
Standards
In
practicing
the invention,
the
mixing
of
the
solid
waste
with
the
pH
buffering
agent
and
anion
contributing
agent
must
be
sufficiently
complete
so
that
a
random
small
sample
(e.g. 100
grams)
of
the waste
mixture
will
have
sufficient
agents
to limit
cadmium
extraction
to below
1.0
mg/L
and
lead
extraction
to
below
5.0
mg/L
when
the
waste
mixture
is analyzed
using
the
Extraction
Procedure Toxicity
Test,
and
to limit
cadmium
leaching
to below
0.01
mg/L
and
lead leaching
to
below
0.05
mg/L
when
the
waste
mixture
is analyzed
using
a
water
leach
test.
For
a specific
solid
waste,
the
appropriate
ratio
of
agents
to
solid
waste
may
be arrived
at
by
experimentation
on
100
gram
samples
of the
waste
having
various
percentages
of the
agents
by
weight.
The
appropriate
ratios
of
agents
to
waste
would
be
ratios
which
result
in
the leaching
of
lead
and
cadmium
at levels
significantly
below
the
hazardous
waste
standards
when
analyzed
using
the
EP
Toxicity
Test
and
leaching
of
lead
and
cadmium
at levels
significantly
below
the
drinking
water
standards
when
analyzed
using
a water
leach
test.
The
appropriate
ratios
will
vary
with
the
concentration
of
leachable
heavy
metals
in
the waste.
Dry
mixing
of
the
agents
with
the
solid
waste
does
not necessarily
cause
the reactions
which
convert
the
lead,
cadmium
and
zinc
into substantially
non-leachable
forms.
This
reaction
may
not
occur
until
the
waste
mixture
is analyzed
using
the
Extraction
Procedure
Toxicity
Test
and
will
result
in the
material
not
being
classified
as
EP
toxic.
The
reactions
may
also not
occur
until
the
waste
mixture is
analyzed
using
a
water
leach
test,
which
is
designed
to simulate
the leaching
conditions
present
in an
industrial
landfill.
In
the
field,
the
reaction
will
probably
occur
slowly
as
the
waste
is
wetted.
The
pH
buffering
agent
and
anion
contributing
agent
may
be
mixed
with
the
solid
waste
in
a
foundry
either
before
or
after
the
dust
is
collected
form
the
waste
gas
stream.
Both
points
of
+5%
TSP
5.0
7.0
16
+10%
TSP
5.0
4.7
1.1
+15%
TSP
5.0
2.5
0.4
+20%
TSP
5.1
1.1
<0.1
+5%
MgO
6.1
6.3
6.4
+10%
MgO
7.4
3.5
3.2
+15%
MgO
9.2
0.044
0.2
10.3
<0.005
+20%
MgO
9.3
0.033
0.3
+5%
TSP
&
7.7
0.24
0.1
20%
MgO
+5%
TSP
& 8.5
0.053
<0.1
10.3
<0.005
+5%
MgO
Not
Tested
0.075
0.014
.033
<0.
003
1.0
0.01
0.05
5.0
addition
have
been
used
in
the past
for addition
of
materials
to foundry
wastes.
For
example,
injection
of
materials,
commonly
lime, into
foundry
ductwork
has
been
used
for
years
as
a
method
of
coating
baghouses
with
materials
to protect
against
sparks
and
improve
dust
collecting.
Such
mixing
may
be
accomplished
by
injection
of
the
pH
buffering
agent
and
anion
contributing
agent
into
foundry
ductwork
before
the baghouse.
This
is
a
preferred
application,
because
treatment
in
the
ductwork
prior
to dust
collection
may
eliminate
the
generation
of a
hazardous
waste
because
such
treatment
is considered
totally
enclosed
treatment
by
the
U.S.
EPA
and
does
not require
permitting.
Because
generators
of hazardous
waste
are subject
to
regulation,
it
is
an ad-vantage
to
avoid
generating
hazardous
waste
in
the first
place.
The
two
agents
used
in the preferred
embodiment
of
the invention,
magnesium
oxide
and triple
superphosphate,
are
both
dry
materials
that
can
be injected
into
ductwork.
Triple
superphosphate,
however,
would
need
to
be pulverized
before
being
injected.
It
is understood
that the
invention
is
not confined
to the
particular
process
disclosed
herein,
nor
to
the
materials
and
substances
described,
but embraces
such
modified
forms
thereof
as
come
within
the
scope
of
the
following
claims.